Abstract
The effect of caffeine and adenine nucleotides on the sarcoplasmic reticulum (SR) Ca2+ release mechanism was investigated in permeabilized frog skeletal muscle fibres. Caffeine was rapidly applied and the resulting release of Ca2+ from the SR detected using fura-2 fluorescence. Decreasing the [ATP] from 5 to 0.1 mm reduced the caffeine-induced Ca2+ transient by 89 ± 1.4 % (mean ± s.e.m., n = 16), while SR Ca2+ uptake was unaffected.
The dependence of caffeine-induced Ca2+ release on cytosolic [ATP] was used to study the relative ability of other structurally related compounds to substitute for, or compete with, ATP at the adenine nucleotide binding site. It was found that AMP, ADP and the non-hydrolysable analogue adenylyl imidodiphosphate (AMP-PNP) partially substituted for ATP, although none was as potent in facilitating the Ca2+-releasing action of caffeine.
Adenosine reversibly inhibited caffeine-induced Ca2+ release, without affecting SR Ca2+ uptake. Five millimolar adenosine markedly reduced the amplitude of the caffeine-induced Ca2+ transient by 64 ± 4 % (mean ± s.e.m., n = 11). The degree of inhibition was dependent upon the cytosolic [ATP], suggesting that adenosine may act as a competitive antagonist at the adenine nucleotide binding site.
These data show that (i) the sensitivity of the in situ SR Ca2+ channel to caffeine activation is strongly dependent upon the cytosolic [ATP], (ii) the number of phosphates attached to the 5′ carbon of the ribose ring influences the efficacy of the ligand, and (iii) removal of a single phosphate group transforms AMP from a partial agonist, to adenosine, which acts as a competitive antagonist under these conditions.
Our current understanding of the mechanisms underlying physiological and pharmacological regulation of the sarcoplasmic reticulum (SR) Ca2+ channel is mostly derived from experiments on membrane vesicles or purified channels reconstituted into planar lipid bilayers (for reviews see Coronado et al. 1994; Sitsapesan et al. 1995; Franzini-Armstrong & Protasi, 1997). It has been shown that a rise in [Ca2+] at the cytosolic face of the channel increases the open probability (Po) by increasing the frequency of opening without affecting the duration (Smith et al. 1986). Millimolar levels of caffeine and adenine nucleotides also activate the SR Ca2+ channel, although the underlying mechanisms differ. In skeletal muscle, physiological levels of ATP increase Po in the absence or presence of Ca2+ (Smith et al. 1986; Rousseau et al. 1988). This effect of ATP is shared by other adenine nucleotides and is characterized by increases in both the frequency and duration of channel openings. In contrast, low levels of caffeine (< 2 mm) appear to sensitize the channel to cytosolic Ca2+. Increases in Po occur only in the presence of Ca2+ and the frequency of channel openings increases without change in the mean open lifetime. Higher concentrations of caffeine induce a Ca2+-independent activation of the channel, with increases in both the frequency and duration of channel openings (Rousseau et al. 1988; McGarry & Williams, 1994; Sitsapesan & Williams, 1995). These differences in activation characteristics, combined with data from competitive binding studies, have led to the conclusion that caffeine and adenine nucleotides act at distinct sites on the SR Ca2+ channel (Rousseau et al. 1988; McGarry & Williams, 1994).
A limited amount of evidence also suggests that the sensitivity of the SR Ca2+ channel to activation by caffeine may depend upon occupancy of the adenine nucleotide site (Rousseau et al. 1988). Such an effect might have important consequences in studies involving skinned or intact muscle preparations, where caffeine is widely used to assess the Ca2+ content of the SR or to investigate other aspects of the excitation-contraction process. However, studies on isolated channels or membrane vesicles are often conducted under ionic conditions that differ markedly from the in vivo state. Furthermore, recent work suggests that the activity of the SR Ca2+ channel is influenced by a number of associated proteins including FK506 binding protein (Mayrleitner et al. 1994; Brillantes et al. 1994), triadin (Brandt et al. 1992) and calsequestrin (Ikemoto et al. 1989). Purification of SR Ca2+ channels or membrane fractions results in loss of these proteins to varying degrees, and this has been linked to alterations in channel gating characteristics, or the sensitivity to physiological or pharmacological activators (Mayrleitner et al. 1994; Brillantes et al. 1994; Wagenknecht et al. 1996; Franzini-Armstrong & Protasi, 1997). It is not clear, therefore, to what extent data obtained on isolated Ca2+ channels or SR vesicles can be applied quantitatively to more complete systems such as skinned fibres or intact muscle. Indeed, a recent study on skinned skeletal muscle fibres found that the Ca2+-releasing action of caffeine was largely independent of the cytosolic [ATP] (Stienen et al. 1993).
In the present study, we have investigated the effects of caffeine and adenine nucleotides on the SR Ca2+-release process in saponin-permeabilized skeletal muscle fibres. In this preparation, the SR remains in situ and the ionic conditions closely match those in intact fibres. Caffeine was rapidly applied and the resulting release of Ca2+ from the SR detected using fura-2 fluorescence. It was found that decreasing the [ATP] from 5 to 0.1 mm reduced the amplitude of the caffeine-induced Ca2+ transient by almost 90 %, without affecting SR Ca2+ uptake. The ATP dependence of caffeine-induced Ca2+ release was then used to study the relative efficacy of other structurally related compounds to substitute for, or compete with, ATP at the adenine nucleotide binding site. It was found that the non-hydrolysable analogue adenylyl imidodiphosphate (AMP-PNP), ADP and AMP partially substituted for ATP, although none was as effective in facilitating the Ca2+-releasing action of caffeine. However, adenosine induced a concentration-dependent inhibition of the caffeine-induced Ca2+ release which was dependent on the cytosolic [ATP]. These results are discussed in relation to previous studies addressing the effects of adenine nucleotides and caffeine on the SR Ca2+ channel. Possible difficulties associated with the use of caffeine to assess SR Ca2+ content are also considered.
