Abstract
The neuroendocrine system of the lungs has no clear function. However, previous studies of one of its products, somatostatin, have implicated it in lung liquid removal at birth. The present study extends this concept by investigating the effects of dopamine, a major product of this system, on lung liquid reabsorption.
The effects of dopamine on fetal lung liquid production and reabsorption were tested on in vitro lungs from fetal guinea-pigs of 60 ± 2 days of gestation (term = 67 days). Dopamine was placed in the outer bathing saline during the middle hour of 3 h incubations. Fluid movements across the pulmonary epithelium were monitored by a dye dilution method, and changes in rates over 1 h intervals were tested for significance by analysis of variance and regression analysis.
Dopamine was able to reduce fluid production or cause reabsorption (based on 42 preparations). Control preparations and those given 10−8m dopamine showed no significant changes; those given higher concentrations showed significant reductions in production or reabsorption (P < 0.025 to P < 0.0005), according to dose (42.6 ± 10.8 % reduction at 10−7m; 75.4 ± 5.9 % reduction at 10−6m; 92.1 ± 7.0 % reduction at 10−5m and 121.4 ± 12.8 % (reabsorption) at 10−4m dopamine). The linear log dose-response curve (r = 0.99) showed a theoretical threshold at 1.7 × 10−9m dopamine.
Effects were mediated through specific dopamine receptors (based on 78 preparations). Dopamine at 10−6m was tested together with each of three dopamine receptor antagonists at 10−5m. The general dopamine receptor antagonist haloperidol and the more specific D2 receptor blocker domperidone both abolished responses, but the D1 receptor antagonist SCH 23390 was without effect. This suggested that D2 dopamine receptors mediated the responses, and that responses were not due to conversion of dopamine to adrenaline or noradrenaline.
There was no evidence that responses involved amiloride-sensitive Na+ transport (based on 54 preparations). Apical amiloride at 10−6, 10−5 or 10−4m, and the more specific Na+ channel blocker benzamil (10−5m), had no effect on responses to dopamine, in contrast to their effects on responses to adrenaline in sheep.
It is suggested that internal release of dopamine by the neuroendocrine system of the lungs may influence lung liquid reabsorption at birth. This system, which also produces somatostatin, another agent active on lung liquid production, is maximally developed and activated at birth; it is also deficient in hyaline membrane disease.
During gestation, fetal lungs produce large quantities of fluid which contribute substantially to the amniotic fluid, particularly in the guinea-pig, where production rates appear to be higher than those for urine (Strang, 1991; Perks et al. 1992). This production is based on a Na+-K+-2Cl− cotransport system, probably located in type II cells (Strang, 1991). However, at birth it is vital for this process to be replaced by fluid reabsorption, and it is generally agreed that this is brought about by an amiloride-sensitive, Na+-based transport system, probably augmented by colloid osmotic effects (Strang, 1991).
The first agent shown to activate fluid reabsorption was adrenaline (sheep: Walters & Olver, 1978; goat: Perks & Cassin, 1989). However, in the guinea-pig, reabsorption can be produced by both adrenaline and noradrenaline, which, unlike the β-receptor activation in sheep, act through α-adrenoreceptors (Doe & Perks, 1998); although the transport mechanisms involved were not investigated, there was little reason to suspect that they were not the same Na+-based reabsorptive system found in other species (Woods et al. 1997; S. Doe, B. A. Woods & A. M. Perks, unpublished observations). Again, it was assumed that in vivo, the catecholamines would come from the adrenal glands. However, there could be sources in the lung. This became a serious possibility when acetylcholine was shown to produce powerful reabsorption in in vitro lungs, apparently by liberation of catecholamines within the pulmonary tissue (Woods et al. 1996). The source of these catecholamines was not known. Although a number of sources could be suggested, an important possibility was the internal endocrine system of the lungs, the complex of scattered neuroepithelial cells and discrete neuroepithelial bodies that show many similarities to the chromaffin cells of the adrenal medulla (Scheuermann, 1991). Like the adrenal medulla, the neuroepithelial bodies receive cholinergic innervation. Significantly, this system is maximally developed around birth, and contains more cell types than in the adult; it appears to be activated at birth, although the function is unknown (Cutz et al. 1974; Hage, 1976; Sorokin et al. 1982; Redick & Hung, 1984; Cutz et al. 1984; Scheuermann, 1991). The cells show immunoreactivity typical of many agents, notably somatostatin, a peptide which appears to be generated after the first breath and which is known to halt lung liquid production in in vitro lungs (Perks et al. 1992). The cells contain chromogranins, typical of the adrenal medulla, but their main catecholamine appears to be dopamine (Scheuermann, 1991). However, dopamine has never been tested for effects on lung liquid production; this omission is rectified in the results presented here, and a number of unusual results were obtained.
METHODS
Animals
Pregnant albino guinea-pigs of an inbred departmental stock were given food and water ad libitum (guinea-pig chow, Ralston-Purina, supplemented with fresh vegetables and vitamin C). Treatment of the animals was in accordance with the Canadian Council for Animal Care, and in conditions approved by the Animal Care Committee of the University of British Columbia. Studies were performed on 144 fetuses of 60 ± 2 days of gestation (term = 67 days) and 81.0 ± 11.4 g body wt (means ±s.d.).
Experimental procedures
The rate of lung liquid production was measured by an impermeant tracer technique, using Blue Dextran 2000 (Pharmacia, Dorval, Québec, Canada; molecular mass, 2000 kDa, Stokes radius, 27 nm (270 Å); radius of gyration, 38 nm (380 Å)). The basis of the method and confirmation of its validity have been given previously; simultaneous use of the Dextran with 125I-labelled albumin tracer showed close agreement in rates, but the dye gave less scatter around the lines of best fit (see below) (Cassin & Perks, 1982; Perks et al. 1990).
Pregnant guinea-pigs were anaesthetized with halothane (Fluothane; Ayers, Montreal, Québec, Canada) until full inhibition of the corneal reflex; final death was produced by severing the carotid arteries. The fetuses were removed by Caesarian section, with their amniotic sacs intact, and transferred to Krebs-Henseleit saline; no fetal breathing movements were seen. A mid-line incision through the thorax exposed the lungs and trachea. The trachea was ligated rostrally and cannulated caudally with 1.5–2.0 cm of polyethylene tubing filled with saline (PE50, Intramedic, Clay Adams, Parsippany, NJ, USA). The cannula was attached to a 1.0 ml tuberculin reservoir syringe via an 18-gauge hypodermic needle and 3-way stopcock. The cannula was tied in place with double ligatures just above the bifurcation of the bronchi; this eliminated the trachea itself from study. The trachea was then severed rostral to the cannula. The lungs were then separated from their vascular attachments and from the oesophagus. The heart was also removed. During all this time the lungs were kept warm and moist by frequent washes with Krebs-Henseleit saline at 37°C. The preparations were then suspended in 100 ml baths of Krebs- Henseleit saline at 37°C, oxygenated and maintained at pH 7.4 with 95 % O2 and 5 % CO2. The baths were protected from light with aluminium foil. It was important to set up the preparations rapidly (within 3–4 min). The lung liquid was withdrawn into the reservoir syringe and 100 μl of Blue Dextran 2000 (50 mg ml−1 in 0.9 % NaCl) were added to this fluid, thoroughly mixed in, and the mixture was replaced into the lung lumen. The preparations were equilibrated for 30 min, and the lung fluid was withdrawn and returned every 5 min throughout this period; this ensured an even distribution of dye throughout the lungs.
