Abstract
The present study has investigated whether fatty acids directly influence peptide release from enteroendocrine cells using STC-1, a mouse intestinal endocrine tumour cell line, previously shown to release cholecystokinin (CCK) in response to other physiological stimuli.
Fatty acids elicited a chain length- and dose-dependent stimulation of CCK secretion. Dodecanoic acid (C12) was most effective, producing up to a 5-fold increase in CCK secretion. Fatty acids with less than ten carbon atoms did not increase secretion. The chain length dependence of these effects mimics closely fatty acid-induced CCK secretion previously observed in humans in vivo.
Esterification of C12 abolished CCK secretion, indicating a critical role for a free carboxyl group in eliciting secretion. In contrast, modification of the methyl terminus had no effect on C12-induced secretion. The non-metabolizable C12 analogue 2-bromododecanoic acid was equally effective.
C12 elicited a marked increase in intracellular calcium levels (200–300 nm) in STC-1 cells which was abolished by the L-type Ca2+ channel antagonist nicardipine. In contrast, C8 produced a smaller and more transient Ca2+ response. C12-induced CCK secretion was also blocked by nicardipine.
These data suggest that fatty acids can interact directly with enteroendocrine cells to stimulate CCK secretion via increases in intracellular calcium mediated primarily by L-type Ca2+ channels.
Cholecystokinin (CCK) is secreted in response to intraluminal nutrients by specialized enteroendocrine cells (I-cells) located predominantly in the proximal small intestinal mucosa. CCK serves a variety of functions which together integrate and optimize the intestinal digestion and absorption of fat and protein. These actions include stimulation of gall bladder emptying, increased pancreatic enzyme secretion, delayed gastric emptying and inhibition of further food intake (Hopman et al. 1985; Liddle et al. 1986; Hildebrand et al. 1990; Ballinger et al. 1995).
The release of CCK is stimulated both by protein and by lipid. While there are trypsin-sensitive CCK-releasing peptides which may mediate the effect of dietary protein (Liddle, 1995), the precise mechanism for lipids is unknown. Dietary lipids stimulate CCK secretion specifically via intraduodenal free fatty acids (Beardshall et al. 1989; Guimbaud et al. 1997). Although it is known that long chain triglycerides but not medium chain triglycerides stimulate release of CCK in humans (Isaacs et al. 1987), the precise structural specificity of the CCK response to luminal fatty acids has not been characterized. We have now reported that fatty acids require a minimum acyl chain length of twelve carbon atoms to cause a measurable increase in peripheral blood CCK concentration in healthy human subjects (McLaughlin et al. 1996). In addition, it has previously been reported that a non-esterified carboxyl group is required for lipid-induced CCK secretion in man (Olsen et al. 1989).
The cellular mechanisms by which fatty acids evoke CCK secretion have not been defined. Although enteroendocrine cells project processes into the lumen (Polak et al. 1975), it is not known whether luminal fatty acids interact directly with these cells to modulate their function. This is a fundamental point, since a different sensory cell type or a lipid-responsive secreted factor could be responsible for lipid-induced CCK release in vivo. For example, an indirect mechanism has been suggested for protein-induced CCK secretion in the rat, via trypsin-sensitive intraluminal mediators such as monitor peptide (Liddle, 1995). However, no evidence for a similar mechanism has been reported for lipid-induced CCK secretion.
A major obstacle to progress in elucidating cellular control of CCK release has been the difficulty in isolating native enteroendocrine cells, since these represent less than 1% of small intestinal epithelial cells, and preparations have generally been of poor specificity and viability. The recent development of an enteroendocrine cell line, STC-1, has begun to allow more detailed characterization of the cellular signalling mechanisms involved in CCK secretion. This cell line is derived from an intestinal endocrine tumour in double transgenic mice (Rindi et al. 1990) and secretes biologically active forms of CCK as major products in response to the types of physiological and pharmacological stimuli that elicit secretion in vivo, including tryptophan, l-phenylalanine and bombesin (Chang et al. 1994; Snow et al. 1994; Mangel, 1995). Fatty acid effects on STC-1 cells have received scant attention, although the long chain mono-unsaturated oleic acid (C18:1) has been shown to induce CCK release (Chang et al. 1994).
The primary objectives of the present experiments were to define whether fatty acids induced CCK secretion in STC-1 cells, to determine whether fatty acid responses exhibit chain length and structural specificity, and to explore the role of calcium as an intracellular mediator of fatty acid-induced CCK release.