METHODS
Preparation
Frogs (Rana temporaria) were killed by stunning and decerebration. The sartorius muscle was removed rapidly and placed in ‘relaxing’ solution approximating to the intracellular milieu (see below). Small muscle fibre bundles (100–150 μm diameter) were then attached between an isometric tension transducer (SensoNor, Norway) and a fixed support using monofilament snares (diameter 30 μm) within stainless steel tubes (inside diameter 100 μm; Goodfellow Metals, Cambridge, UK). Small bundles of muscle fibres were used because it was found that the perturbation associated with rapid caffeine application produced deterioration in single fibres. Preparations were permeabilized by exposure to an ATP-containing solution with 10 μg ml−1 saponin for 10–15 min. Frog skeletal muscle fibres contain roughly equal amounts of RyR1 and RyR3 (α and β) isoforms of the ryanodine receptor (Franzini-Armstrong & Protasi, 1997). We assume, therefore, that the results of this study reflect the average behaviour of the α- and β-isoforms of the ryanodine receptor in the in situ state, under conditions commonly used in skinned muscle studies.
Apparatus
The apparatus for simultaneous measurement of tension and SR Ca2+ release has been described previously (Duke & Steele, 1998a). Briefly, the mounted preparation was lowered within 5 μm of the bottom of a shallow bath with a coverslip base. A Perspex column (5 mm diameter) was lowered close to the surface of the muscle to minimize the volume of the solution above the preparation. Throughout the experimental protocols, preparations were perfused at 0.8 ml min−1 via a narrow duct (200 μm diameter) passing through the centre of the column. Waste solution was collected continuously at the column edge. The volume of solution between the coverslip and the base of the column (i.e. the effective bath volume) was approximately 6 μl. The basic perfusing solution was changed using a series of valves positioned above the column. Using this method, the solution within the bath could be exchanged within 10–15 s. The comparatively slow solution exchange reflects the mixing of solutions in the tubing between the valves and the column. Solutions containing caffeine were rapidly applied (20 ml min−1) for 2 s duration via a narrow plastic tube connected to the injection duct at the column base. The higher flow rate and the smaller dead space allowed a more rapid exchange of solutions within the bath. Previous measurements based on quench of indo-1 fluorescence by caffeine (O'Neill et al. 1990) under similar conditions have shown that the caffeine concentration within the bath typically increased to 50 % of the concentration injected within 8–10 ms (Smith & Steele, 1993).
The bath was placed on the stage of an S200 Nikon Diaphot inverted microscope. The muscle was viewed via a × 20 Fluor objective (Nikon CF Fluor, NA 0.75) and the length was increased to approximately 20 % above slack length. However, in control experiments, we have found that length does not have a direct effect on SR Ca2+ release. The preparation was alternately illuminated with light of wavelengths 340 nm and 380 nm at 50 Hz frequency using a spinning wheel spectrophotometer (Cairn Research, Faversham, Kent, UK). The average [Ca2+] within the visual field containing the preparation was indicated by the ratio of light intensities emitted at > 500 nm. Light emitted from areas of the field not occupied by the muscle image was reduced using a variable rectangular diaphragm on the side port of the microscope.
Solution composition and Ca2+ detection
All chemicals were purchased from Sigma unless otherwise stated. In all experiments, the ionic composition of the solution was adjusted to maintain the [Ca2+], [Mg2+], [Na+], [K+] and pH constant. In brief, for most experiments a basic solution was prepared containing KCl (60 mm), Hepes (25 mm), EGTA (0.2 mm) phosphocreatine (10 mm) and fura-2 (5 μm). MgCl2 and CaCl2 were added (from 1 m BDH stock) to produce free concentrations of 1.4 mm and 100 nm respectively. This solution was then split and ATP or other adenine nucleotides added to each fraction. The amount of additional Mg2+ added to each solution to compensate for the variable amount of binding to adenine nucleotides was calculated using a computer program and binding constants as previously described (Fabiato & Fabiato, 1979; Miller & Smith, 1984). Corrections for ionic strength, details of pH measurement, allowance for EGTA purity and the principles of the calculations are described in Fabiato & Fabiato (1979). In some experiments, the Mg2+ levels were also checked using Mg fura-2. As some nucleotides were added as disodium salts, an equivalent amount of NaCl was added to other solutions to maintain a constant level of Na+. The pH was adjusted to 7.0 by addition of KOH. Following this procedure, the total concentration of K+ and Na+ was 40 and 90 mm, respectively. The Cl− concentration varied between 76 and 108 mm. In control experiments, it was found that differences in [Cl−] within this range had no apparent effect on SR function or fura-2 fluorescence. In some experiments, azide (5 mm) was added to inhibit possible mitochondrial activity. However, 5 mm azide had no apparent effect on the results obtained. In some experiments involving AMP or ADP, the myokinase inhibitor 0.1 mm diadenosine pentaphosphate was also included. However, there was no significant difference between results obtained in the absence or presence of diadenosine pentaphosphate. All experiments were done at room temperature (22–24°C). Details of the experimental set-up and methods relating to Ca2+ measurement in skinned skeletal muscle preparations have been described elsewhere (Duke & Steele, 1998a).
Data recording and analysis
In all experiments, the ratio and individual wavelength intensities and the isometric tension signals were low-pass filtered (−3 dB at 30 Hz) and digitized for later analysis using an IBM-compatible 80486 computer with a Data Translation 2801A card.
RESULTS
Protocol to assess the effects of ATP on caffeine-induced Ca2+ release
Figure 1 shows simultaneous recordings of the 340 nm/380 nm fluorescence ratio (lower panel) and isometric tension (upper panel) from a saponin-permeabilized fibre bundle. In this experiment the preparation was perfused constantly with solutions containing 100 nm Ca2+, and caffeine (20 mm) was briefly applied for 1.5 s at 2 min intervals. Each caffeine application resulted in a transient increase in the fluorescence ratio due to SR Ca2+ release, and an associated tension response. Application of caffeine for 1.5 s was sufficient to produce responses of maximal amplitude under control conditions, i.e. a more prolonged application did not increase the amplitude of the Ca2+ or tension transients. We have shown previously that (i) the descending phase of the Ca2+ transient following brief caffeine application is dependent upon re-accumulation of Ca2+ by the SR and (ii) the rate of rise of the Ca2+ transient appears to be limited by the inward diffusion of caffeine (Duke & Steele, 1998a). Hence, the rate of decline of the Ca2+ transient can be used to assess changes in net Ca2+ uptake (see below), while the amplitude can be used as an index of Ca2+ release.
