Abstract
Concomitant application of the cholinergic agonist carbachol and nanomolar doses of kainate can elicit persistent gamma frequency oscillations in all layers of the mouse somatosensory cortex in vitro. Receptor pharmacology with bath-applied antagonists indicated that oscillatory network activity depended crucially on the participation of cholinergic muscarinic, (S)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate and GABAA receptors.
The timing of action potentials and the occurrence of excitatory as well as inhibitory postsynaptic events was highly correlated with the phasic change of extracellularly recorded population activity. Firing probability was lowest during the peak negativity of IPSPs and gradually increased during their ensuing decay. In conjunction with the effect of a barbiturate to decrease the frequency of gamma oscillations, this suggests a crucial role of IPSPs in phasing the suprathreshold activity of principal neurons.
At nearby (< 1 mm) sites contained within any given cortical layer, oscillatory extra- and intracellular activity was highly synchronous with no apparent phase lag. However, interlaminar mapping experiments demonstrated a phase reversal of both extra- and intracellularly recorded activity near the lower border of thalamo-recipient layer 4, thus corroborating findings that have been obtained in vivo.
In conclusion, a modest increase of tonic excitatory drive in conjunction with the activation of cholinergic muscarinic receptors can elicit persistent gamma frequency network oscillations in the rodent somatosensory cortex. These findings (re)emphasize the role of the cholinergic ascending system in the cortical processing of sensory information.
Neuronal populations in mammalian sensory cortices have the ability to engage in stimulus-specific synchronous oscillations in the gamma frequency range (∼30–70 Hz; for review, see Singer & Gray, 1995). Such temporally coherent activity may arise in a variety of cortical areas subserving the entire range of sensory modalities, and is likely to subserve an important role in cortical information processing (Gray & Singer, 1989; Jones & Barth, 1997; Ritz & Sejnowski, 1997). Gamma oscillations may appear, as an emergent property, within a network of mutually connected and tonically excited cortical interneurons (Whittington et al. 1995), which, in turn, may pace the sub- and suprathreshold activity of principal neurons (Traub et al. 1997).
Although the functional relevance of sensory stimuli in the induction of gamma oscillations appears to be well established, it is equally clear that the operational mode of cortical neurons is also governed by several ascending modulatory systems originating in a variety of brain regions (Steriade et al. 1993; Munk et al. 1996). Amongst these, the cholinergic input has gained some prominence, as it may play a role in the formation of associative memories (Hasselmo et al. 1992). Moreover, the stimulation of cholinergic subcortical nuclei has been shown to evoke coherent cortical rhythms in the gamma frequency band (Steriade et al. 1991) and, in the absence of fast inhibition, cholinergic agonists can induce robust theta frequency oscillations in the neocortex in vitro (Lukatch & MacIver, 1997). It may thus be that cholinergic activation primes cortical areas to generate oscillatory activity in response to an increase of excitatory drive in the cortico-thalamic feedback loop. We have therefore proceeded to test this hypothesis by investigating network mechanisms in the mouse somatosensory cortex in vitro following the activation of cholinergic receptors in conjunction with an increase in tonic excitatory drive.
METHODS
Adult BALB/c mice were anaesthetized with inhaled isoflurane, immediately followed by an intramuscular injection of ketamine (≥ 100 mg kg−1) and xylazine (≥ 10 mg kg−1). When all responses to noxious stimuli, such as tail pinch, had completely subsided, the animals were perfused intracardially with ∼10 ml of modified artificial cerebrospinal fluid (ACSF), which was composed of (mm): 252 sucrose, 3.0 KCl, 1.25 NaH2PO4, 24 NaHCO3, 2.0 MgSO4, 2.0 CaCl2 and 10 glucose. Following brain removal, 450 μm thick slices were cut in either the horizontal or coronal plane, respectively. Those sections containing somatosensory cortex were readily identified with the aid of a binocular microscope, trimmed and transferred to either a holding or a recording chamber, where they were maintained at either room temperature or, for recording purposes, at 34–35°C at the interface between oxygenated ACSF and a mixture of 95% O2 and 5% CO2. After 30–45 min in modified ACSF, all sucrose was replaced with equiosmolar (126 mm) NaCl, and recordings began following an additional recovery period of ∼45 min.