METHODS
Test agents
All chemicals were obtained from Sigma unless otherwise stated. Saturated fatty acids (FAs) used were C3 (proprionic), C6 (hexanoic), C8 (octanoic), C10 (decanoic), C12 (dodecanoic), C14:1 (cis-9-tetradecenoic) and C18:1 (cis-9-octadecenoic). The ethyl ester of C12 (ethyl laurate), a branched chain C12 (11-methyl undecanoic or isolauric acid) and a non-metabolizable C12 analogue (2-bromododecanoic acid) were also used. Fatty acids were prepared in Hanks' balanced salts solution (HBSS) with 5 mm Hepes as described below. Nicardipine was dissolved in polyethylene glycol (PEG; molecular weight, 400) to a stock concentration of 5 mm and diluted in HBSS immediately before use.
STC-1 cell culture
STC-1 cells (supplied by D. Hanahan, University of California, San Francisco) were grown in Dulbecco's modified Eagle's medium (DMEM) containing 15% horse serum, 2.5% fetal bovine serum, 50 i.u. ml−1 penicillin and 500 μg ml−1 streptomycin in a humidified 5% CO2 atmosphere at 37°C. Cells were routinely subcultured by trypsinization upon reaching 80–90% confluency. For experiments, cells were seeded at a density of 1.5 × 105 cells cm−2 in twelve-well plates and used at sub-confluency. For fluorescence imaging of intracellular Ca2+, cells were grown on polylysine-coated coverslips. Cells were used between passages 43 and 55.
Preparation of fatty acid media
Fatty acids were dispersed into HBSS buffered with 5 mm Hepes by ultrasonication for 2 min at room temperature to produce initial fatty acid concentrations equivalent to 10–50 mm. These were then serially diluted with HBSS and again sonicated to disperse fatty acids to concentrations of 50 μm to 5 mm. The pH of the fatty acid solution remained at 7.4 across this concentration range; fatty acid solutions were prepared fresh on the day of the experiment and re-sonicated briefly prior to use.
Studies of fatty acid-induced CCK secretion
Cells were washed twice with HBSS at 37°C to remove growth medium, and 2 ml of HBSS containing the sonicated fatty acid suspensions was added. Cells were incubated at 37°C for 2–120 min after which 1.5 ml of medium was removed and centrifuged immediately to remove any intact cells, and the supernatant stored at −70°C for analysis of CCK and lactate dehydrogenase (LDH). Where applicable, the removed medium was replaced with fresh, warmed medium and the incubation continued. At the end of the experiment, culture plates were washed free of medium, and the adherent cells dissolved in 0.1 m NaOH for subsequent determination of cell protein. To assess the effects of nicardipine on CCK secretion, cells were preincubated in 5 μm nicardipine for 15 min prior to fatty acid exposure, using 500 μm C12 as the stimulus in the continued presence of nicardipine. Values reported are the means of at least three separate experiments with duplicate or triplicate wells used in each experiment.
CCK radioimmunoassay
CCK was measured using a radioimmunoassay using antibody L425, raised by immunizing rabbits with sulphated CCK octapeptide. This is highly specific for the C-terminal of CCK or gastrin. Since gastrin was undetectable in STC-1 cells using a highly specific antigastrin antibody, L2, which does not cross-react with CCK, all immunoreactivity detected by L425 can be considered to be CCK. Fatty acids did not interfere in this assay. Reference CCK standards were used, with either 125I-CCK8 or 125I-non-sulphated gastrin heptadecapeptide as radiolabelled tracer. The binding characteristics of L425 are identical using both radiolabelled peptides, allowing them to be used interchangeably.
Calcium imaging
Intracellular Ca2+ ([Ca2+]i) was measured in STC-1 cells loaded with the Ca2+-sensitive dye fura-2. Cells grown on polylysine-coated coverslips were incubated with 10 μm fura-2 AM in HBSS containing Pluronic F-127 (0.025%) for 45 min at room temperature. After repeated washing in HBSS, coverslips were placed in a specially constructed chamber on the stage of a Nikon Diaphot inverted microscope which allowed continuous and rapid (2 ml min−1) perfusion. Cells were epi-illuminated alternately at 340 and 380 nm and the emitted light above 520 nm was captured through a ×40, 1.3 NA objective by an extended ISIS-M video camera (Photonic Science, Robertsbridge, UK) and digitized by a PT50 frame grabber (Perceptics Corporation, Knoxville, TN, USA). Consecutive frames obtained during 340 nm and 380 nm excitation were analysed pixel by pixel using Ionvision software (Improvision, Coventry, UK) to give ratio images every 5 s. [Ca2+]i was calculated with reference to a calibration curve relating Ca2+ (in nanomoles) to the 340/380 ratio using sigmoid curve-fitting software. The calibration curve was constructed using commercially available Ca2+-EGTA buffer solutions yielding known free Ca2+ concentrations in the range 0–39.8 μm (Molecular Probes Inc.) and containing 50 μm fura-2.