After equilibration, experiments continued for 3 h. During this time fluid was withdrawn as before, every 10 min, and 10 μl samples were taken from the upper cup of the stopcock with a 1701 NCH gas-tight fixed volume syringe (Hamilton Co., Reno, NV, USA). Fluid was also withdrawn and returned to the lungs midway between sampling; this ensured proper mixing within the lungs. Mixing was also aided by the gentle but continuous movements of the lungs in the bubbled saline. Samples were placed in 250 μl polyethylene Eppendorf micro test-tubes, diluted 1 : 20 with distilled water, sealed and vortexed. Samples were then centrifuged at 250 g for 10 min. The supernatants were estimated for Blue Dextran by spectrophotometry using 250 μl quartz microcells (λ= 620 nm). The experiments followed an ABA design. Samples taken during the first hour after equilibration gave the resting rate of fluid production. The lungs, still attached to their reservoir syringe, were then transferred to fresh Krebs-Henseleit saline which contained one of the following: (a) dopamine hydrochloride at 10−8, 10−7, 10−6, 10−5 or 10−4m (Sigma); (b) 10−6m dopamine with 10−5m haloperidol (Sigma); (c) 10−5m haloperidol alone; (d) 10−6m dopamine with 10−5mR(+)-SCH23390 hydrochloride (RBI); (e) 10−5m SCH23390 alone; (f) 10−6m dopamine with 10−5m domperidone (RBI); (g) 10−5m domperidone alone; (h) 10−4m dopamine with 10−6, 10−5 or 10−4m amiloride hydrochloride (Sigma); (i) 10−6, 10−5 or 10−4m amiloride alone; (j) 10−4m dopamine with 10−5m benzamil hydrochloride (RBI); (k) 10−5m benzamil alone; (l) 0.01 % ascorbic acid control, as appropriate (Sigma); (m) 1 % methanol control, as appropriate; (n) Krebs-Henseleit saline with no drugs (untreated controls; these preparations received changes of saline on each hour, as for experimental preparations). In all studies involving dopamine, 0.01 % ascorbic acid was placed in the outer saline during the treatment, since this agent prevents the rapid degradation of dopamine in solutions containing sodium bicarbonate at above pH 6.8; appropriate controls of ascorbic acid alone were also tested. In all studies of amiloride and benzamil, the agents were placed directly into the lung liquid (apically) just before the treatment hour; they were also placed in the outer saline, at the same concentration, in order to reduce possible loss of the small molecules down an outward concentration gradient. In all studies of domperidone, the agent was dissolved initially in methanol, and then diluted down to a final concentration of 10−5m with Krebs-Henseleit saline; appropriate controls of 1 % methanol were also tested. Concentrations of dopamine antagonists were based on preliminary experiments, and on studies on in vitro tissues of guinea-pigs by Takahashi et al. (1991) and Capasso & Sorrentino (1997). Concentrations of Na+ channel blockers were based on earlier studies and Olver et al. (1986) and O'Brodovich et al. (1990). In all experiments, the preparations were returned to Krebs-Henseleit saline for the final hour. Experimental runs never exceeded 3 h, and preparations were never used more than once.
Quantification of results and statistical methods
The rates of fluid production were calculated from the fall in concentration of Blue Dextran, as described previously (Cassin & Perks, 1982; Perks et al. 1990). Rates were estimated from plots of the total volume of fluid against time, with readings recorded every 10 min; the total volume of fluid was the sum of that within the lungs and that removed for study. Appropriate sequential adjustments were made every 10 min for the removal of both fluid and Dextran during incubation. The rates of production of fluid over 1 h intervals were calculated from the volume plots, using the slopes of their regressions, fitted by the method of least squares (Steel & Torrrie, 1970; Hewlett-Packard program SD-03A, or by Apple II Plus computer). In groups of similar experiments, differences in rates in successive hours were analysed by analysis of variance and Scheffé's contrast test (ANOVA) (Zar, 1984). When plots from similar experiments were combined, the volumes were expressed as a percentage of the volume present at the end of the first hour, just prior to transfer to test solutions or fresh saline; the values were then averaged. The significance of changes in rate were also assessed from the combined graphs by submitting the changes in slope to a test for differences between two regressions (regression analysis); this test utilized all values for volumes from all experiments in the group. While both ANOVA and regression analysis considered the magnitude of the changes seen, ANOVA took into consideration the repeatability of the responses, and regression analysis allowed for scatter around the lines of best fit, a variability not included in ANOVA. All mean values are given with their standard errors, unless otherwise stated. Statistical significance was accepted at or below P < 0.05.
RESULTS
The effect of dopamine on lung liquid production
Studies were carried out on forty-two fetuses of 61 ± 1 days of gestation and 81.9 ± 9.5 g body wt (means ±s.d.). Results are shown in Fig. 1. Untreated controls, controls given 0.01 % ascorbic acid alone (see Methods) and preparations given 10−8m dopamine (all n = 6) showed no significant changes (ANOVA, regression analysis; Fig. 1A, B and C). All six preparations given 10−7m dopamine showed significant reductions in fluid production (Fig. 1D; 42.6 ± 10.8 % reduction; P < 0.025, both ANOVA and regression analysis). All six preparations given 10−6m dopamine showed significant reductions in production, with one stopping production (Fig. 1E; 75.4 ± 5.9 % reduction; P < 0.0005, both ANOVA and regression analysis). At the higher concentration of 10−5m dopamine, one preparation reabsorbed fluid, three stopped production, and two reduced to a slight production only; the overall effect was a significant and almost complete arrest of production (Fig. 1F; 92.1 ± 7.0 % fall; P < 0.0005, both ANOVA and regression analysis). Of six preparations treated with the highest concentration of dopamine, 10−4m, three showed strong reabsorption and three stopped production; the overall effect was fluid reabsorption (Fig. 1G; 121.4 ± 12.8 % reduction; P < 0.0005, both ANOVA and regression analysis). In most studies there was some tendency towards recovery in the final hour, but it was less marked than in earlier work with adrenaline and noradrenaline (Doe & Perks, 1998). When the responses were quantified as percentage reductions between the hours before and during treatment (as above), the responses showed a linear relationship with the log concentration of dopamine (Fig. 2; r = 0.99); the theoretical threshold was 1.7 × 10−9m dopamine.
Figure 1. Effects of dopamine on lung liquid production by in vitro lungs from fetal guinea-pigs.