Extracellular recording electrodes were pulled from borosilicate glass tubing and filled with ACSF, with pipette resistances generally ranging between 2 and 5 MΩ. Those micropipettes manufactured for intracellular recordings were filled with 1.5 M KCH3SO4 and had resistances varying from 70 to 130 MΩ. Both extra- and intracellular recordings were made with an Axoprobe amplifier (Axon Instruments) and permanently acquired with a DTR-1402 digital audio tape recorder (Biologic, Claix, France). Data analysis was continued off-line by (re) digitizing the data at 1–10 kHz, low-pass filtering the traces between 0.2 and 2 kHz and employing both RC Electronics Computerscope (RC Electronics, Santa Barbara, CA, USA) and Axograph (Axon Instruments) software. For further technical details, see Tamás et al. (1998).
RESULTS
When vertical slices of mouse somatosensory cortex were exposed to nanomolar concentrations of the potent (S)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor agonist kainic acid (200–1000 nm), addition of the cholinergic agonist carbachol (10–50 μm) could elicit gamma frequency oscillatory activity in ∼30–50% of the slices (Fig. 1A). Activity at nearby sites (< 1 mm) within any of the cortical layers was highly synchronous with no apparent phase-lag. Once initiated, network oscillations were relatively stable and could remain unabated for periods in excess of 5 h. Although in the presence of carbachol considerably higher concentrations of AMPA (∼10 μm) were occasionally effective in fostering the induction of gamma oscillations, kainate proved to be more potent, perhaps due to its non-desensitizing action on AMPA receptors (Mayer & Westbrook, 1987). Furthermore, neither kainate (100–2000 nm; n = 3 slices; Fig. 1B) nor carbachol (1–200 μm; n = 3 slices; not shown) were able to elicit oscillatory network activity on their own. With pilot experiments indicating a higher success rate with a slightly elevated level of extracellular potassium (), and the concentration of kainate being the most variable factor, we standardized the majority of subsequent experiments by generally starting with raised to 5 mm and a carbachol concentration of 50 μm (but see Fig. 1A and C) and proceeded by gradually increasing the concentration of kainate (typically to 300–750 nm) until oscillatory activity became apparent. In contrast, cholinergic activation solely with an elevated (range 3–10 mm), so as to increase the overall level of network excitability, proved to be ineffective in triggering gamma frequency network activity.
Figure 1. Pharmacological induction and receptor mechanisms of cortical gamma oscillations in vitro.
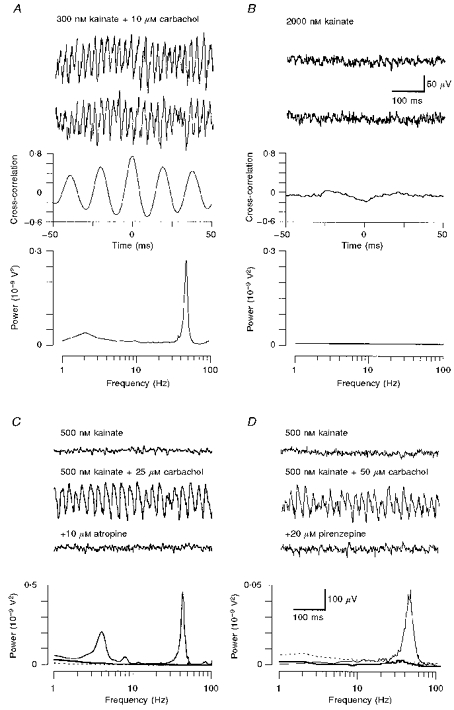
A, extracellular recordings of two neighbouring layer 4 sites showing the induction of oscillatory network activity due to the conjoint action of kainate and carbachol. Cross-correlograms reveal a high degree of synchrony, whereas the power spectrum shows a prominent peak in the gamma frequency range. B, control experiment demonstrating that within layer 4, kainate alone failed to induce gamma oscillations. C, the muscarinic antagonist atropine potently blocked oscillatory activity elicited by carbachol in layer 5/6. D, likewise, the specific M1 receptor antagonist pirenzepine was also effective in reversibly antagonizing gamma frequency network oscillations. The interrupted lines in the corresponding power spectra (bottom panels) denote baseline activity (kainate only); continuous lines (addition of carbachol) reveal a clear increase of power in the gamma frequency spectrum, whereas both muscarinic antagonists (thick lines) reduce spectral activity back to baseline levels. Calibration bars in B are for panels A and B, those in D are for C and D.