Assessment of FA cytotoxicity
Possible cytotoxic effects of fatty acids on STC-1 were assessed by measuring their effects on the release of the cytosolic enzyme lactate dehydrogenase (LDH). Aliquots of media collected from cells following incubation with fatty acids were assayed for LDH activity using a commercial kit (Boehringer Mannheim). Results are expressed as the percentage of the total LDH activity present in the cells which is released into the extracellular medium in a given time. Total LDH was measured after detergent lysis of the cells with 1% Triton X-100. Studies indicated that fatty acid did not interfere directly in the LDH assay system.
Statistical analysis
Results are expressed as means ±s.e.m. All statistical comparisons were made using Student's unpaired, two-tailed t test. Differences were considered significant when P < 0.05.
RESULTS
Chain length dependence of FA-induced CCK release
STC-1 cells exhibit a low basal rate of CCK secretion in fatty acid-free HBSS of 213 ± 30 fmol (mg cell protein)−1 over 15 min (mean of n = 3 experiments), which represents 1% of the total cellular CCK content (23.6 ± 2.8 pmol (mg protein)−1).
Replacement of culture medium with HBSS containing 500 μm dodecanoic acid (C12) resulted in a marked stimulation of CCK secretion, with the most rapid increase occurring during the initial 5 min following C12 addition (Fig. 1). Stimulation of CCK secretion by FAs was dependent on both acyl chain length and fatty acid concentration (Fig. 2). First, FAs with chain lengths of eight carbon atoms or less were unable to elicit CCK secretion from STC-1 cells at any concentration in the range 50–1000 μm. Second, while both C10 and C12 were able to stimulate CCK secretion over this range, C12 was significantly more effective, with a threshold at 100 μm and maximal secretion occurring at 500 μm with an EC50 of ∼280 μm. Third, longer chain length fatty acids (C14:1 and C18:1) at 500 μm had a comparable effect to C12 (C14:1, 614 ± 50%; C18:1, 660 ± 201%; n = 3–4).
Figure 1. Time course of FA-induced CCK secretion in STC-1 cells.
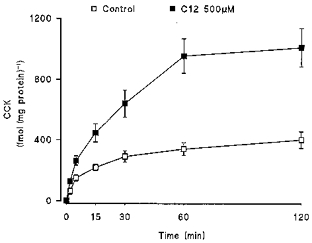
Cells were challenged with 500 μm C12 or HBSS and medium sampled at the time points indicated to 120 min. CCK secretion is presented cumulatively and expressed in relation to the total protein content in representative wells. Data are the means ± s.e.m. of 3 separate experiments.
Figure 2. Dose and chain length dependence of FA-induced CCK secretion.
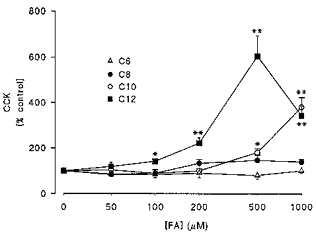
Cells were challenged with increasing concentrations of C6 (n = 4), C8 (n = 4), C10 (n = 5) and C12 (n = 5) saturated fatty acids over 15 min. Results are expressed as a percentage of CCK secretion in control (untreated) cells over the same period. Data are means ± s.e.m. *P < 0.01, **P < 0.001.
Fatty acids may have cytotoxic effects, so to exclude non-specific damage as a contributory factor in fatty acid-induced secretion of CCK in STC-1 cells, the effect of C12 on the release of the cytosolic enzyme LDH was measured. Under control conditions, a low level of LDH activity (1–2% of total cellular activity) is released into the medium (Fig. 3). However, C12 over the concentration range associated with increased CCK secretion (50–1000 μm) produced no further LDH release. In contrast, high concentrations (10 mm) of C12 were clearly cytotoxic being associated with almost total release of LDH activity during a 15 min incubation.
Figure 3. Relationship between C12-induced CCK secretion and LDH release.
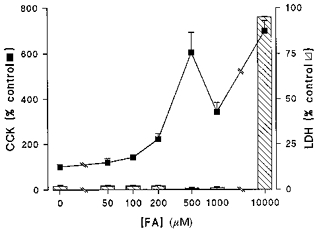
STC-1 cells were exposed to varying concentrations (0–10 mm) of C12 FA over 15 min after which the medium was assayed for CCK and LDH activity. CCK results are expressed as a percentage of secretion in the absence of C12. LDH is expressed as the percentage of total cell-associated activity. Results are means + s.e.m. from n = 5 experiments in each group except 10 mm C12 where n = 3.