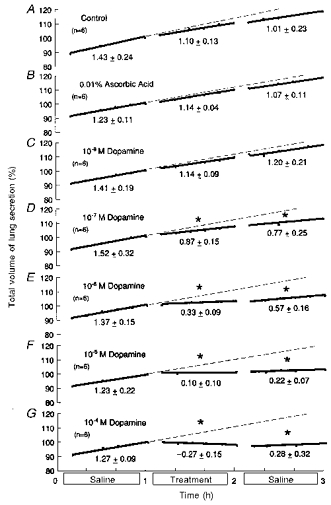
Based on 42 fetuses of 61 ± 1 days of gestation and 81.9 ± 9.5 g body wt (means ± s.d.). During the middle hour, lungs were immersed in saline containing: saline alone (untreated preparations) (A); 0.01 % ascorbic acid control (B); 10−8 m dopamine (C); 10−7 m dopamine (D); 10−6 m dopamine (E); 10−5 m dopamine (F); or 10−4 m dopamine (G). Ordinates: total volume of lung liquid expressed as a percentage of that present at the end of the first hour, immediately prior to treatment, where 100 % was: 0.91 ± 0.23 ml (means ± s.d.) (A); 0.92 ± 0.11 ml (B); 1.04 ± 0.12 ml (C); 1.08 ± 0.27 ml (D); 1.05 ± 0.16 ml (E); 0.97 ± 0.07 ml (F); and 0.94 ± 0.13 ml (G). Abscissae: time in hours. All regressions are lines of best fit; slopes represent production rates. Values below the lines are average production rates in ml (kg body wt)−1 h−1. Asterisks above the lines show significant changes from the rate for production in the first hour (ANOVA). Standard errors of the mean are omitted for clarity, but they averaged: 1.21 ± 0.12 (A); 0.96 ± 0.09 (B); 1.25 ± 0.13 (C); 1.09 ± 0.12 (D); 1.09 ± 0.13 (E); 1.02 ± 0.09 (F); and 1.22 ± 0.17 (G). Corresponding coefficients of variation were: 2.90 ± 0.31 % (A); 2.26 ± 0.20 % (B); 2.96 ± 0.29 % (C); 2.59 ± 0.27 % (D); 2.61 ± 0.30 % (E); 2.56 ± 0.23 % (F); and 3.05 ± 0.41 % (G).
Figure 2. Relationship between log concentration of dopamine and the response of in vitro lungs from fetal guinea-pigs.
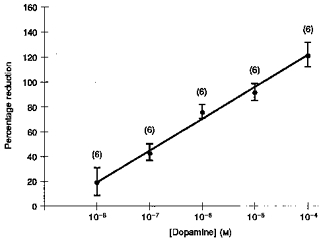
Ordinate: responses quantified as the percentage reduction of resting fluid production (first hour) during the hour of treatment (second hour). Vertical bars show ± s.e.m.; numbers above the bars give the number of preparations tested. Abscissa: concentrations of dopamine (M).
The effect of dopamine receptor antagonists on responses to dopamine
The effects of haloperidol
The importance of the above effects would be strongly supported if they were shown to involve specific dopamine receptors. Therefore, the effects of the general dopamine receptor antagonist haloperidol were investigated (Capasso & Sorrentino, 1997). Studies were carried out on twenty-four fetuses of 61 ± 1 days of gestation and 85.6 ± 7.9 g body wt (means ±s.d.). Results are shown in Fig. 3.
Figure 3. Effects of haloperidol on responses to dopamine.
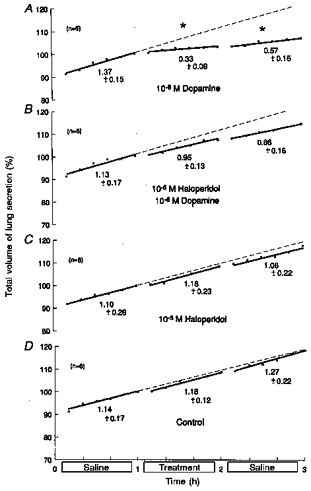
Based on 24 fetuses, 61 ± 1 days of gestation and 85.6 ± 7.9 g body wt. During the middle hour, lungs were immersed in saline containing: 10−6 m dopamine alone (A); 10−6 m dopamine with 10−5 m haloperidol (B); 10−5 m haloperidol alone (C); or saline alone (untreated controls) (D). Ordinates: total volume of lung liquid expressed as a percentage of that immediately before treatment, where 100 % was: 1.05 ± 0.16 ml (means ± s.d.) (A); 0.95 ± 0.11 ml (B); 0.98 ± 0.17 ml (C); and 1.01 ± 0.17 ml (D). Abscissae: time in hours. All regressions are lines of best fit; slopes represent production rates. Values below the lines are average production rates in ml (kg body wt)−1 h−1. Asterisks above the lines show significant changes from the rate of production in the first hour (ANOVA). Standard errors of the mean are omitted for clarity, but they averaged: 1.05 ± 0.16 (A); 0.77 ± 0.09 (B); 2.12 ± 0.22 (C); and 1.08 ± 0.12 (D). Corresponding coefficients of variation were: 2.61 ± 0.30 % (A); 1.82 ± 0.22 %(B); 4.92 ± 0.47 % (C); and 2.53 ± 0.26 % (D).
The significant reductions in fluid production produced by 10−6m dopamine (Fig. 3A; P < 0.0005, ANOVA and regression analysis; n = 6) were abolished by 10−5m haloperidol (Fig. 3B; n = 6). Haloperidol (10−5m) alone and untreated controls showed no significant changes (ANOVA, regression analysis; Fig. 3C and D; n = 6, respectively). It was concluded that the effects of dopamine were mediated through dopamine receptors.
The effects of SCH23390
To determine the subtype of receptor activated by dopamine, the more specific D1 receptor antagonist SCH23390 was investigated (Takahashi et al. 1991; Capasso & Sorrentino, 1997). Studies were carried out on twenty-four fetuses of 60 ± 1 days of gestation and 79.0 ± 11.8 g body wt (means ±s.d.). Results are shown in Fig. 4.
Figure 4. Effects of SCH23390 on responses to dopamine.
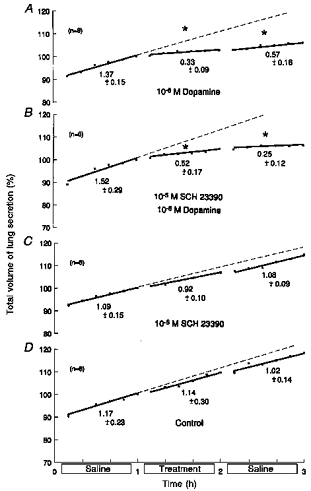
Based on 24 fetuses, 60 ± 1 days of gestation and 79.0 ± 11.8 g body wt (means ± s.d.). During the middle hour, lungs were immersed in saline containing: 10−6 m dopamine alone (A); 10−6 m dopamine with 10−5 m SCH23390 (B); 10−5 m SCH23390 alone (C); or saline alone (untreated controls) (D). Ordinates: total volume of lung liquid expressed as a percentage of that immediately before treatment, where 100 % was: 1.05 ± 0.16 ml (means ± s.d.) (A); 0.90 ± 0.13 ml (B); 0.89 ± 0.15 ml (C); and 0.73 ± 0.12 ml (D). Abscissae: time in hours. All regressions are lines of best fit; slopes represent production rates. Values below the lines are average production rates in ml (kg body wt)−1 h−1. Asterisks above the lines show significant changes from the rate of production in the first hour (ANOVA). Standard errors of the mean were omitted for clarity, but they averaged: 1.05 ± 0.16 (A); 0.76 ± 0.09 (B); 0.76 ± 0.08 (C); and 0.81 ± 0.09 (D). Corresponding coefficients of variation were: 2.61 ± 0.30 % (A); 1.85 ± 0.24 % (B); 1.82 ± 0.19 % (C); and 1.93 ± 0.23 % (D).