Figure 1. Steady-state caffeine-induced responses at 2 and 4 min loading periods.
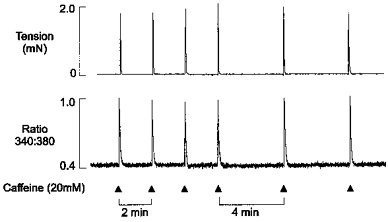
Simultaneous records of the 340 nm/380 nm fluorescence ratio (lower panel) and isometric tension (upper panel) from a saponin-permeabilized muscle fibre bundle in the presence of 100 nm bathing Ca2+. Caffeine (20 mm) was briefly applied (for 1.5 s) at 2 min intervals as indicated. Each application resulted in a transient increase in the fluorescence ratio and a corresponding tension response. After 4 control responses, the time between caffeine applications was increased from 2 to 4 min.
Increasing the Ca2+ loading period (i.e. the interval between caffeine applications) from 2 to 4 min had no effect on the amplitude of the Ca2+ and tension transients. This shows that at a bathing [Ca2+] of 100 nm, the SR Ca2+ content reaches a steady state within 2 min under these conditions. Therefore, in subsequent experiments, caffeine was applied at 4 min intervals and the level of adenine nucleotides (or related compounds) altered 2 min after a caffeine application. In most circumstances (see below), this procedure ensured that caffeine-induced Ca2+ release was triggered at the same SR Ca2+ content during each intervention.
Effects of cytosolic ATP on caffeine-induced Ca2+ release
Figure 2A shows simultaneous records of isometric tension (upper panel), the 340 nm/380 nm fluorescence ratio (middle panel) and the individual 340 nm and 380 nm fluorescence signals (lower panel). Caffeine was briefly applied at 4 min intervals in the presence of 5 mm ATP, until reproducible steady-state Ca2+ and tension transients were obtained. After the second steady-state response, a further 2 min was allowed to fully load the SR with Ca2+. The [ATP] in the perfusing solution was then reduced from 5 mm to 0.1 mm, and in this example, the subsequent caffeine-induced Ca2+ transient was almost completely abolished. On average, the caffeine-induced response was reduced by 89 ± 1.4 % (mean ±s.e.m., n = 16) in the presence of 0.1 mm ATP.
Figure 2. The effect of cytosolic [ATP] on caffeine-induced Ca2+ release.
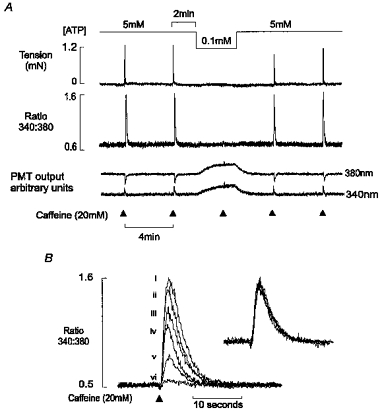
A, simultaneous records of the isometric tension (upper panel), the 340 nm/380 nm fluorescence ratio (middle panel) and the 340 and 380 nm fluorescence signals are shown. Reduction in the bathing [ATP] caused a marked decrease in the caffeine-induced Ca2+ transient. The decrease in [ATP] was associated with a parallel increase in the 340 nm and 380 nm signals, with no effect on the ratio. B, superimposed caffeine-induced Ca2+ transients in the presence of [ATP] (mm): (i) 5, (ii) 4, (iii) 3, (iv) 2, (v) 1 and (vi) 0.1. Inset shows normalized responses. All data are from the same preparation.
Resting tension was unaffected by the reduction in [ATP], suggesting that 0.1 mm ATP was sufficient to prevent the formation of rigor bridges under these conditions. We have shown previously that complete ATP withdrawal results in a slow maintained release of Ca2+ from the SR which appears to reflect passive Ca2+ efflux via a leak pathway (Steele & Duke, 1997; Duke & Steele, 1998a). The fact that the [Ca2+] did not rise after [ATP] was decreased to 0.1 mm confirms that sufficient substrate was present to support the activity of the Ca2+ pump, preventing passive loss of Ca2+ prior to caffeine application.
Withdrawal of ATP was associated with an increase in the 340 and 380 nm fluorescence signals. This suggests that the millimolar levels of ATP normally present in the cytosol quench fura-2 fluorescence. However, neither the 340 nm/380 nm ratio (middle panel) nor the apparent Kd of the dye for Ca2+ was affected by the change in [ATP] (data not shown). Therefore, the changes in emitted fluorescence can be used to monitor the rate of change of [ATP] within the preparation following a solution change, without affecting the Ca2+ signal.
Relationship between [ATP] and caffeine-induced Ca2+ release
Figure 2B shows superimposed caffeine-induced Ca2+ transients at a range of [ATP]. The protocol used was similar to that in Fig. 1, such that each release was induced at a constant SR Ca2+ load. Reducing the [ATP] from 5 to 0.1 mm produced a concentration-dependent decrease in the caffeine-induced Ca2+ transient. The superimposed (Fig. 2B) and normalized responses (inset) demonstrate that the time course of the Ca2+ transient was unaffected by the decrease in [ATP]. The lack of effect on the descending phase of the Ca2+ transient is consistent with previous findings that decreasing the [ATP] from millimolar levels to 100 μm does not influence Ca2+ re-uptake by the SR (Stienen et al. 1993).
Accumulated data showing the relationship between cytosolic [ATP] and the amplitude of the caffeine-induced Ca2+ transient are given in Fig. 3. The abscissa indicates the [ATP] and the ordinate, the mean steady-state amplitude of caffeine-induced Ca2+ transients, expressed as a percentage of control values obtained in the presence of 5 mm ATP. It is apparent that caffeine-induced Ca2+ release decreased markedly as the [ATP] was reduced below 5 mm. However, increasing the [ATP] from 5 to 10 mm only increased Ca2+ release by a further 14.6 ± 5 % (mean ±s.e.m., n = 5).
Figure 3. Cumulative data showing the relationship between the cytosolic [ATP] and the amplitude of the caffeine-induced Ca2+ transient.