Influence of fatty acid structure
The effect of structural modifications on the ability of C12 to stimulate CCK secretion in STC-1 was also investigated. 11-Methylundecanoic (isolauric) acid, a C12 fatty acid with a branched methyl terminus was found to be as effective as linear C12 (dodecanoic acid) in stimulating a CCK secretory response (500 μm dodecanoic C12, 636 ± 117 fmol (mg protein)−1; 500 μm isolauric C12, 674 ± 163 fmol (mg protein)−1; over 15 min, n = 3–5). In contrast, ethyl laurate, a C12 FA with an esterified carboxyl terminus was ineffective in stimulating CCK release (< 100 fmol (mg protein)−1) in STC-1 cells. Metabolism of the FA does not appear to be important in stimulating CCK secretion in these cells since 2-bromo-C12, which is resistant to metabolism, was as effective as C12 in inducing CCK secretion (787 ± 21 fmol (mg protein)−1, n = 3).
Tachyphylaxis of C12-induced CCK release
The effect of repeat stimulation with C12 on CCK secretion was also investigated (Fig. 4). This was achieved by an initial 15 min exposure to 500 μm C12, after which the FA was removed and cells were left in control medium for varying periods (0.25–4 h) before re-exposure to 500 μm C12 (Fig. 4). The initial exposure to C12 for 15 min elicited the characteristic increase in CCK secretion shown in Fig. 2. Repeat addition of C12 after a wash period of 15 min resulted in a significantly smaller increase in CCK secretion suggesting that the effect of FA is refractory in these cells. Reduction of the FA response was also evident when cells were re-challenged after 60 and 120 min. However, by 240 min there was marked recovery of the FA-induced CCK response to ∼70% of that seen following the initial challenge with C12 (Fig. 4).
Figure 4. CCK secretion following repeat challenge with C12 FA.
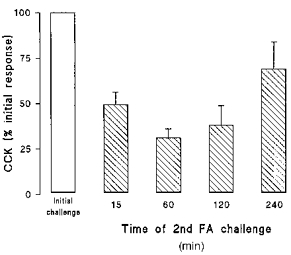
Cells were challenged with 500 μm C12 for 15 min, after which the FA was removed and cells bathed in control medium for varying times (15–240 min) prior to a second challenge with C12. Results show CCK secretion resulting from the second exposure to C12 expressed as a percentage of the initial response. Data are means + s.e.m. for 9 observations in each group.
Role of intracellular calcium in FA response
In view of the evidence that increases in intracellular calcium ([Ca2+]i), are an important regulatory signal for CCK secretion in enteroendocrine cells, the effects of C8 and C12 fatty acids on [Ca2+]i were investigated (Fig. 5). Mean basal [Ca2+]i levels in STC-1 were 92 ± 2 nm (n = 125 cells). In four experiments, addition of C8 produced a small increase in [Ca2+]i above basal with a mean of 51 ± 8 nm (n = 77 cells) compared with a mean increase of 230 ± 16 nm with C12 (Fig. 5). In addition the C8 response was transient, peaking ∼30 s after addition and returning rapidly to the basal level even in the continued presence of the FA, while the response to C12 was more sustained. There was evidence of cell-to-cell variability in [Ca2+]i responses, particularly to the C8 fatty acid. In over half the cells studied (39/77), C8 elicited an increase in [Ca2+]i of less than 10% of that seen with C12. However, in a small proportion of cells (8/77), the C8-induced increase in [Ca2+]i was equal to or greater than the C12 response.
Figure 5. Influence of C8 and C12 on intracellular Ca2+ levels in STC-1.
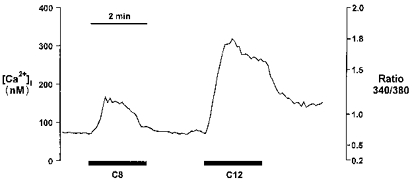
C8 (500 μm) and C12 (500 μm) added sequentially to fura-2-loaded STC-1 cells as indicated by the bars. The figure shows a single experiment with averaged data from 11 cells. Mean data from several experiments are given in the text.