The significant reductions in fluid production produced by 10−6m dopamine (Fig. 4A; n = 6) were unaffected by 10−5m SCH23390 (Fig. 4B; n = 6). Reductions in the presence of the antagonist were significant by ANOVA (P < 0.0025) and regression analysis (P < 0.0005), and ANOVA showed no significant differences between responses with or without the blocker, quantified as percentage reductions, as above. SCH23390 (10−5 M) alone and untreated controls showed no significant changes (ANOVA, regression analysis; Fig. 4C and D, respectively). It was concluded that there was no evidence that D1 receptors were involved in responses to dopamine.
The effect of domperidone
Since D1 receptors did not appear to be involved, D2 receptors were investigated. Use was made of the specific D2 receptor antagonist domperidone (Takahashi et al. 1991). Studies were based on thirty fetuses of 61 ± 2 days of gestation and 81.4 ± 11.8 g body wt (means ±s.d.). Results are shown in Fig. 5.
Figure 5. Effects of domperidone on responses to dopamine.
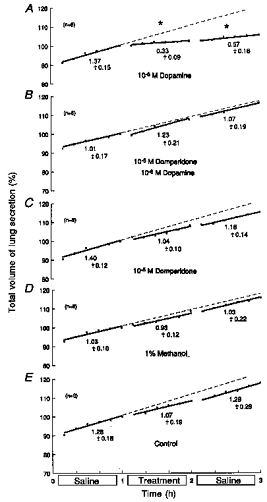
Based on 30 fetuses of 61 ± 2 days of gestation and 81.4 ± 11.8 g body wt (means ± s.d.). During the middle hour, lungs were immersed in saline containing: 10−6 m dopamine alone (A); 10−6 m dopamine with 10−5 m domperidone (B); 10−5 m domperidone alone (C); 1 % methanol (solvent) alone (D); or saline alone (untreated controls) (E). Ordinates: total volume of lung liquid expressed as a percentage of that immediately before treatment, where 100 % was: 1.05 ± 0.16 ml (means ± s.d.) (A); 0.91 ± 0.22 ml (B); 1.04 ± 0.17 ml (C); 1.01 ± 0.08 ml (D); and 1.05 ± 0.28 ml (E). Abscissae: time in hours. All regressions are lines of best fit; slopes represent production rates. Values below the lines are average production rates in ml (kg body wt)−1 h−1. Asterisks above the lines show significant changes from the rate of production in the first hour (ANOVA). Standard errors of the mean are omitted for clarity, but they averaged: 1.05 ± 0.16 (A); 1.48 ± 0.20 (B); 0.71 ± 0.11 (C); 0.95 ± 0.12 (D); and 0.94 ± 0.14 (E). Corresponding coefficients of variation were: 2.61 ± 0.30 % (A); 3.38 ± 0.41 % (B); 1.66 ± 0.24 % (C); 2.18 ± 0.26 % (D); and 2.19 ± 0.30 % (E).
The significant reductions in fluid production produced by 10−6m dopamine (Fig. 5A; n = 6) were completely abolished by 10−5m domperidone; in fact, production rose slightly (Fig. 5B; n = 6). Domperidone (10−5m) alone had no significant effect as judged by ANOVA, although the slight fall in production was significant by regression analysis (P < 0.01) (Fig. 5C; n = 6). Methanol (1 %; carrier) and untreated controls showed no significant changes (Fig. 5D and E; n = 6, respectively). The results suggest that the effects of dopamine were mediated through D2 receptors.
The effects of Na+ channel blockers on responses to dopamine
Since all previous investigations had shown that reabsorptions depended on Na+-based, amiloride-sensitive mechanisms, the effects of the Na+ channel blockers amiloride and benzamil were tested.
The effects of amiloride
Studies were carried out on forty-two fetuses of 60 ± 1 days of gestation and 78.6 ± 12.1 g body wt (means ±s.d.). They investigated amiloride at 10−6, 10−5 and 10−4m, but all investigations used 10−4m dopamine, since this produced clear reabsorptions. Initial studies of 10−6m amiloride were based on four groups of six preparations, but were supplemented by six extra experiments, since the results obtained were unexpected.
The significant reabsorptions produced by 10−4m dopamine (Fig. 6A; n = 6) were unaffected by 10−6m amiloride (Fig. 6B; n = 12). In the presence of the blocker, reabsorptions were still significant by ANOVA and regression analysis (P < 0.0005, both tests), and ANOVA showed no significant differences between responses with or without amiloride, quantified as before. Amiloride (10−6m) alone and untreated controls showed no significant changes (both tests; Fig. 6C and D; n = 6, respectively).
Figure 6. Effects of 10−6 m amiloride on responses to dopamine.
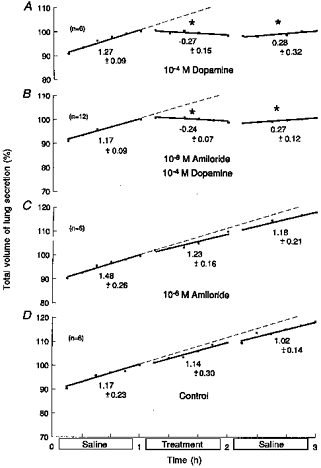
Based on 30 fetuses, 61 ± 2 days of gestation and 79.9 ± 12.5 g body wt (means ± s.d.). During the middle hour, lungs were immersed in saline containing: 10−4 m dopamine alone (A); 10−4 m dopamine with 10−6 m amiloride (B); 10−6 m amiloride alone (C); or saline alone (untreated controls) (D). Ordinates: total volume of lung liquid expressed as a percentage of that immediately before treatment, where 100 % was: 0.94 ± 0.13 ml (means ± s.d.) (A); 0.81 ± 0.03 ml (B); 1.06 ± 0.31 ml (C); and 0.73 ± 0.12 ml (D). Abscissae: time in hours. All regressions are lines of best fit; slopes represent production rates. Values below the lines are average production rates in ml (kg body wt)−1 h−1. Asterisks above the lines show significant changes from the rate of production in the first hour (ANOVA). Standard errors of the mean are omitted for clarity, but they averaged: 1.22 ± 0.17 (A); 0.88 ± 0.10 (B); 1.09 ± 0.1 (C); and 0.81 ± 0.09 (D). Corresponding coefficients of variation were: 3.05 ± 0.41 %(A); 3.07 ± 0.33 %(B); 2.59 ± 0.24 %(C); and 1.93 ± 0.23 %(D).
Since these studies were negative, the concentration of amiloride was increased to 10−5 and 10−4m. Amiloride was still ineffective (Table 1). Significant, even increased reabsorptions persisted, and at all concentrations of amiloride ANOVA was unable to show any significant differences between responses with or without amiloride, quantified as before. Appropriate amiloride controls showed no significant changes (Table 1).
Table 1.