With carbachol playing a crucial role in the generation of oscillatory activity, we tested the hypothesis that the induction process may be dependent on the activation of muscarinic cholinergic receptors. Indeed, the broad-spectrum muscarinic antagonist atropine (10 μm; n = 3), as well as the specific M1 receptor antagonist pirenzepine (10–20 μm; n = 5), were effective in abolishing network activity induced by adding 25–50 μm carbachol to the kainate-containing control solution (Fig. 1C and D). Occasionally, power spectra revealed an additional prominent peak in the theta frequency range, which was also eliminated by the addition of muscarinic antagonists (Fig. 1C).
In view of the known agonist profile of kainate, it also appeared to be likely that its action on AMPA and kainate receptors may play a crucial role in the induction of gamma frequency oscillations. However, it is also conceivable that due to the substantial increase of network activity endogenously released glutamate could also have an effect on NMDA and/or metabotropic glutamate receptors that, in turn, may play a crucial role in the induction of rhythmic network activity. We therefore bath-applied the metabotropic glutamate receptor antagonist (S)-α-methyl-4-carboxyphenylglycine ((S)-MCPG; 200–500 μm) followed by the NMDA receptor antagonist d-2-amino-5-phosphonopentanoic acid (d-AP5; 50 μm), neither of which was effective in either reducing the spectral power of the oscillations or inducing a frequency change. However, subsequent addition of the AMPA/kainate receptor antagonist 6-nitro-7-sulphamoylbenzo (f) -quinoxaline-2, 3-dione (NBQX; 20 μm; n = 4) abolished all oscillatory activity (Fig. 2).
Figure 2. The effect of glutamate receptor antagonists on neocortical gamma oscillations in vitro.
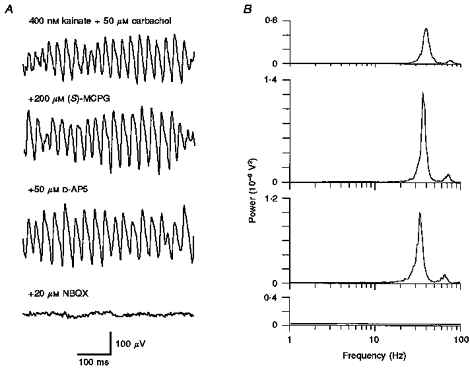
A, oscillatory activity was induced by superfusing mouse somatosensory cortex (layer 5/6) with both kainate and carbachol. Neither the metabotropic glutamate antagonist (S)-MCPG ((S)-α-methyl-4-carboxyphenylglycine) nor the NMDA receptor antagonist D-AP5 (D-2-amino-5-phosphonopentanoic acid) were effective in diminishing gamma oscillations, whereas the AMPA/kainate receptor blocker NBQX (6-nitro-7-sulphamoylbenzo (f) quinoxaline-2, 3-dione) eliminated all rhythmic activity. B, the corresponding power spectra reveal modest changes in spectral amplitude following the addition of (S)-MCPG and D-AP5, whereas neither of the drugs had an effect on oscillatory peak frequency.
With other experimental and computational paradigms of gamma oscillations (Whittington et al. 1995; Wang & Buzsáki 1996) indicating a crucial involvement of GABAA receptors, we tested the effect of low doses of the competitive GABAA receptor antagonist bicuculline (1–2 μm). Indeed, bicuculline consistently and reversibly blocked all oscillatory activity in the gamma frequency range (Fig. 3A; n = 3). Further increases of the bicuculline concentration (10 μm) only led to the emergence of synchronized epileptiform activity. Thus a modest dose of the GABAA receptor antagonist is effective in abolishing gamma frequency network synchrony, but still too low so as to precipitate the development of disinhibitory hypersynchrony. Moreover, these data also indicate that, in view of their presynaptic action on inhibitory terminals (Behrends & ten Bruggencate, 1993; Clarke et al. 1997), neither carbachol nor kainate were sufficiently potent to trigger epileptic activity.
Figure 3. A prominent role of GABAergic mechanisms in the generation of gamma oscillations.