The likely source of the FA-induced rise in [Ca2+]i was investigated using the L-type Ca2+ channel blocker nicardipine. Figure 6 shows the [Ca2+]i responses to subsequent additions of 500 μm C12. Under control conditions (upper trace), C12 generated a mean increase in [Ca2+]i of 367 ± 35 nm (n = 109 cells from 4 experiments). Re-application of C12 after a 15 min wash period produced a similar increase in [Ca2+]i of 297 ± 33 nm. Similar studies in a further sixty cells (from 4 experiments), but with 5 μm nicardipine present during the second application of fatty acid (Fig. 6, lower trace), resulted in a marked (∼90%) inhibition of the [Ca2+]i response (P < 0.001 compared with control 2nd response) suggesting the involvement of voltage-gated L-type Ca2+ channels in the response to FA. In separate studies, 5 μm nicardipine abolished the increase in CCK secretion stimulated by 500 μm C12 (Fig. 7).
Figure 6. Effect of L-type Ca2+ channel blockade on C12-induced increases in intracellular Ca2+.
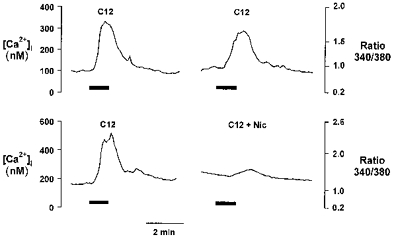
Upper trace, Ca2+ response to sequential addition of 500 μm C12 in STC-1 cells. Lower trace, response to sequential addition of C12 where cells have been pretreated with 5 μm nicardipine for 5 min following the initial C12 challenge. Addition of C12 is shown by the bars. The break in each trace represents 5 min during which the cells were perfused with buffer (upper) or buffer + nicardipine (lower) but no fluorescence images or Ca2 +measurements were taken. Figures show results of single experiments with averaged data from 12 (upper trace) and 36 (lower trace) cells. Mean data from several experiments are given in the text.
Figure 7. Inhibition of C12-induced CCK secretion by nicardipine.
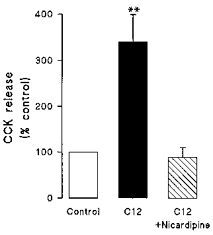
Effects of 500 μm C12 on CCK secretion in cells pretreated with 5 μm nicardipine ( ) or vehicle (▪) for 5 min. Data are shown as the percentage of CCK released in the absence of C12 and are means + s.e.m. of 9 observations in each group. **P < 0.01.
) or vehicle (▪) for 5 min. Data are shown as the percentage of CCK released in the absence of C12 and are means + s.e.m. of 9 observations in each group. **P < 0.01.
DISCUSSION
Recent evidence from in vivo studies indicates that dietary fatty acids with a minimum acyl chain length of twelve carbon atoms cause a marked increase in peripheral blood CCK concentration in healthy human subjects (McLaughlin et al. 1996). Acyl chain length dependence of FAs for elevation of plasma CCK levels may be important in terms of the differential handling of FAs by the digestive tract. Longer chain fats are more resistant to hydrolysis and require a slower presentation to the small intestine, along with bile salt delivery for efficient assimilation (Tso, 1994). Since CCK is needed to achieve these optimal conditions by stimulation of gall bladder contraction and pancreatic secretion and inhibition of gastric emptying, the ability of longer chain FAs to stimulate CCK release would facilitate their digestion.
The present study using STC-1 cells demonstrates that FAs can act directly on CCK-producing cells to stimulate CCK secretion via a mechanism involving an increase in [Ca2+]i mediated by the activation of L-type Ca2+ channels. Importantly, the chain length dependence for these effects in STC-1 cells appears to be similar to that observed in humans in vivo with FAs of ≥ C12 being the most effective stimulators of CCK secretion. Minor differences in FA-induced secretion between STC-1 and human systems were apparent, notably that C10 was able to stimulate CCK secretion in STC-1 cells, albeit at 5-fold higher concentrations than C12, whereas in humans C10 was reported to be completely ineffective (McLaughlin et al. 1996). These differences may reflect species variation though currently the in vivo murine response to FA is not known.
The present study in the STC-1 cell line provides some insight into the physicochemical characteristics of chain length-dependent FA-induced CCK secretion. Aqueous solubility, which would be expected to correlate with the level of monomeric FAs in solution, does not appear to be a primary factor in determining the ability of FAs to stimulate responses in STC-1 cells. The longer chain FAs, C12 and above, have much poorer aqueous solubility (Weast, 1976) than short chain FAs but are the only ones effective in stimulating CCK secretion. This is reinforced by the fact that longer chain, unsaturated FAs (C14:1 and C18:1) while more soluble than saturated C12 exhibit a similar potency in stimulating CCK secretion.