Effect of different concentrations of amiloride and 10−5 m benzamil on responses to 10−4 m dopamine
| Rate of production of lung liquid (ml (kg body wt)−1 h−1) | Sig. of reductions during treatment | Sig. of reductions aafter treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dopamine m | Na+ channel blockers m | n | Hour before treatment | Hour during treatment | Hour after treatment | ANOVA | Regression analysis | ANOVA | Regression analysis |
| 10−4 | 0 | 6 | 1.27 ± 0.09 | −0.27 ± 0.15 | 0.28 ± 0.32 | P < 0.0005 | P < 0.0005 | P < 0.005 | P < 0.0005 |
| Untreated controls | 6 | 1.17 ± 0.23 | 1.14 ± 0.30 | 1.02 ± 0.14 | n.s. | n.s. | n.s. | n.s. | |
| 10−4 | 10−6 amiloride | 12 | 1.17 ± 0.09 | −0.24 ± 0.07 | 0.27 ± 0.12 | P < 0.0005 | P < 0.0005 | P < 0.005 | P < 0.0005 |
| 0 | 10−6 amiloride | 6 | 1.48 ± 0.26 | 1.23 ± 0.16 | 1.18 ± 0.21 | n.s. | n.s. | n.s. | n.s. |
| 10−4 | 10−5 amiloride | 3 | 1.14 ± 0.24 | −0.39 ± 0.09 | −0.39 ± 0.18 | P < 0.025 | P < 0.0005 | P < 0.025 | P < 0.0005 |
| 0 | 10−4 amiloride | 3 | 0.97 ± 0.17 | 0.98 ± 0.12 | 0.70 ± 0.17 | n.s. | n.s. | n.s. | n.s. |
| 10−4 | 10−4 amiloride | 3 | 0.90 ± 0.21 | −0.50 ± 0.11 | −0.09 ± 0.03 | P < 0.005 | P < 0.0005 | P < 0.01 | P < 0.0005 |
| 0 | 10−4 amiloride | 3 | 1.40 ± 0.12 | 1.44 ± 0.46 | 1.18 ± 0.36 | n. s. | n.s. | n.s. | n.s. |
| 10−4 | 10−5 benzamil | 6 | 1.17 ± 0.15 | −0.22 ± 0.16 | −0.08 ± 0.14 | P < 0.0005 | P < 0.0005 | P < 0.0005 | P < 0.0005 |
| 0 | 10−5 benzamil | 6 | 1.06 ± 0.22 | 1.03 ± 0.11 | 1.07 ± 0.15 | n.s. | n.s. | n.s. | n.s. |
All values are means ±s.e.m. Sig., significance.
The effects of 10−5m benzamil
Since amiloride appeared to be ineffective, the results were checked with the more specific Na+ channel blocker, benzamil (O'Brodovich et al. 1990). Studies were based on twenty-four fetuses of 61 ± 1 days of gestation and 89.9 ± 13.4 g body wt (means ±s.d.). Results are shown in Fig. 7.
Figure 7. Effects of benzamil on responses to dopamine.
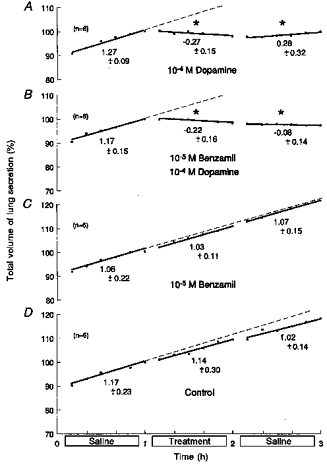
Based on 24 fetuses of 61 ± 1 days of gestation and 89.9 ± 13.4 g body wt (means ± s.d.). During the middle hour, lungs were immersed in saline containing: 10−4 m dopamine (A); 10−4 m dopamine with 10−5 m benzamil (B); 10−5 m benzamil alone (C); or saline alone (untreated controls) (D). Ordinates: total volume of lung liquid expressed as a percentage of that immediately before treatment, where 100 % was: 0.94 ± 0.13 ml (means ± s.d.) (A); 0.87 ± 0.06 ml (B); 0.78 ± 0.11 ml (C); and 0.73 ± 0.12 ml (D). Abscissae: time in hours. All regressions are lines of best fit; slopes represent production rates. Values below the lines are average production rates in ml (kg body wt)−1 h−1. Asterisks above the lines show significant changes from the rate of production in the first hour (ANOVA). Standard errors of the mean are omitted for clarity, but they averaged: 1.22 ± 0.17 (A); 0.87 ± 0.06 (B); 0.98 ± 0.08 (C); and 0.81 ± 0.09 (D). Corresponding coefficients of variation were: 3.05 ± 0.41 % (A); 2.88 ± 0.41 % (B); 2.29 ± 0.20 % (C); and 1.93 ± 0.23 % (D).
The significant reabsorptions produced by 10−4m dopamine (P < 0.0005, ANOVA and regression analysis; Fig. 7A; n = 6) were unaffected by the presence of 10−5m benzamil (Fig. 7B; n = 6). The reabsorptions were still significant by ANOVA and regression analysis (P < 0.0005, both tests), and ANOVA showed no significant differences in responses with or without benzamil, quantified as before. Benzamil (10−5 M) alone and untreated controls showed no significant changes (Fig. 7C and D; n = 6, respectively).
DISCUSSION
The above studies showed that dopamine at 10−5m and 10−4m could cause strong reabsorption of lung liquid from in vitro lungs from fetal guinea-pigs, and at 10−7m and 10−6m there were clear reductions in production; 10−8m dopamine produced no significant responses. Responses showed a linear log dose-response curve (r = 0.99), with a theoretical threshold at 1.7 × 10−9m dopamine. The effect was dependent on the activation of specific dopamine receptors. It was blocked by the general dopamine receptor antagonist haloperidol, and tests with the D1 blocker SCH23390 and D2 antagonist domperidone suggested that D2 receptors were involved. Tests involving fifty-four fetuses and 10−6, 10−5 or 10−4m amiloride and 10−5m benzamil failed to produce any evidence for the involvement of amiloride-sensitive Na+ transport in responses to dopamine.
It must be remembered that these results depend on an in vitro method, and no such method, whether by tissue culture or isolated organ, is entirely physiological. However, no method is free of problems. Although these studies should be extended to the whole animal, in vivo work has problems from complicating influences, such as reflexes, anaesthesia and ‘stress’. Problems with the diffuse and deeply integrated neuroendocrine system are even greater. However, this in vitro model forms a good basis for further work, and has many assets. It allows elimination of external influences, such as reflexes, and a simplification of the many complex variables. It allows the use of agents and antagonists at precise concentrations, free of placental destruction or loss, and the use of agents toxic or with widespread effects in the whole animal. A number of these assets were of use here.