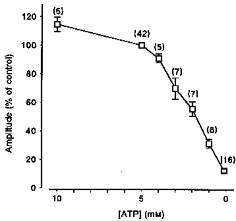
Each point is the mean (± s.e.m.) expressed as a percentage of the control response obtained in the presence of 5 mm ATP using the protocol shown in Fig. 2A. The number of preparations is indicated in parentheses.
Substitution of ATP with other nucleotides
As ATP facilitates caffeine-induced Ca2+ release from the SR, the ability of other structurally related nucleotides to substitute for ATP was investigated. Again, steady-state responses were obtained in the presence of 5 mm ATP and a further 2 min period was allowed to fully load the SR. However, the solution was then changed to one containing 0.1 mm ATP and a 4.9 mm concentration of the substituted nucleotide. ATP was retained at 0.1 mm in the perfusing solution to support the activity of the SR Ca2+ pump and myofilaments. The cumulative data (Fig. 4) show the mean control Ca2+ transient amplitudes obtained in the presence of 5 mm ATP or 0.1 mm ATP. Also shown are responses obtained in the presence of 0.1 mm ATP in combination with 4.9 mm AMP-PNP, ADP, AMP, adenosine or CTP. A substantial caffeine-induced release was observed in the presence of the non-hydrolysable ATP analogue AMP-PNP. The adenine nucleotides ADP and AMP also facilitated caffeine-induced Ca2+ release, although none of these compounds was as effective as 5 mm ATP alone. Interpretation of the results obtained with ADP was complicated by the finding that the introduction of the compound resulted in a slow ryanodine-insensitive efflux of Ca2+ from the SR, which probably resulted from reversal of the SR Ca2+ pump (not shown). However, the loss of Ca2+ from the SR prior to addition of caffeine was limited due to the slow rate of efflux on addition of ADP (see Discussion). CTP and adenosine failed to facilitate the action of caffeine. Indeed, the mean amplitudes of responses obtained in the presence of 4.9 mm adenosine or CTP (and 0.1 mm ATP) were significantly smaller than responses obtained in the presence of 0.1 mm ATP alone. This suggests that CTP, and to a greater extent adenosine, inhibit caffeine-induced Ca2+ release under these conditions. Furthermore, it was found that complete substitution of 5 mm ATP with 5 mm adenosine consistently abolished caffeine-induced Ca2+ release (n = 4, data not shown).
Figure 4. Effect of substituting 5 mm ATP with selected nucleotides.
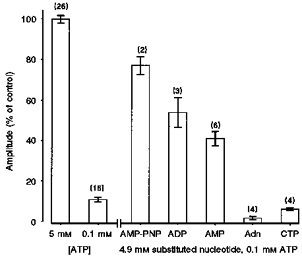
Each point is the mean ± s.e.m. expressed as a percentage of the control response obtained in the presence of 5 mm ATP using the protocol shown in Fig. 2A. Mean Ca2+ transient amplitude in the presence of 5 mm ATP or 0.1 mm ATP is shown. Responses obtained in the presence of 0.1 mm ATP and 4.9 mm AMP-PNP, ADP, AMP, adenosine (Adn) or CTP are also given. Responses in the presence of adenosine and CTP are significantly smaller than those obtained in the presence of 0.1 mm ATP alone (P < 0.05). The number of preparations is indicated in parentheses.
Competition between adenine nucleotides at the SR Ca2+ channel
One possible explanation for the inhibitory action of adenosine and CTP is that these compounds bind to the adenine nucleotide site on the SR Ca2+ channel, but do not induce activation or facilitate the action of caffeine. The possibility of competitive antagonism at the ATP binding site was investigated by application of caffeine in the presence of equal concentrations of ATP and other nucleotides. Figure 5 shows accumulated data illustrating the effect of 5 mm concentrations of selected nucleotides on the amplitude of the caffeine-induced response, in the presence of 5 mm ATP. All responses are expressed relative to the mean Ca2+ transient amplitude in the presence of 5 mm ATP alone. Again, a 2 min period was allowed to fully load the SR before introduction of 5 mm nucleotide in the continued presence of 5 mm ATP (not shown). Under these conditions, AMP and CTP produced only a small decrease in the caffeine-induced Ca2+ transient, which was not significant. A more pronounced decrease in the amplitude of the response occurred in the presence of ADP, although this may partially reflect a decrease in SR Ca2+ content due to the Ca2+ efflux which occurred prior to application of caffeine. However, 5 mm adenosine markedly reduced the amplitude of the caffeine-induced Ca2+ transient to 36 ± 4 % (mean ±s.e.m., n = 11) of controls.
Figure 5. Effect of 5 mm nucleotide in the presence of 5 mm ATP.
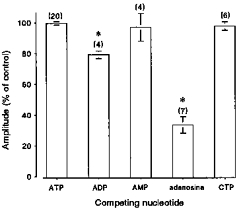
Each point is the mean ± s.e.m. expressed as a percentage of the control response obtained in the presence of 5 mm ATP alone, using the protocol shown in Fig. 2A. Mean Ca2+ transient amplitude in the presence of 5 mm ATP or 5 mm ATP in combination with 5 mm ADP, AMP, adenosine or CTP. * Responses significantly different (P < 0.05) from those obtained in the presence of 5 mm ATP. The number of preparations is indicated in parentheses.
The effect of adenosine on caffeine-induced Ca2+ release
The inhibitory effect of adenosine on caffeine-induced Ca2+ release was investigated in more detail, as shown in Fig. 6A. This record shows caffeine-induced Ca2+ transients obtained in a saponin-treated preparation in the constant presence of 5 mm ATP. Caffeine was briefly applied at 4 min intervals, and after an initial control Ca2+ response, the adenosine concentration was increased in a stepwise manner over the range 2–10 mm. The progressive increase in adenosine concentration resulted in corresponding decreases in the amplitude of the caffeine-induced Ca2+ transient, and this effect was fully and rapidly reversible. The fact that the introduction of adenosine was not associated with a release of Ca2+ suggests that the decline in the SR Ca2+ transient reflects inhibition of the release process, rather than a decrease in the SR Ca2+ content. Figure 6B shows superimposed Ca2+ transients from the same preparation in the absence (i) and presence (ii-iv) of increasing adenosine levels. These data, and the normalized responses (inset), show that adenosine reduces the amplitude of the caffeine-induced Ca2+ transient without affecting the time course of the response. This suggests that adenosine reduces the amount of released Ca2+, without affecting the kinetics of the release process, or the activity of the SR Ca2+ pump. Figure 7A shows accumulated data illustrating the concentration dependence of the inhibitory action of adenosine on caffeine-induced Ca2+ release, in the presence of 5 mm ATP. On average, 10 mm adenosine reduced the amplitude of the response by 81.2 ± 3.46 % (mean ±s.e.m., n = 5).