In the present study FAs were routinely presented as an aqueous suspension to mimic more closely the environment in the gut lumen initially after feeding a pure fatty acid meal, as used in our in vivo studies (McLaughlin et al. 1996). In many studies on the cellular effects of FAs, albumin is utilized as a FA carrier since it functions in this role in the circulation and interstitial fluids, and also allows free fatty acid concentrations to be controlled (Spector et al. 1971; Elsing et al. 1995). However, albumin is unlikely to play a physiological role in fatty acid presentation in the gut lumen even after a normal meal. Moreover, the binding relationships for FAs of short and medium chain lengths in the range used in these studies are unknown.
In addition to chain length, the current studies show that FA-stimulated CCK secretion requires a non-esterified FA carboxyl group. The reasons for this structural requirement are unclear but could include binding to a key ligand in the secretory signalling pathway. This is not without precedent, for example, a similar requirement has been noted for CCK secretion in man, the CCK secretory effect of oleic acid not being shared by oleyl alcohol (Olsen et al. 1989). Moreover, binding to fatty acid binding protein also appears to occur via the carboxyl group (Thumser et al. 1996). However, not all enterocyte responses to FA require a free carboxyl group: Barber et al. (1991) showed that neurotensin release from canine enteric endocrine cells was stimulated by oleic methyl ester.
The preliminary evidence that a poorly metabolizable C12 analogue was equally effective in stimulating CCK secretion is interesting given that FA-mediated insulin secretion from the islet β-cell requires their metabolism to influence ATP:ADP ratios and thereby modulate KATP channel activity (Prentki, 1996). While KATP channels have been demonstrated in STC-1 cells (Mangel et al. 1994; Mangel, 1995), the data make it unlikely that ATP:ADP ratios are a key factor in the FA-induced regulation of CCK release.
Throughout this study we were particularly concerned to eliminate the possibility that FA-induced secretion of CCK resulted primarily from non-specific damage to STC-1 cells. Several pieces of evidence point to a specific FA effect on the CCK secretory process in this system. First, maximal stimulation of CCK secretion by C12 was not accompanied by an increased release of the cytosolic enzyme LDH. C12 did elicit LDH release but only at concentrations severalfold higher (5–10 mm) than those required to induce CCK secretion. Second, C12 produced only a transient increase in the rate of CCK secretion even during prolonged exposure, leaving the greater part of total cellular CCK content unsecreted. It would be expected that, under these conditions, the unsecreted CCK fraction would continue to be liberated if significant cell damage were occurring. Third, while CCK secretion did become transiently refractory to a second FA challenge, the responsiveness to C12 returned to near normal after a delay of 4 h. The delay in recovery may reflect the time required to replenish fully processed secretory vesicles following their depletion by the initial FA challenge. These observations argue strongly that FA modulates CCK secretion in STC-1 cells via a highly specific mechanism.
Elucidating the molecular interaction between specific FA and the Ca2+-signalling process will be an important aim of future studies. FA-induced increases in [Ca2+]i were recently demonstrated in an enterocyte cell line (IEC6) in response to oleic acid (C18:1) but not to octanoic acid (C8:0) (Shintani et al. 1995). A free carboxyl group was also required for this response. Calcium has long been recognized as a key mediator in stimulus-secretion coupling and recent studies (Mangel, 1995; Mangel et al. 1995) have shown it to be a final common mediator for several activators of CCK secretion in STC-1 cells including phenylalanine, glucose and bombesin. The data presented here argue strongly that FA-induced CCK secretion from STC-1 enteroendocrine cells involves an elevation of [Ca2+]i associated with the influx of extracellular calcium, through L-type voltage-gated calcium channels. In other types of endocrine cell, depolarization-evoked Ca2+ influx through Ca2+ channels has been found to be a more effective stimulus for exocytosis than the release of Ca2+ from intracellular stores (Schlegel & Mollard, 1995).
At this stage, the nature of the recognition system that couples specific chain length FA to the Ca2+-signalling process is unknown. FA may act at the plasma membrane to gate ion channels directly in a chain length-dependent manner (Ordway et al. 1991; Petrou et al. 1995). In this respect it is interesting that FAs with a chain length of twelve carbon atoms or more were recently shown to activate voltage-dependent Ca2+ currents in cardiac myocytes (Huang et al. 1992). Alternatively, FA may interact with an, as yet, unidentified membrane receptor coupled to the Ca2+-signalling process. FAs can also enter the cell via passive diffusion or in a specific manner by facilitated transport, from where they have been reported to modulate directly second messenger processes including those involving protein kinases A and C, adenylate cyclase and phospholipases, as well as generating indirect effects via protein acylation or metabolites such as eicosanoids (Graber et al. 1994). Resolving these questions will be an important aim of future studies.