At first sight, dopamine appears an unlikely candidate for control of lung liquid reabsorption. In adult sheep, it only represents about 2 % of the catecholamines in the adrenal medulla (Holzbauer & Sharman, 1972). However, it does enter the blood, and reaches average plasma levels of (1.2 ± 0.3) × 10−9m (means ±s.d.) in late fetal sheep, and rises to (1.2 ± 0.4) × 10−8m just before delivery (calculated from Jones, 1980; Faucher et al. 1987; Reid et al. 1990). The highest levels reported in fetal plasma are seen in rats, where, despite some decline in late gestation, they remain at 1.3 × 10−8m at birth, 2–3 times those of the mother, and constitute about 20–30 % of the total plasma catecholamines (Ben-Johnathan & Maxson, 1978). In late fetal guinea-pigs of the same age used here, plasma concentrations are only (2.8 ± 0.4) × 10−10m (Jelinek & Jensen, 1991); although these are closely similar to those for noradrenaline, they are completely inadequate for influencing lung liquid. Even if, close to birth, there was a 10-fold increase similar to that in sheep, plasma levels would only reach about 3 × 10−9m. Therefore, values in most species only just reach the threshold of our log dose-response curve (1.7 × 10−9m); others fail to reach the 10−7m concentration needed for significant responses, and none show the 10−5 or 10−4m levels necessary for reabsorption.
Despite this, it would be unwise to reject any possible importance of dopamine. Firstly, we do not know the plasma levels during or soon after delivery, when other catecholamines reach their highest levels (Brown et al. 1983); prior to birth, dopamine usually follows the changes in other catecholamines (Jones, 1980). It may be significant that there is a dramatic rise in dopamine in amniotic fluid in rats close to birth, possibly due to passage in the lung liquid (Ben-Johnathan & Maxson, 1978; Gardey-Levassort et al 1981). Secondly, it is possible that responses would increase close to birth, as they do for adrenaline, arginine vasopressin (AVP) and expansion of the lungs (Perks & Cassin, 1989; Walters et al. 1990; Strang, 1991; Garrad Nelson & Perks, 1996); our preparations were almost a week prior to birth. Thirdly, dopamine could be expected to add its effects to those of other catecholamines, but by acting through different receptors, and possibly different mechanisms. However, the most interesting possibility is that dopamine does not act primarily through plasma levels, but by local release within the lungs.
Dopamine is not only found within the lungs of many species, but its levels are unusually high. In the human, but not in every species, concentrations are above those in the adrenal glands, and in lungs of adult guinea-pigs they are greater than those of noradrenaline (Holzbauer & Sharman, 1972). In adult sheep, the lungs and caudate nucleus show the highest concentrations of many tissues studied; both have similar values, close to 10 μg (g fresh tissue)−1 (Juorio & Chedrese, 1990). Although there are no values for lungs of fetal guinea-pigs, the sheep study allows us to make a reasonable estimate. As in sheep, the caudate nucleus of fetal guinea-pigs has the highest levels in the brain, with values not far from those in sheep (5.6 ± 1.9 μg (g frozen tissue)−1; Jelinek & Jensen, 1991). Therefore, if distribution follows that in sheep, the lung would be expected to be rich in dopamine, with about 5–6 μg (g tissue)−1, or 25 times that found in lungs of adult guinea-pigs. This could be an underestimate. Studies in the rat show that dopamine in lungs surges to twice its former level at delivery, and reaches the maximum found in an extended period of study (5 days prenatal to 60 days postnatal); it was suggested that this was related to the remarkable prenatal rise in dopamine in amniotic fluid (Ben-Johnathan & Maxson, 1978; Gardey-Levassort et al. 1981). Clearly, our knowledge is limited, but it all points to high levels of dopamine extractable from lungs, and a surge in concentrations at birth. Local liberation within the lungs could raise high concentrations close to alveolar cells, and potentially influence lung liquid. Such a possibility finds support from morphological and histochemical work.
Histochemical studies suggest that dopamine is produced by decarboxylation of L-DOPA (L-3,4-dihydroxyphenylalanine) by the cells of the internal neuroendocrine system of the lungs (Cutz et al. 1974; Hage, 1976; Scheuermann, 1991). This system, which has many similarities to the adrenal medulla, is both activated and at its maximal development at birth (Scheuermann, 1991). In the human, these neuroendocrine cells can be seen throughout the second half of gestation; they appear to reach their greatest development at or near delivery, and then decline in the months after birth (Hage, 1976; Johnson et al. 1982). The same system is seen in guinea-pigs, mice and rabbits, but in the rabbit a number of independent studies have shown a dramatic but temporary loss of amine fluorescence just prior to birth (Hage, 1976; Sorokin et al. 1982; Redick & Hung, 1984). This suggests a rapid release of amines at a time when dopamine is known to surge. In the light of our results, this would be consistent with a possible role for dopamine and the neuroendocrine system in lung liquid drainage at birth.
The studies with dopamine antagonists showed that the effects of dopamine were mediated by its own receptors, and not due to conversion of dopamine to adrenaline or noradrenaline. However, the apparent use of D2 receptors was unexpected, and raised the first unusual aspect of these studies. D2 receptors reduce liberation of cAMP, and cAMP is known to stimulate reabsorption (DeCamilli et al. 1979; Kindler et al. 1992). However, the situation is not unusual for the guinea-pig preparation. Adrenaline stimulates α-adrenoreceptors, which also inhibit cAMP in most other tissues, yet also cause fluid reabsorption in these lungs (Bylund, 1988; Doe & Perks, 1998).
The second unusual aspect of these studies was the failure to demonstrate the importance of amiloride-sensitive Na+ transport as the basis of fluid reabsorption. This system has been well established in fetal sheep and goats, where it is activated by adrenaline and AVP, and probably by other factors (Olver et al. 1986; Cassin & Perks, 1993). Its importance has been demonstrated by the use of amiloride placed on the apical (luminal) surface of the alveolar cells. Despite this, the system is not notably sensitive to amiloride; the most acceptable concentration, 10−6m amiloride, which only blocks sensitive Na+ channels, produces only partial inhibition. However, use of 10−4m amiloride has produced consistent results, and there is little doubt that an amiloride-sensitive Na+ system is involved (Olver et al. 1986; Cassin & Perks, 1993). The same system exists in fetal guinea-pigs. In in vivo studies, O'Brodovich and his co-workers have shown that amiloride at 10−5 or 10−4m (but not at 10−6m) can impair water clearance from lungs of newborn guinea-pigs (O'Brodovich et al. 1990). In our own in vitro studies, amiloride at the lower concentration of 10−6m greatly reduced responses to falls in temperature (Garrad & Perks, 1990), expansion of the lungs (Garrad Nelson & Perks, 1996) and dinitrophenol (Perks et al. 1993); in all cases, reductions in production were smaller, and reabsorptions were abolished. Amiloride at the same low concentration reduced or abolished effects of aldosterone, and eliminated responses to AVP in these in vitro lungs (Perks et al. 1993, 1997). In contrast, in the many experiments reported here, amiloride used in the same way on the same preparation failed to change responses to dopamine, and raising the amiloride concentration to 10−5 or 10−4m had no effect. Tests with new batches of amiloride gave the same results. Further, the more specific Na+ channel blocker benzamil, used at 10−5m, a concentration known to be effective on fetal pulmonary cells in other situations, also failed to change responses to dopamine (see O'Brodovich et al. 1990; Matalon et al. 1993). It is possible that dopamine acts through amiloride-insensitive Na+ channels, which are known to exist in the pulmonary epithelium. O'Brodovich and his co-workers have shown that the fetal rat lung contains both ‘H-type’ Na+ channels with high affinity for amiloride and benzamil, and ‘L-type’ channels with low affinity; of these, the L-type channels were the more abundant, and responsible for most Na+ uptake (Matalon et al. 1993). As pointed out earlier, even in fetal sheep the Na+ channels in the lungs are not remarkable for their amiloride sensitivity, and need 10−4m concentrations for clear results. If amiloride-insensitive Na+ transport exists in lungs of fetal guinea-pigs, it must be present alongside amiloride-sensitive mechanisms activated by agents such as AVP. However, we must still keep an open mind on the possibility of Na+-independent, or partially independent reabsorption mechanisms. If sodium is not the primary factor, Cl− ions might be considered. The high levels of Cl− ions in lung liquid were of great interest in the early years of study, and they still represent the greatest gradient towards the blood plasma; some mechanism to facilitate their return to the blood, such as insertion of Cl− channels into the basal membrane of the pulmonary cells, might well incapacitate the Na+-K+-2Cl− secretory system, and allow movement of fluid from the lungs. However, it is too early to resort to such new mechanisms, and Na+ channels with a low sensitivity to amiloride may still be the simplest explanation.