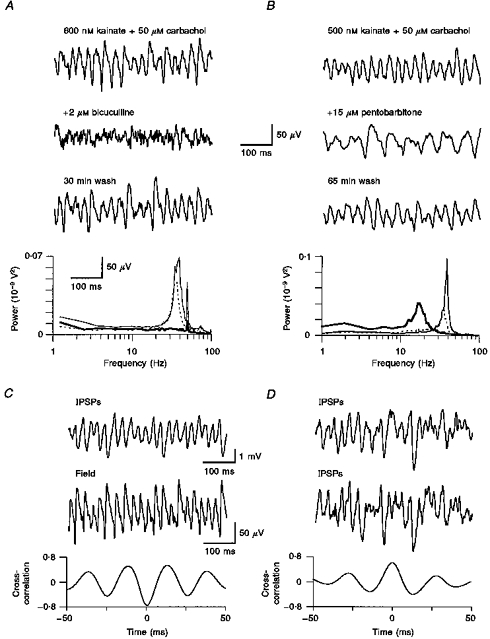
A, non-saturating doses of the competitive GABAA receptor antagonist bicuculline abolished gamma oscillatory activity in layer 2/3 (thick line in power spectrum). Note that the corresponding spectra reveal a disinct notch at 50 Hz, resulting from a modest degree of contamination with mains hum. During washout, oscillatory activity reappeared at the same frequency as during the control period (thin line). B, in contrast, the GABAA receptor modulator pentobarbitone was effective in dramatically decreasing the oscillation frequency (thick line in spectrum). The effect was reversible following washout (interrupted line). C, intracellular recording of a layer 2/3 cell at a depolarized holding potential (−40 mV) revealed fast hyperpolarizing events, presumably IPSPs that were tightly correlated, albeit in antiphase, with concomitantly recorded extracellular field activity. D, dual intracellular impalements of regular spiking neurons in layer 2/3 revealed synchronous activity in both recordings.
We then tested whether rhythmic GABAA receptor-mediated inhibitory postsynaptic potentials (IPSPs) played a role in phasing the activity of principal neurons. If so, drugs that significantly increase the duration of inhibitory events should therefore shift the spectral peak of gamma oscillations towards lower frequencies. As predicted, increasing doses of pentobarbitone (Fig. 3B; only 15 μm shown; n = 3) progressively decelerated oscillatory activity. Furthermore, if fast, synchronous IPSPs were to have a crucial involvement in phasing population activity, it follows that IPSPs and extracellular field recordings must also have a fixed phase relationship. This prediction was confirmed by monitoring intracellularly recorded IPSPs at depolarized holding potentials (≥−40 mV) concomitantly with extracellular field potentials, with cross-correlations showing that both traces were phase-locked, generally close to antiphase (Fig. 3C). A more direct confirmation for the presumed synchronicity of IPSPs was therefore obtained with dual intracellular recordings showing the temporal coincidence of gamma frequency IPSPs in two simultaneously recorded neurons (Fig. 3D; n = 5). Although extra- and intracellularly recorded neurons can generate action potentials (see below), they are generally absent in such IPSP recordings, this being simply due to the near-complete inactivation of sodium channels at depolarized holding potentials. In this respect, the injection of depolarizing current proved advantageous not only to ‘eliminate’ the contamination of intracellular traces by action potentials, but also to increase the chloride driving force so as to resolve IPSPs more clearly.
In order to determine the putative role of IPSPs in phasing the discharge of principal neurons, we assessed the phase relationship of evoked rheobasic action potentials with concomitantly recorded extracellular field activity. Using spike-triggered averaging techniques, it was apparent that action potentials tended to occur near the peak negativity of the extracellular field (Fig. 4A; n = 3). However, a more informative picture of the temporal probability of action potential generation was obtained by setting the event trigger on the negative slope of field oscillations (t0) and measuring the temporal interval between any spike occurring within a set time-window either prior to or succeeding t0. When computing spike probability histograms it thus appeared that action potentials could essentially occur at any phase of concomitantly recorded gamma oscillatory activity, albeit with a very uneven distribution and spikes generally clustering near the peak negativity of the extracellular field (Fig. 4B). These observations were confirmed with extracellular single-unit recordings (n = 3; data not shown). Accordingly, action potentials are most likely to occur during the peak positivity of intracellular voltage recordings at depolarized holding potentials, as shown in Fig. 3C, where the extra- and intracellular traces were shown to be in antiphase. This notion was corroborated further by setting the event trigger on the negative slope of the extracellular field and averaging concomitantly recorded IPSPs (Fig. 4E). As predicted, the peak positivity of the intracellular voltage trace was near the trough of the extracellular field recording, and vice versa. Thus, taken together, these findings provide clear evidence that action potentials are least probable during the trough of hyperpolarizing IPSPs and become increasingly frequent during their ensuing decay.