Figure 6. Effect of adenosine on caffeine-induced Ca2+ transient.
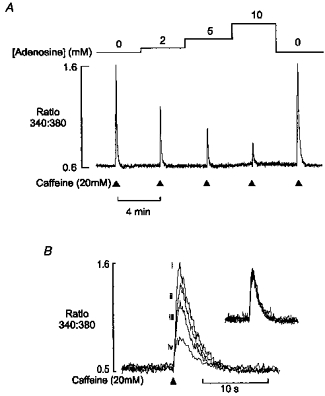
A, continuous record of the 340 nm/380 nm fluorescence ratio from a saponin-permeabilized fibre in the presence of 100 nm Ca2+ and 5 mm ATP. Caffeine (20 mm) was briefly applied at 4 min intervals as indicated. Following an initial steady-state control response, the adenosine concentration was increased in a stepwise manner from 2 to 10 mm. This resulted in a corresponding decrease in the amplitude of the caffeine-induced Ca2+ transient. Removal of adenosine resulted in a rapid return to control levels. B, superimposed Ca2+ transients from the same preparation, in the presence of (i) 0, (ii) 2, (iii) 5 and (iv) 10 mm adenosine. Normalized responses (inset) show that the time course of the Ca2+ transient was unaffected.
Figure 7. Interactions between caffeine and adenosine.
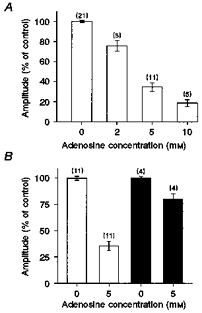
A, relationship between adenosine concentration and caffeine-induced release. Mean (± s.e.m.) amplitude of the caffeine-induced Ca2+ transient in the absence and presence of 2, 5 or 10 mm adenosine. All responses were obtained in the presence of 5 mm ATP using the protocol shown in Fig. 6A. The number of preparations is indicated in parentheses. B, ATP dependence of adenosine-induced release inhibition. Each point is the mean (± s.e.m.) amplitude of the caffeine-induced Ca2+ transient in the presence or absence of 5 mm adenosine, at 5 (□) or 10 mm (▪) ATP. All results are expressed relative to the mean response in the presence of 5 mm ATP, 0 mm adenosine. The number of preparations is indicated in parentheses.
On balance, the data suggest that adenosine inhibits caffeine-induced Ca2+ release indirectly by antagonizing the binding of ATP to the adenine nucleotide site on the SR Ca2+ channel rather than direct inhibition of the caffeine-induced release process. This possibility is further supported by experiments involving the application of adenosine in the presence of differing levels of ATP. If adenosine acts by competitive antagonism of ATP binding to the SR Ca2+ channel, then the relationship shown in Fig. 3 suggests that the degree of inhibition should be more pronounced at 5 mm ATP than in the presence of 10 mm ATP. This was confirmed in experiments which showed that 5 mm adenosine reduced the caffeine-induced Ca2+ transient by 64 ± 4 % (mean ±s.e.m., n = 11) in the presence of 5 mm ATP. However, in the presence of 10 mm ATP, the transient was reduced by only 20 ± 5.19 % (mean ±s.e.m., n = 4) of the control response (Fig. 7B).
DISCUSSION
Effects of ATP on caffeine-induced Ca2+ release in skinned muscle preparations
The present study has demonstrated that in permeabilized muscle fibres, with an intact SR, the caffeine-sensitive efflux pathway is markedly influenced by cytosolic ATP. Decreasing the [ATP] from 10 to 0.1 mm reduced the amount of Ca2+ released in response to 20 mm caffeine. This is consistent with previous studies on heavy SR vesicles from rabbit skeletal muscle, where caffeine-activated 45Ca2+ efflux was potentiated by addition of millimolar levels of ATP to the bathing medium. Similar results were obtained in SR Ca2+ channels incorporated into lipid bilayers, where the increase in Po induced by 20 mm caffeine was more than doubled by addition of 2 mm ATP (Rousseau et al. 1988).
The present results, however, differ from previous work on saponin-permeabilized skeletal muscle fibres from the toad (Stienen et al. 1993). In this preparation, the amount of Ca2+ released by caffeine appeared relatively unaffected by a large decrease in [ATP] from 5 mm to 10 μm. Indeed, it was reported that 5 mm caffeine released more than 90 % of the total SR Ca2+ content, in the complete absence of ATP. However, the onset of Ca2+ release was delayed at low levels of ATP, and it was concluded that the rate of Ca2+ efflux was reduced. This contrasts with the present study, where decreasing the [ATP] from 10 mm to 100 μm markedly reduced the amplitude of the caffeine-induced Ca2+ transient, without effect on the time course of Ca2+ release (Fig. 2B).
This apparent discrepancy might be explained by differences in the experimental techniques and protocols as follows. (i) Stienen and colleagues used tension as an indirect index of SR Ca2+ release. Low levels of ATP have pronounced effects on myofilament Ca2+ sensitivity and peak force, which complicated the interpretation of the results. (ii) Responses obtained at low levels of ATP may also be influenced by changes in the activity of the SR Ca2+ pump. Previous work on skinned fibres has shown that re-uptake of Ca2+ by the SR can attenuate the rise in cytosolic [Ca2+] following caffeine application (Makabe et al. 1996; Steele & Duke, 1997). Therefore, at low levels of ATP, where the rate of Ca2+ uptake is reduced (see below), a given amount of Ca2+ released from the SR would be expected to cause a larger Ca2+ and tension transient. (iii) When ATP is absent completely, a low level of channel activation combined with efflux via the Ca2+ leak pathway will fully deplete the SR given sufficient time. Hence, a large fraction of total SR Ca2+ may be released, even if caffeine is much less effective. (iv) If SR Ca2+ uptake is impaired, local accumulation of Ca2+ at the surface of the SR might also result in a progressive increase in the Ca2+ efflux rate, due to Ca2+-induced Ca2+ release. Such an effect could explain the rise in tension after a very prolonged (6 s) delay, as observed by Stienen and colleagues. In the present study, complications associated with the effects of ATP on the myofilaments were avoided by direct measurement of SR Ca2+ release. We have also limited our study to ATP levels above that to support the activity of the SR Ca2+-ATPase (∼100 μm). Under these conditions, a clear relationship between caffeine-induced Ca2+ release and cytosolic [ATP] is observed.