Clearly, the importance of the pulmonary neuroendocrine system in the control of lung liquid removal is a growing possibility. This system has no known function, and suggestions that it is linked to responses of blood vessels and airways to hypoxia and hypercapnia have received only mixed support (Scheuermann, 1991). However, its notable development and apparent activation at the time of birth have already led to suggestions of an importance in adaptations of the perinatal lung, although these adaptations were not made clear (Redick & Hung, 1984; Scheuermann, 1991). Now we can add physiological information. At least two active agents in neuroendocrine cells, somatostatin and dopamine, are capable of influencing lung liquid production, although in different ways. We have shown previously that somatostatin more than doubles its concentration in lung tissues after the first breath, and that, at levels considered physiological, it can halt lung liquid production - but never cause reabsorption (Perks et al. 1992). In this present paper, dopamine, already known to double its levels in lung tissues at birth (Gardey-Levassort et al. 1981), is shown to be able to reduce lung liquid production or produce powerful reabsorption, possibly by new mechanisms. However, does this system really work at birth? Final proof would be particularly difficult to find, because of the diffuse and deeply integrated nature of the pulmonary neuroendocrine cells. However, in a situation where direct investigation would be difficult, we have fascinating indications from clinical studies. Work on newborn infants has shown that those with respiratory problems, such as hyaline membrane disease, show lack of neuroendocrine cells in the lungs at birth, as compared with normal infants (Johnson et al. 1982). Therefore, we must seriously consider the possibility that the neuroendocrine system and its active agents are concerned with withdrawal of liquid from the newborn lung, and that its failure to function may be a factor in respiratory distress syndrome.
Acknowledgments
We wish to thank the Department of Zoology of the University of British Columbia for financial support, and the National Research Council of Canada for Operating Grant 582–584 (A. M. P.).
References
- Ben-Johnathan N, Maxson RE. Elevation of dopamine in fetal plasma and the amniotic fluid during gestation. Endocrinology. 1978;102:649–652. doi: 10.1210/endo-102-2-649. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Olver RE, Ramsden CA, Strang LB, Walters DV. Effects of adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. The Journal of Physiology. 1983;344:137–152. doi: 10.1113/jphysiol.1983.sp014929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund DB. Subtypes of α2-adrenoreceptors: pharmacological and molecular biological evidence converge. Trends in Pharmacological Sciences. 1988;9:356–361. doi: 10.1016/0165-6147(88)90254-4. [DOI] [PubMed] [Google Scholar]
- Capasso A, Sorrentino L. Differential influence of D1 and D2 dopamine receptors on acute opiate withdrawal in guinea-pig isolated ileum. British Journal of Pharmacology. 1997;120:1001–1006. doi: 10.1038/sj.bjp.0700995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassin S, Perks AM. Studies of factors which stimulate lung fluid secretion in fetal goats. Journal of Developmental Physiology. 1982;4:311–325. [PubMed] [Google Scholar]
- Cassin S, Perks AM. Amiloride inhibits arginine vasopressin-induced decrease in fetal lung liquid secretion. Journal of Applied Physiology. 1993;75:1925–1929. doi: 10.1152/jappl.1993.75.5.1925. [DOI] [PubMed] [Google Scholar]
- Cutz E, Chan W, Wong V, Conen PE. Endocrine cells in rat fetal lungs. Ultrastructural and histochemical study. Laboratory Investigation. 1974;30:458–464. [PubMed] [Google Scholar]
- Cutz E, Gillian JE, Track NS. Pulmonary endocrine cells in the developing human lung and during neonatal adaptation. In: Becker KL, Gazdar AF, editors. The Endocrine Lung in Health and Disease. Philadelphia: W. B. Saunders Co.; 1984. pp. 210–231. [Google Scholar]
- De camilli P, Macconi D, Spada A. Dopamine inhibits adenylate cyclase in human prolactin-secreting pituitary adenomas. Nature. 1979;278:252–254. doi: 10.1038/278252a0. [DOI] [PubMed] [Google Scholar]
- Doe S, Perks AM. α-Adrenoreceptor influences on liquid movements by in vitro lungs from fetal guinea-pigs. Journal of Applied Physiology. 1998;84:746–753. doi: 10.1152/jappl.1998.84.2.746. [DOI] [PubMed] [Google Scholar]
- Faucher DJ, Lowe TW, Magness RR, Laptook AR, Porter JC, Rosenfeld CR. Vasopressin and catecholamine secretion during metabolic acidemia in the ovine fetus. Pediatric Research. 1987;21:38–43. doi: 10.1203/00006450-198701000-00010. [DOI] [PubMed] [Google Scholar]
- Gardey-Levassort C, Richard M-O, De lauture D, Thiroux G, Olive G. Catecholamines in developing rat lung. Life Sciences. 1981;28:331–337. doi: 10.1016/0024-3205(81)90076-x. 10.1016/0024-3205(81)90076-X. [DOI] [PubMed] [Google Scholar]
- Garrad P, Perks AM. The effects of temperature change on lung liquid production by in vitro lungs from fetal guinea-pigs. Journal of Developmental Physiology. 1990;14:109–114. [PubMed] [Google Scholar]
- Garrad nelson P, Perks AM. The effects of lung expansion on lung liquid production by in vitro lungs from fetal guinea-pigs: I. Basic studies and the effects of amiloride and propranolol. Reproduction, Fertility and Development. 1996;8:335–346. doi: 10.1071/rd9960335. [DOI] [PubMed] [Google Scholar]
- Hage E. Endocrine-like cells of the pulmonary epithelium. In: Coupland RE, Fujeta T, editors. Chromaffin, Enterochromaffin and Related Cells. Amsterdam: Elsevier Scientific Publishing Co.; 1976. pp. 317–332. [Google Scholar]
- Holzbauer M, Sharman DF. The distribution of catecholamines in vertebrates. Handbook of Experimental Pharmacology. 1972;33:110–185. (new series) [Google Scholar]
- Jelinek J, Jensen A. Catecholamine concentrations in plasma and organs of the fetal guinea-pig during normoxemia, hypoxemia and asphyxia. Journal of Developmental Physiology. 1991;15:145–152. [PubMed] [Google Scholar]