Figure 4. The relationship of oscillatory field activity with the sequential timing of intracellularly recorded sub- and suprathreshold events.
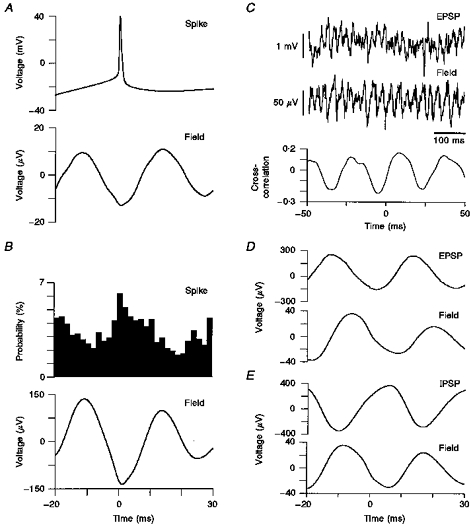
A, rheobasic action potentials were elicited in a regular spiking neuron of an oscillating slice. Spike-triggered averages of the concomitantly recorded extracellular field activity revealed action potentials to be located near the peak negativity of an oscillatory cycle. B, when using field triggered data acquisition and constructing spike latency histograms, it was apparent that action potentials could occur during any part of an oscillatory cycle, but with the peak of their probability distribution overlapping with the trough of the averaged extracellular field. C, when adjusting the membrane potential during intracellular recordings to the apparent point of IPSP reversal (−70 mV), it was feasible to discriminate depolarizing events, presumably EPSPs, which were clearly correlated with the extracellular population activity. D, field triggered averages of such recordings were nearly matching, albeit with a slight precession of the intracellular waveform. E, field triggered acquisition of intracellular recordings displaying numerous IPSPs at depolarized holding potentials showed the peak positivity of the intracellular trace to coincide with the trough of the averaged field. All recordings in A-E were obtained within layer 5/6.
With action potentials of principal neurons occurring with some degree of synchrony, it is conceivable that this may be reflected in their correspondingly rhythmic recurrent excitatory feedback to neighbouring neurons. To address such a putative relationship of excitatory postsynaptic potentials (EPSPs) and extracellular field activity, we obtained recordings in which the membrane potential of neurons was carefully adjusted to the point of IPSP reversal (∼-70 mV; n = 4). Thus numerous fast, small-amplitude (1–2 mV) membrane potential fluctuations were apparent which we presumed to be EPSPs (Fig. 4C). When computing field-triggered averages of the intracellular trace, it was apparent that the peak positivity of the averaged signal coincided with the rising slope of the extracellular field (Fig. 4D). Thus within an oscillatory cycle, EPSPs tend to cluster at a point of time closely following rather than preceding the peak probability of action potential generation.
With all of the previously described results that originated from two distinct recording sites, utmost care was take to ensure that their respective positions remained aligned horizontally and thus presumably within a given cortical lamina. Extra- and intracellular recordings were obtained in all cellular layers of the somatosensory cortex, with the experimental data being reassuringly consistent. Regarding the vertical placement of electrodes, layer 4 invariably served as a prominent landmark due to its high content of myelinated axons. Recording sites in layer 2/3 were normally located approximately 100 μm above the border with layer 4. Conversely, electrode positions in the infragranular layers were usually 50–100 μm above the white matter border, and thus presumably most of the time within layer 6. However, without histological verification and with layer 5 remaining relatively ill-defined, we preferred to group both infragranular laminae as layer 5/6.