Although not addressed in detail, one other interesting aspect of this study is that altering the cytosolic [ATP] over the range 0.1–5 mm had no effect on the SR Ca2+ content. Experiments on isolated SR vesicles suggest that increasing the [ATP] would be expected to progressively activate the SR Ca2+ channel (Smith et al. 1986). In skinned fibres, this should result in a release of Ca2+ from the SR and a decrease in the SR Ca2+ content. However, the present data suggest that under the conditions of this study, changes in the cytosolic [ATP] do not have major direct effects on the activity of the in situ SR Ca2+ channel. Instead, the occupancy of the adenine nucleotide site by ATP or other agonists appears to have a marked influence on the physiological release process (Owen et al. 1996) and the sensitivity of the SR Ca2+ channel to activation by drugs such as caffeine.
The marked dependence of caffeine-induced Ca2+ release on cytosolic [ATP] may have implications for experiments on intact and skinned skeletal muscle, where the amplitude of the Ca2+ or tension transient is commonly used as an index of SR Ca2+ content. The present results suggest that any intervention causing a local decrease in [ATP] at the junctional surface of the SR will reduce the fraction of the total Ca2+ released by caffeine. Such an effect might be misinterpreted as a decrease in SR Ca2+ content. The hydrolysis products substitute partially at the adenine nucleotide binding site (see below), limiting this potential difficulty. However, ADP releases Ca2+ from the SR via a ryanodine-insensitive efflux pathway which might further complicate interpretation of the data in circumstances where ATP is weakly buffered.
Interaction between caffeine and other nucleotides
A number of difficulties were encountered in experiments involving ADP which complicate the interpretation of the results. First, introduction of ADP was associated with a slow Ca2+ release from the SR. ADP-induced Ca2+ release was found to be insensitive to Ruthenium Red or ryanodine, but was abolished by the SR Ca2+ pump inhibitor cyclopiazonic acid (not shown). This is consistent with ADP-induced efflux via the SR Ca2+ pump (Hasselbach, 1978). If significant SR Ca2+ efflux occurs prior to addition of caffeine, a smaller release will occur and the effect of ADP and its ability to substitute for ATP may be underestimated. However, we estimate that this factor is unlikely to influence the Ca2+ transient by more than 10–20 % because the slow nature of the ADP-induced Ca2+ release allowed caffeine to be applied before significant loss of SR Ca2+ occurred. A second potential difficulty is that a fraction of the ADP diffusing into the preparation will be converted to ATP via the creatine phosphokinase (CPK) or the adenylate kinase reactions. Inhibition of the myokinase with diadenosine pentaphosphate had no apparent effect on the responses obtained, suggesting that synthesis via this pathway is not of major importance under these conditions. However, it was not possible to study the effects of ADP in the absence of creatine phosphate (CrP) because it was found that SR Ca2+ uptake and release mechanisms were markedly impaired in the absence of CrP (Korge et al. 1993; Duke & Steele, 1998b). Despite this, it seems unlikely that the CPK reaction could reduce the ADP levels in the vicinity of the SR Ca2+ channel significantly for the following reasons. (i) ADP is free to diffuse from the effectively infinite volume of surrounding solution into the muscle. (ii) Any ATP produced is free to diffuse out of the muscle. (iii) The fact that ADP-induced reversal of the SR Ca2+ pump occurs in the presence of CrP demonstrates that high levels of ADP are present in the vicinity of the SR. (iv) Ca2+ transients are not observed in the presence of solutions containing millimolar ADP with CrP, but lacking ATP (not shown). This suggests that insufficient ATP is produced from ADP to support the activity of the SR Ca2+ pump and the release mechanism. Thus, it seems reasonable to assume that the effect of adding ADP to the perfusing solution is not a secondary consequence of ATP synthesis. However, these uncertainties remain a limitation of the method.
The experiments involving substitution of ATP with other nucleotides showed that AMP-PNP, ADP and AMP also facilitate the action of caffeine on the SR Ca2+ channel, although none was as effective as ATP (Fig. 4). These results are consistent with data on isolated SR Ca2+ channels and vesicle preparations showing that the relative potency to induce activation of the Ca2+ channel is AMP-PNP > ADP > AMP (Morii & Tonomura, 1983; Meissner, 1984; Pessah et al. 1986). This structure-activity relationship of the in situ SR confirms previous suggestions that the number of phosphates attached to the 5′ carbon of the ribose ring determines the efficacy of the ligand. Failure of CTP to substitute for ATP is also consistent with previous studies showing that non-adenine triphosphates do not activate the skeletal SR Ca2+ channel (Meissner, 1984). Indeed, there was some evidence of a weak competitive antagonism when the [CTP] is much in excess of the [ATP] (Fig. 4) as has been reported in isolated SR (Morii & Tonomura, 1983).
Effects of adenosine
The effects of adenosine on the skeletal SR Ca2+ channel have not been characterized in detail previously. However, recent experiments on isolated channels have shown that adenosine is a potent activator of the cardiac SR Ca2+ channel (McGarry & Williams, 1994). It was found that both adenosine and caffeine maximally activated the channel in the presence of micromolar Ca2+, although adenosine was more potent with an EC50 of approximately 0.75 mm. Application of 1 mm ATP to channels activated by 1 mm adenosine resulted in a decrease in Po. This was interpreted as evidence that adenosine acts at the same site as ATP, but with greater potency. A disproportionate increase in Po occurred when both caffeine and adenosine were introduced together, suggesting a synergistic interaction.