In summary, the STC-1 cell line provides a useful model for further detailed study of FA-induced CCK secretion. It is intrinsically capable of responding to FAs in a pattern similar to that in vivo and so may permit understanding of the mechanisms by which the gut detects, discerns and responds appropriately to its lipid content, at both a cellular and molecular level.
Acknowledgments
These studies were supported by grants from the Biotechnology and Biological Sciences Research Council and The Wellcome Trust.
References
- Ballinger A, McLoughlin L, Medbak S, Clark M. Cholecystokinin is a satiety hormone in humans at physiological postprandial conditions. Clinical Science. 1995;89:375–381. doi: 10.1042/cs0890375. [DOI] [PubMed] [Google Scholar]
- Barber DL, Cacace AM, Raucci DY, Ganz MB. Fatty acids stereospecifically stimulate neurotensin release and increase [Ca2+]i in enteric endocrine cells. American Journal of Physiology. 1991;268:G497–503. doi: 10.1152/ajpgi.1991.261.3.G497. [DOI] [PubMed] [Google Scholar]
- Beardshall K, Frost G, Morarji Y, Domin J, Bloom SR, Calam J. Saturation of fat and cholecystokinin release. Lancet. 1989;ii:1008–1010. doi: 10.1016/s0140-6736(89)91017-9. [DOI] [PubMed] [Google Scholar]
- Chang CH, Chey WY, Sun Q, Leiter A, Chang T. Characterization of the release of cholecystokinin from a murine neuroendocrine tumor cell line, STC-1. Biochimica et Biophysica Acta. 1994;1221:339–347. doi: 10.1016/0167-4889(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Elsing C, Winn-Borner U, Stremmel W. Confocal analysis of hepatocellular long-chain fatty acid uptake. American Journal of Physiology. 1995;269:G842–851. doi: 10.1152/ajpgi.1995.269.6.G842. [DOI] [PubMed] [Google Scholar]
- Graber R, Sumida C, Nunez EA. Fatty acids and cell signal transduction. Journal of Lipid Mediators and Cell Signalling. 1994;9:91–116. [PubMed] [Google Scholar]
- Guimbaud R, Moreau JA, Bouisson M, Durand S, Escorrou J, Vaysse N, Frexinos J. Intraduodenal free fatty acids rather than triglycerides are responsible for the release of CCK in humans. Pancreas. 1997;14:76–82. doi: 10.1097/00006676-199701000-00012. [DOI] [PubMed] [Google Scholar]
- Hildebrand P, Beglinger C, Gyr K, Jansen JBMJ, Rovati LC, Zuercher M, Lamers CBHW, Setnikar I, Stalder GA. Effects of a cholecystokinin receptor antagonist on intestinal phase of biliary and pancreatic responses in man. Journal of Clinical Investigation. 1990;85:640–646. doi: 10.1172/JCI114486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopman WPM, Kerstens PJSM, Jansen JBMJ, Rosenbusch G, Lamers CBHW. Effect of graded physiologic doses of cholecystokinin on gallbladder contraction measured by ultrasonography. Gastroenterology. 1985;89:1242–1247. doi: 10.1016/0016-5085(85)90639-0. [DOI] [PubMed] [Google Scholar]
- Huang JM, Xian H, Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proceedings of the National Academy of Sciences of the USA. 1992;89:6452–6456. doi: 10.1073/pnas.89.14.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs PF, Lardas S, Forgacs IC, Dowling RH, Ellam SL, Adrian TC, Bloom SR. Comparison of effects of ingested medium- and long-chain triglycerides on gallbladder volume and release of cholecystokinin and other gut peptides. Digestive Diseases and Sciences. 1987;32:451–480. doi: 10.1007/BF01296030. [DOI] [PubMed] [Google Scholar]
- Liddle RA. Regulation of cholecystokinin secretion by intraluminal releasing factors. American Journal of Physiology. 1995;32:G319–327. doi: 10.1152/ajpgi.1995.269.3.G319. [DOI] [PubMed] [Google Scholar]
- Liddle RA, Morita ET, Conrad CK, Williams JA. Regulation of gastric emptying in humans by cholecystokinin. Journal of Clinical Investigation. 1986;77:992–996. doi: 10.1172/JCI112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JT, Lucauca MG, Jones MN, Thompson DG, Dockray GJ. CCK is released by C12 but not C10 saturated fatty acyl chain fatty acid in humans: evidence for a critical acyl chain length. Gastroenterology. 1996;110(S4):A1099. [Google Scholar]