When conducting cross-laminar mapping experiments it was apparent that gamma oscillatory activity in layers 2/3 and 4 was highly correlated and generally showed little or no phase-lag (Fig. 5A). However, around the layer 4/5 border region, a dramatic phase shift occurred when leaving a reference electrode in layer 4 and gradually shifting the position of a second site into the infragranular layers (Fig. 5B). Accordingly, oscillatory activity in layers 2/3 and 5/6 was also found to be in antiphase (Fig. 5C), raising the intriguing question of whether the noted phase-reversal was due solely to a polarity change of the extracellularly recorded field or, alternatively, a genuine phase-shift of the sub- and suprathreshold activity pattern. If the latter assumption was correct, it follows that within a given cortical layer extra- and intracellular activity should have an invariant phase relationship. Indeed, in all layers, presumed IPSPs and extracellular gamma oscillations were consistently found to be in antiphase (Fig. 5D; for layer 2/3, see Fig. 3C). Consequently, with the phase reversal occurring at the layer 4/5 border, intracellular events in layer 5/6 and extracellular activity in layer 4 were accordingly found to be in phase (Fig. 5E), whereas intracellular activity in layers 2/3 and 5/6 were in antiphase (Fig. 5F). We thus conclude that the prominent phase-shift of extracellular field activity is mirrored by a similar transition of intracellular events. Hence gamma activity across layers 2/3 and 4 is both phase-locked and synchronous, whereas the oscillatory cycle in layer 5/6 shows a prominent phase-shift, when compared with the upper layers. Therefore intralaminar synaptic links must assume a vital but, as yet, enigmatic functional role by differentially processing the vertical transfer of synaptic information in the temporal domain.
Figure 5. Interlaminar correlation of extra- and intracellular oscillatory activity.
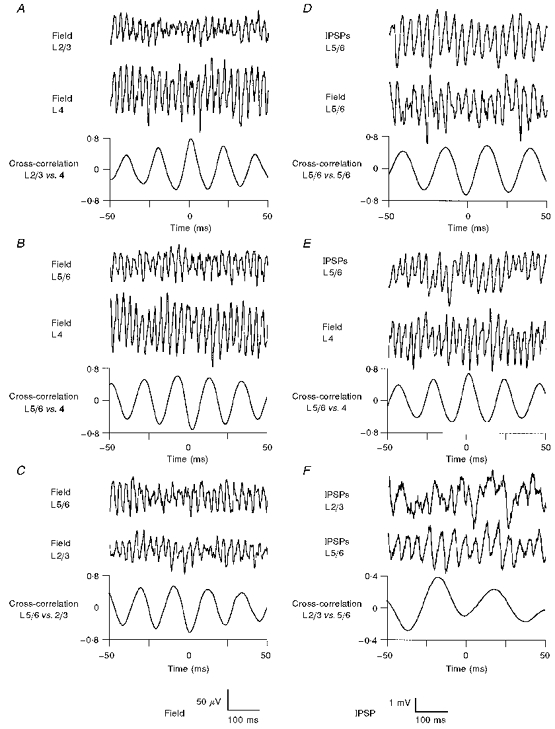
A, two-site mapping experiments along vertical strips of cortex revealed gamma oscillations in layers (L) 2/3 and 4 to occur without appreciable phase-lag. B, however, when leaving a reference electrode in layer 4 and shifting the position of a roving second electrode across the layer 4/5 border, a near-complete phase reversal became apparent. C, consequently, oscillatory activity in layers 2/3 and 5/6 was also found to be in antiphase. In contrast to their antiphasic relationship within a given cortical layer (panel D; for upper layers, see Fig. 3C) extra- and intracellular activity across the layer 4/5 border were found to be near synchronous (E). F, the prominent extracellular phase shift was also corroborated with dual intracellular recordings at depolarized holding potentials, here showing IPSPs in layers 2/3 and 5/6 being in antiphase (for dual impalements within a given layer, see Fig. 3D).
DISCUSSION
Muscarinic cholinergic activation in conjunction with an increase in tonic excitatory drive were shown to elicit sustained gamma frequency network oscillations in the rodent somatosensory cortex. Conceivably, the resonant properties of intrinsically oscillating neurons, such as the ‘chattering cells’ in cat visual cortex, may contribute to the generation of such synchronous oscillations (Chagnac-Amitai & Connors, 1989; Llinás et al. 1991; Gray & McCormick, 1996). Nevertheless, temporally coherent network activity can only emerge provided individual neurons are suitably interconnected with chemical and/or electrical synapses (Jefferys et al. 1996). In this respect, the data presented above provide three lines of evidence to suggest a pivotal role of GABAergic neurotransmission in the generation of gamma-band synchronous population activity. First, the pharmacological block of GABAA receptor-mediated conductances abolishes gamma frequency oscillations (Whittington et al. 1995). Second, the effect of barbiturates indicates that the network oscillation frequency appears to be governed, at least in part, by the decay kinetics of GABAergic synaptic events (Whittington et al. 1996). Third, the firing probability of principal neurons increases concomitantly with the ensuing decay of IPSPs, an observation that is commensurate with rhythmic IPSPs entraining neuronal firing, by virtue of phasing the generation of action potentials (Lytton & Sejnowski, 1991; Cobb et al. 1995; Whittington et al. 1995; Traub et al. 1996a).