Based on these observations, adenosine might be expected to substitute for ATP at the adenine nucleotide binding site and facilitate the action of caffeine on skeletal muscle. However, adenosine failed to substitute for ATP or facilitate the Ca2+-releasing action of caffeine (Fig. 4). Indeed, introduction of adenosine was associated with a marked concentration-dependent inhibition of caffeine-induced Ca2+ release in the presence of ATP (Figs 6 and 7A). The inhibitory effect of adenosine on caffeine-induced release was much less pronounced in the presence of 10 mm than 5 mm ATP. Given the relationship between caffeine-induced release and [ATP] (Fig. 3), this suggests that adenosine may competitively antagonize ATP binding to the SR Ca2+ channel.
The apparent difference between the present work and previous studies on isolated channels from cardiac muscle probably reflects intrinsic differences in the activation characteristics of cardiac and skeletal SR Ca2+ channels. It is known that RyR1 receptors are more sensitive to activation by adenine nucleotides (Meissner & Henderson, 1987). Experiments on rabbit skeletal muscle have also shown that the base compound adenine inhibits rather than potentiates caffeine-induced Ca2+ efflux in the presence of ATP (Rousseau et al. 1988). The present study suggests that in skeletal muscle (but not cardiac muscle) removal of a single phosphate group may reduce AMP from a moderately potent agonist at the adenine nucleotide site to adenosine, which acts a competitive antagonist.
The ability of adenosine to antagonize the action of ATP may be useful in determining whether other endogenous substances or drugs act at the adenine nucleotide binding site. However, further work is required to establish whether adenosine has similar effects in mammalian skeletal muscle. Frog skeletal muscle fibres contain roughly equal amounts of RyR1 and RyR3 (α and β) isoforms of the ryanodine receptor whereas mammalian muscle contains a much greater (although variable) proportion of RyR1 relative to RyR3 (Franzini-Armstrong & Protasi, 1997). Preliminary experiments suggest that in rat extensor digitorum longus muscle fibres, which contain virtually no RyR3, caffeine-induced Ca2+ release is similarly affected by cytosolic ATP and adenosine (not shown).
Physiological significance
Previous work on mechanically skinned rat skeletal muscle fibres has shown that the physiological, depolarization-induced Ca2+-releasing process, is inhibited by a reduction in the cytosolic [ATP] (Owen et al. 1996). One possible explanation for this is that ATP directly facilitates the interaction between the t-tubule voltage sensor and the SR Ca2+ channel. However, the fact that caffeine-induced Ca2+ release is similarly affected by ATP, suggests that (i) caffeine-induced Ca2+ release and depolarization-induced Ca2+ release share a common pathway and (ii) ATP may influence the excitation-contraction coupling process by acting at a point subsequent to the initial voltage-dependent step. One possibility is that caffeine-induced Ca2+ release and depolarization-induced Ca2+ release both involve a component of positive feedback via Ca2+-induced Ca2+ release, the sensitivity of which is modulated the cytosolic [ATP].
It has been suggested that a local reduction in [ATP] contributes to the rapid decline in SR Ca2+ release during the latter stages of fatigue by inhibiting the SR Ca2+ channel (Owen et al. 1996; Allen et al. 1997). One argument against this is that the ATP hydrolysis products may also bind to the adenine nucleotide binding site, thereby maintaining the sensitivity of the SR Ca2+ channel. However, this study has shown that the products of ATP breakdown are less potent than ATP in facilitating the action of caffeine in the intact SR. Assuming that depolarization-induced Ca2+ release is similarly affected, a local decrease in [ATP] and accumulation of hydrolysis products would be expected to inhibit release.
Another interesting aspect of the present study is that adenosine was found to act as a competitive antagonist at the adenine nucleotide binding site. It has been shown that adenosine is released from intact frog skeletal muscle following stimulation (Rodrigo et al. 1998). There is also some evidence of a small rise in the average intracellular adenosine concentration following intense exercise or hypoxia in human skeletal muscle (Tullson et al. 1998). It has been argued that, although the mean fall in cytosolic [ATP] during fatigue is comparatively small (∼20 %), the local decrease in the vicinity of the SR Ca2+ channel might be much larger. Similarly, if local adenosine levels in the vicinity of the SR Ca2+ channel increased significantly during intense activity, the sensitivity of the SR Ca2+ channel would be further decreased by competitive antagonism at the adenine nucleotide binding site.
Conclusions
The results of this study demonstrate that caffeine-induced Ca2+ release exhibits a marked dependence on cytosolic [ATP] in saponin-permeabilized skeletal muscle fibres. Other adenine nucleotides partially substituted for ATP while adenosine inhibited caffeine-induced Ca2+ release in a manner consistent with competitive antagonism at the adenine nucleotide binding site. These data suggest that the number of phosphates attached to the 5′ carbon of the ribose ring determines the efficacy of the ligand and that removal of a single phosphate group transforms AMP from a partial agonist to adenosine, which acts as a competitive antagonist under these conditions.
Acknowledgments
Financial support for this work was provided by The Wellcome Trust and the British Heart Foundation.