- Mangel AW. Electrophysiology of intestinal cholecystokinin secretion. Regulatory Peptides. 1995;56:121–129. doi: 10.1016/0167-0115(95)00017-6. 10.1016/0167-0115(95)00017-6. [DOI] [PubMed] [Google Scholar]
- Mangel AW, Prpic V, Scott L, Liddle RA. Inhibitors of ATP-sensitive potassium channels stimulate intestinal CCK secretion. Peptides. 1994;15:1565–1566. doi: 10.1016/0196-9781(94)90134-1. 10.1016/0196-9781(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Mangel AW, Prpic V, Wong H, Basavappa S, Hurst LJ, Scott L, Garman RL, Hayes JS, Sharara AI, Snow ND, Walsh JH, Liddle RA. Phenylalanine-stimulated secretion of cholecystokinin is calcium dependent. American Journal of Physiology. 1995;268:G90–94. doi: 10.1152/ajpgi.1995.268.1.G90. [DOI] [PubMed] [Google Scholar]
- Olsen O, Ainsworth M, Schaffalitzky DE, Muckadell OB, Cantor P. Effects of oleic acid and oleyl alcohol on cholecystokinin and secretin in plasma and pancreatobiliary secretion. Scandinavian Journal of Gastroenterology. 1989;24:529–532. doi: 10.3109/00365528909093084. [DOI] [PubMed] [Google Scholar]
- Ordway RW, Singer JJ, Walsh JV. Direct regulation of ion channels by fatty acids. Trends in Neurosciences. 1991;14:96–100. doi: 10.1016/0166-2236(91)90069-7. 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- Petrou S, Ordway RW, Kirber MT, Dopico AM, Hamilton JA, Walsh JV, Singer JJ. Direct effects of fatty acids and other charged lipids on ion channel activity in smooth muscle cells. Prostaglandins, Leukotrienes and Essential Fatty Acids. 1995;52:173–178. doi: 10.1016/0952-3278(95)90018-7. 10.1016/0952-3278(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Polak JM, Pearse AGE, Bloom SR, Buchan AJM. Identification of cholecystokinin secreting cells. Lancet. 1975;ii:1016–1018. doi: 10.1016/s0140-6736(75)90297-4. 10.1016/S0140-6736(75)90297-4. [DOI] [PubMed] [Google Scholar]
- Prentki M. New insights into pancreatic cell metabolic signalling in insulin secretion. European Journal of Endocrinology. 1996;134:272–286. doi: 10.1530/eje.0.1340272. [DOI] [PubMed] [Google Scholar]
- Rindi G, Grant SGN, Yiangou Y, Ghatei M, Bloom SR, Bautch VL, Solcia E, Polak J. Development of neuroendocrine tumors in the gastrointestinal tract of transgenic mice. American Journal of Pathology. 1990;136:1349–1363. [PMC free article] [PubMed] [Google Scholar]
- Schlegel W, Mollard P. The Electrophysiology of Neuroendocrine Cells. Boca Raton, FL, USA: CRC Press; 1995. Electrical activity and stimulus secretion coupling in neuroendocrine cells; pp. 23–38. [Google Scholar]
- Shintani T, Takahashi N, Fushiki T, Sugimoto E. The recognition system of dietary fatty acids by the rat small intestinal cells. Bioscience Biotechnology and Biochemistry. 1995;59:479–481. doi: 10.1271/bbb.59.479. [DOI] [PubMed] [Google Scholar]
- Snow ND, Prpic V, Mangel AW, Sharara AI, McVey DC, Hurst LJ, Vigna SR, Liddle RA. Regulation of cholecystokinin secretion by bombesin in STC-1 cells. American Journal of Physiology. 1994;267:G859–865. doi: 10.1152/ajpgi.1994.267.5.G859. [DOI] [PubMed] [Google Scholar]
- Spector AA, Fletscher JE, Ashbrook JD. Analysis of long chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry. 1971;10:3229–3232. doi: 10.1021/bi00793a011. [DOI] [PubMed] [Google Scholar]
- Thumser AEA, Voysey J, Wilton DC. Mutations of recombinant rat liver fatty acid binding proteins at residues 102 and 122 alter its structural integrity and affinity for physiological ligands. Biochemical Journal. 1996;314:943–949. doi: 10.1042/bj3140943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso P. Intestinal lipid absorption. In: Johnson LR, Alpers DH, Christensen J, Jacobson ED, Walsh JH, editors. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 1867–1907. [Google Scholar]
- Weast RC. Handbook of Chemistry and Physics. 57. Boca Raton, FL, USA: CRC Press; 1976. [Google Scholar]