Previous work has demonstrated that tonic excitation mediated by metabotropic glutamate receptors may condition networks of hippocampal and neocortical interneurons to entrain each other into rhythmic firing (Whittington et al. 1995). Likewise, modelling data indicate that pools of mutually connected interneurons may generate gamma frequency rhythms and thus govern network activity by virtue of their divergent output onto principal neurons (Traub et al. 1997). In support of this notion it was demonstrated that neocortical interneurons do indeed show a high degree of interconnectivity (Kisvarday et al. 1993; Tamás et al. 1998). It is thus conceivable that the cholinergic activation of GABAergic neurons (Kawaguchi, 1997) in conjunction with an increase in tonic excitatory drive may foster the emergence of synchronous activity within the interneuronal network. However, since GABAergic IPSPs phase rather than evoke suprathreshold activity in postsynaptic neurons, the conjoint action of kainate and an elevated were presumably also necessary to raise the membrane potential of principal neurons beyond firing threshold. Only then will the generation of rhythmic IPSPs be effective in pacing the discharge of spontaneously firing pyramidal cells and thus lead to the phase encoding of their activity (Buzsáki & Chrobak, 1995). In turn, due to recurrent feedback loops, pyramidal cells will generate phase-locked EPSPs, as demonstrated above. Interestingly, however, such EPSPs are associated with a relatively low firing probability in principal neurons (Fisahn et al. 1998), suggesting a minor role in pacing their activity. In order to address the, as yet, unknown functional significance of recurrent EPSPs (for other models, see Traub et al. 1996b), it will thus be necessary to obtain interneuron recordings and assess how the recurrent output of principal cells will affect information processing in the interneuronal network.
Relevance to gamma oscillations observed in vivo
Using physiologically relevant stimuli, such as the manual stimulation of individual vibrissae, gamma frequency oscillations can be reliably evoked in the somatosensory cortex of unanaesthetized and unrestrained rats (Jones & Barth, 1997), thus confirming their well-known association with the processing of sensory information (Gray & Singer, 1989). Although these data emphasize the relevance of sensory input for the emergence of gamma oscillations, our findings demonstrate that the cortical network can generate synchronous 40 Hz activity in the absence of the cortico-thalamic feedback loop. Likewise, the dramatic phase-shift at the lower border of layer 4, occurring both in vitro and in vivo (Murthy & Fetz, 1996), is consequently also a phenomenon arising within the isolated cortical network. In this respect, the temporal sequence of events within an oscillatory cycle was shown to be essentially the same within the spatial domain, and gamma oscillations were found to be phase-locked across all cortical layers. However, it also emerged that a fixed phase relationship does not necessarily imply synchronous activity, and it will be interesting to determine the functional consequences resulting from the differential timing of synaptic information linking neuronal ensembles embedded within the (supra) granular and infragranular layers. Furthermore, apart from the well-known phase shift, the in vitro model also replicates the finding that during stimulus-evoked gamma oscillations in the visual cortex, neurons preferentially discharge in phase with the peak negativity of the locally recorded field potential (Gray & Singer, 1989). Further parallels are also apparent in the fast oscillatory synaptic activity of neurons in the sensory cortex and hippocampus of intact animals (Jagadeesh et al. 1992; Soltesz & Deschênes, 1993).
It thus appears, that the above described pharmacological paradigm elicits a pattern of extra- and intracellular network activity, which shows a striking resemblance to what has been observed in the intact animal. This may even hold for the transformation of slow EEG rhythms into fast gamma oscillations known to occur during arousal and alertness (Steriade et al. 1996). Interestingly, similar changes in cortical population activity can be evoked following the stimulation of cholinergic brainstem nuclei (Steriade et al. 1991) and it appears likely that the bath-applied cholinergic agonist carbachol may have replicated this effect in vitro. It thus seems reasonable to conclude that the cholinergic induction of cortical gamma oscillations is likely to serve as a suitable model system to address those synaptic network mechanisms that subserve sensory cortical processing during a physiologically relevant state of cortical activation.