References
- Allen DG, Lannergren J, Westerblad H. The role of ATP in the regulation of intracellular Ca2+ release in single fibres of mouse skeletal muscle. The Journal of Physiology. 1997;498:587–600. doi: 10.1113/jphysiol.1997.sp021885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt NR, Caswell AH, Brunschwig JP, Kang JJ, Antoniu B, Ikemoto N. Effects of anti-triadin antibody on calcium release from sarcoplasmic reticulum. FASEB Journal. 1992;6:A431. doi: 10.1016/0014-5793(92)80100-u. [DOI] [PubMed] [Google Scholar]
- Brillantes AMB, Ondrias K, Scott A, Korbinsky E, Ondriasova E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium-release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Coronado R, Morrissette J, Sukhareva M, Vaughan DM. Structure and function of ryanodine receptors. American Journal of Physiology. 1994;266:C1485–1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Effects of cyclopiazonic acid on Ca2+ regulation by the sarcoplasmic reticulum in saponin permeabilized skeletal muscle fibres. Pflügers Archiv. 1998a;436:104–111. doi: 10.1007/s004240050610. [DOI] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Effects of phosphocreatine on Ca2+ regulation by the sarcoplasmic reticulum in isolated mechanically skinned rat skeletal muscle fibres. The Journal of Physiology. 1998b;509.P:45P. doi: 10.1111/j.1469-7793.1999.0447t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. Journal de Physiologie. 1979;75:463–505. [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiological Reviews. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Hasselbach W. The reversibility of the sarcoplasmic calcium pump. Biochimica et Biophysica Acta. 1978;515:23–53. doi: 10.1016/0304-4157(78)90007-2. [DOI] [PubMed] [Google Scholar]
- Ikemoto N, Ronjat M, Meszaros LG, Koshita M. Postulated role of calsequestrin in the regulation of calcium release from the sarcoplasmic reticulum. Biochemistry. 1989;28:6764–6771. doi: 10.1021/bi00442a033. [DOI] [PubMed] [Google Scholar]
- Korge P, Byrd SK, Campbell KB. Functional coupling between sarcoplasmic reticulum bound creatine kinase and Ca2+-ATPase. European Journal of Biochemistry. 1993;213:973–980. doi: 10.1111/j.1432-1033.1993.tb17842.x. [DOI] [PubMed] [Google Scholar]
- McGarry SJ, Williams AJ. Adenosine discriminates between the caffeine and adenine nucleotide sites on the sheep cardiac sarcoplasmic reticulum calcium release channel. Journal of Membrane Biology. 1994;137:169–177. doi: 10.1007/BF00233486. [DOI] [PubMed] [Google Scholar]
- Makabe M, Werner O, Fink RHA. The contribution of the sarcoplasmic reticulum Ca2+-transport ATPase to caffeine-induced Ca2+ transients of murine skinned skeletal muscle. Pflügers Archiv. 1996;423:717–726. doi: 10.1007/s004240050190. [DOI] [PubMed] [Google Scholar]
- Mayrleitner M, Timerman AP, Wiederrecht G, Fleischer S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506 binding protein - effect of FKBP-12 on single channel activity of the skeletal muscle ryanodine receptor. Cell Calcium. 1994;15:99–108. doi: 10.1016/0143-4160(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. Journal of Biological Chemistry. 1984;259:2365–2374. [PubMed] [Google Scholar]
- Meissner G, Henderson JS. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. Journal of Biological Chemistry. 1987;262:3065–3073. [PubMed] [Google Scholar]
- Miller DJ, Smith GL. EGTA purity and the buffering of calcium ions in physiological solutions. American Journal of Physiology. 1984;246:C160–166. doi: 10.1152/ajpcell.1984.246.1.C160. [DOI] [PubMed] [Google Scholar]
- Morii H, Tonomura Y. The gating behavior of a channel for Ca2+ induced Ca2+ release in fragmented sarcoplasmic-reticulum. Journal of Biochemistry. 1983;93:1271–1285. doi: 10.1093/oxfordjournals.jbchem.a134261. [DOI] [PubMed] [Google Scholar]
- O'Neill SC, Donoso P, Eisner DA. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. The Journal of Physiology. 1990;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen VJ, Lamb GD, Stephenson DG. Effect of low [ATP] on depolarization-induced Ca2+ release in skeletal muscle fibres of the toad. The Journal of Physiology. 1996;493:309–315. doi: 10.1113/jphysiol.1996.sp021385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Stambuk RA, Casida JE. Ca2+ activated ryanodine binding: Mechanisms of sensitivity and intensity modulation by Mg, caffeine and adenine nucleotides. Molecular Pharmacology. 1986;31:232–238. [PubMed] [Google Scholar]
- Rodrigo A, Cunha A, Sebastiao M. Adenosine and adenine nucleotides are independently released from both nerve terminals and muscle fibres upon electrical stimulation of innervated skeletal muscle of the frog. Pflügers Archiv. 1998;424:503–510. doi: 10.1007/BF00374914. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Ladine J, Liu Q, Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Archives of Biochemistry and Biophysics. 1988;267:75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R, McGarry SJ, Williams AJ. Cyclic ADP-ribose, the ryanodine receptor and Ca2+ release. Trends in Pharmacological Sciences. 1995;16:386–390. doi: 10.1016/s0165-6147(00)89080-x. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Cyclic ADP-ribose activation of the sheep skeletal ryanodine receptor Ca2+-release channel is dependent upon luminal Ca2+ British Journal of Pharmacology. 1995;114:P246. [Google Scholar]
- Smith GL, Miller DJ. Potentiometric measurements of stoichiometric and apparent affinity constants of EGTA for protons and divalent ions including calcium. Biochimica et Biophysica Acta. 1985;839:287–299. doi: 10.1016/0304-4165(85)90011-x. [DOI] [PubMed] [Google Scholar]
- Smith GL, Steele DS. Effects of 2,3 butanedione monoxime on sarcoplasmic reticulum of saponin treated rat cardiac muscle. American Journal of Physiology. 1993;265:H1493–1500. doi: 10.1152/ajpheart.1993.265.5.H1493. [DOI] [PubMed] [Google Scholar]
- Smith JS, Coronado R, Meissner G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum - activation by Ca2+ and ATP and modulation by Mg2+ Journal of General Physiology. 1986;88:573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele DS, Duke AM. Effects of cyclopiazonic acid on caffeine-induced Ca2+ release in isolated saponin skinned frog skeletal muscle fibres. The Journal of Physiology. 1997;501.P:144P. [Google Scholar]
- Stienen GJM, Grass IA, Elzinga G. Uptake and caffeine induced release of calcium in fast muscle fibres of Xenopus laevis: effects of MgATP and Pi. American Journal of Physiology. 1993;265:C650–657. doi: 10.1152/ajpcell.1993.265.3.C650. [DOI] [PubMed] [Google Scholar]
- Tullson CP, Bangsbo J, Hellsten Y, Richter EA. IMP metabolism in human skeletal muscle after exhaustive exercise. Journal of Applied Physiology. 1998;87:146–152. doi: 10.1152/jappl.1995.78.1.146. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T, Grassucci R, Berkowitz J, Wiederrecht GJ, Xin HB, Fleischer S. Cryoelectron microscopy resolves fk506-binding protein sites on the skeletal muscle ryanodine receptor. Biophysical Journal. 1996;70:1709–1715. doi: 10.1016/S0006-3495(96)79733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