- Johnson DE, Lock JE, Elde RP, Thompson TR. Pulmonary neuroendocrine cells in hyaline membrane disease and bronchopulmonary dysplasia. Pediatric Research. 1982;16:446–454. doi: 10.1203/00006450-198206000-00009. [DOI] [PubMed] [Google Scholar]
- Jones CT. Circulating catecholamines in the fetus, their origin, actions and significance. In: Parvez H, Parvez S, editors. Biogenic Amines in Development. Amsterdam: Elsevier/North-Holland Biomedical Press; 1980. pp. 63–86. [Google Scholar]
- Juorio AV, Chedrese PJ. The concentration of dopamine and related monoamines in arteries and some other tissues of the sheep. Comparative Biochemistry and Physiology. 1990;95C:35–37. doi: 10.1016/0742-8413(90)90079-o. [DOI] [PubMed] [Google Scholar]
- Kindler PM, Ziabakhsh S, Perks AM. Effects of cAMP, its analogues and forskolin on lung fluid production by in vitro lung preparations from fetal guinea-pigs. Canadian Journal of Physiology and Pharmacology. 1992;70:330–337. doi: 10.1139/y92-041. [DOI] [PubMed] [Google Scholar]
- Matalon S, Bauer ML, Benos DJ, Kleyman TR, Lin C, Cragoe EJ, O'Brodovich H. Fetal lung epithelial cells contain two populations of amiloride-sensitive Na+ channels. American Journal of Physiology. 1993;264:L357–364. doi: 10.1152/ajplung.1993.264.4.L357. [DOI] [PubMed] [Google Scholar]
- O'Brodovich H, Hannam V, Seear M, Mullen JBM. Amiloride impairs lung liquid clearance in newborn guinea-pigs. Journal of Applied Physiology. 1990;68:1758–1762. doi: 10.1152/jappl.1990.68.4.1758. [DOI] [PubMed] [Google Scholar]
- O'Brodovich H, Rafii B, Post M. Bioelectric properties of fetal alveolar epithelial monolayers. American Journal of Physiology. 1990;258:L201–206. doi: 10.1152/ajplung.1990.258.4.L201. [DOI] [PubMed] [Google Scholar]
- Olver RE, Ramsden CA, Strang LB, Walters DV. The role of amiloride-blockable sodium transport in adrenaline-induced lung liquid reabsorption in the fetal lamb. The Journal of Physiology. 1986;376:321–340. doi: 10.1113/jphysiol.1986.sp016156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perks AM, Cassin S. The effects of arginine vasopressin and epinephrine on lung liquid production in fetal goats. Canadian Journal of Physiology and Pharmacology. 1989;67:491–498. doi: 10.1139/y89-078. [DOI] [PubMed] [Google Scholar]
- Perks AM, Dore JJ, Dyer J, Thom J, Marshall JK, Ruiz T, Woods BA, Vanderhorst E, Ziabakhsh S. Fluid production byin vitro lungs from fetal guinea-pigs. Canadian Journal of Physiology and Pharmacology. 1990;68:505–513. doi: 10.1139/y90-072. [DOI] [PubMed] [Google Scholar]
- Perks AM, Kindler PM, Marshall J, Woods B, Craddock M, Vonder muhll I. Lung liquid production by in vitro lungs from fetal guinea-pigs: effects of arginine vasopressin and arginine vasotocin. Journal of Developmental Physiology. 1993;19:203–212. [PubMed] [Google Scholar]
- Perks AM, Kwok YN, McIntosh CHS, Ruiz T, Kindler PM. Changes in somatostatin-like immunoreactivity in lungs from perinatal guinea-pigs, and the effects of somatostatin-14 on lung liquid production. Journal of Developmental Physiology. 1992;18:151–159. [PubMed] [Google Scholar]
- Perks AM, Ruiz T, Chua B, Vonder muhll I, Kindler PM, Blair W. Reabsorption of lung liquid produced by 2,4-dinitrophenol in in vitro lungs from fetal guinea-pigs. Canadian Journal of Physiology and Pharmacology. 1993;71:1–11. doi: 10.1139/y93-001. [DOI] [PubMed] [Google Scholar]
- Perks AM, Stockbrocks M, Chuang DC, Vonder muhll I, Kindler PW. Lung liquid production in vitro by lungs from fetal guinea-pigs: effects of amiloride on responses to aldosterone. Canadian Journal of Zoology. 1997;75:1147–1154. [Google Scholar]
- Redick ML, Hung K-S. Quantitation of pulmonary neuroepithelial bodies in pre- and postnatal rabbits. Cell and Tissue Research. 1984;238:583–587. doi: 10.1007/BF00219875. [DOI] [PubMed] [Google Scholar]
- Reid DL, Jensen A, Phernetton TM, Rankin JHG. Relationship between plasma catecholamine levels and electrocortical state in the mature fetal lamb. Journal of Developmental Physiology. 1990;13:75–79. [PubMed] [Google Scholar]
- Scheuermann DW. Neuroendocrine cells. In: Crystal RG, West JB, Barnes PJ, Cherniack NS, Weibel ER, editors. The Lung: Scientific Foundations. New York: Raven Press Ltd.; 1991. pp. 289–299. [Google Scholar]
- Sorokin SP, Hoyt RF, Grant MM. Development of neuroepithelial bodies in fetal rabbit lungs. I. Appearance and functional maturation as demonstrated by high-resolution light microscopy and formaldehyde-induced fluorescence. Experimental Lung Research. 1982;3:237–259. doi: 10.3109/01902148209069656. [DOI] [PubMed] [Google Scholar]
- Steel RGD, Torrie JH. Principles and Procedures of Statistics. New York: McGraw-Hill; 1970. [Google Scholar]
- Strang LB. Fetal lung liquid: secretion and reabsorption. Physiological Reviews. 1991;71:991–1016. doi: 10.1152/physrev.1991.71.4.991. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kurosawa S, Wiley JW, Owyang C. Mechanism for the gasterokinetic action of domperidone. In vitro studies in guinea-pigs. Gastroenterology. 1991;101:703–710. doi: 10.1016/0016-5085(91)90528-s. [DOI] [PubMed] [Google Scholar]
- Walters DV, Olver RE. The role of catecholamines in lung liquid absorption at birth. Pediatric Research. 1978;12:239–242. doi: 10.1203/00006450-197803000-00017. [DOI] [PubMed] [Google Scholar]
- Walters DV, Ramsden CA, Olver RE. Dibutyryl cAMP induces a gestation-dependent absorption of fetal lung liquid. Journal of Applied Physiology. 1990;68:2054–2059. doi: 10.1152/jappl.1990.68.5.2054. [DOI] [PubMed] [Google Scholar]
- Woods BA, Doe S, Perks AM. Effects of epinephrine on lung liquid production by in vitro lungs from fetal guinea-pigs. Canadian Journal of Physiology and Pharmacology. 1997;75:772–780. [PubMed] [Google Scholar]
- Woods BA, Ng W, Thakorlal D, Perks AM. The effects of acetylcholine on lung liquid production by in vitro lungs from fetal guinea-pigs. Canadian Journal of Physiology and Pharmacology. 1996;74:918–927. doi: 10.1139/cjpp-74-8-918. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 2. Englewood Cliffs, NJ, USA: Prentice-Hall Inc.; 1984. [Google Scholar]