Acknowledgments
We are grateful to Mr P. Jays and Ms A. A. Robson for expert technical assistance. This work was funded in part by EC Grant BIO4-CT96–0585 and the James McDonnell Foundation. E. H. B. also holds a Medical Research Fellowship at Corpus Christi College, Oxford. G. T. was supported by the European Blaschko Research Scholarship and the Z. Magyary Scholarship.
References
- Behrends JC, ten Bruggencate G. Cholinergic modulation of synaptic inhibition in the guinea pig hippocampus in vitro - excitation of GABAergic interneurons and inhibition of GABA release. Journal of Neurophysiology. 1993;69:626–629. doi: 10.1152/jn.1993.69.2.626. [DOI] [PubMed] [Google Scholar]
- Buzsuzsákiaacute;ki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Current Opinion in Neurobiology. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Connors BW. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. Journal of Neurophysiology. 1989;62:1149–1162. doi: 10.1152/jn.1989.62.5.1149. [DOI] [PubMed] [Google Scholar]
- Clarke VRJ, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike F, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274:109–113. doi: 10.1126/science.274.5284.109. 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proceedings of the National Academy of Sciences of the USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Anderson BP, Bower JM. Cholinergic modulation of cortical associative memory function. Journal of Neurophysiology. 1992;67:1230–1246. doi: 10.1152/jn.1992.67.5.1230. [DOI] [PubMed] [Google Scholar]
- Jagadeesh B, Gray CM, Ferster D. Visually evoked oscillations of membrane potential in cells of cat visual cortex. Science. 1992;257:552–554. doi: 10.1126/science.1636094. [DOI] [PubMed] [Google Scholar]
- Jefferys JGR, Traub RD, Whittington MA. Neuronal networks for induced ‘40 Hz’ rhythms. Trends in Neurosciences. 1996;19:202–208. doi: 10.1016/s0166-2236(96)10023-0. 10.1016/S0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- Jones MS, Barth DS. Sensory-evoked high-frequency (gamma-band) oscillating potentials in somatosensory cortex of the unanesthetized rat. Brain Research. 1997;768:167–176. doi: 10.1016/s0006-8993(97)00639-2. 10.1016/S0006-8993(97)00639-2. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. Journal of Neurophysiology. 1997;78:1743–1747. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- Kisvarday ZF, Beaulieu C, Eysel UT. Network of GABAergic large basket cells in cat visual cortex (area 18): implication for lateral disinhibition. Journal of Comparative Neurology. 1993;327:398–415. doi: 10.1002/cne.903270307. [DOI] [PubMed] [Google Scholar]
- Llinlinás RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proceedings of the National Academy of Sciences of the USA. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukatch HS, Maciver MB. Physiology, pharmacology, and topography of cholinergic neocortical oscillations in vitro. Journal of Neurophysiology. 1997;77:2427–2445. doi: 10.1152/jn.1997.77.5.2427. [DOI] [PubMed] [Google Scholar]
- Lytton WW, Sejnowski TJ. Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. Journal of Neurophysiology. 1991;66:1059–1079. doi: 10.1152/jn.1991.66.3.1059. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Progress in Neurobiology. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Munk MHJ, Roelfsema PR, König P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272:271–274. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. Journal of Neurophysiology. 1996;76:3949–3967. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- Ritz R, Sejnowski TJ. Synchronous oscillatory activity in sensory systems: new vistas on mechanisms. Current Opinion in Neurobiology. 1997;7:536–546. doi: 10.1016/s0959-4388(97)80034-7. 10.1016/S0959-4388(97)80034-7. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annual Review of Neuroscience. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Descheschênesecirc;nes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. Journal of Neurophysiology. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. Journal of Neuroscience. 1996;16:392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Currurró Dossioacute; dossi R, Pararéeacute; D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proceedings of the National Academy of Sciences of the USA. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Tamamás G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. Journal of Neuroscience. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Jefferys JGR, Whittington MA. Simulation of gamma rhythms in networks of interneurons and pyramidal cells. Journal of Computational Neuroscience. 1997;4:141–150. doi: 10.1023/a:1008839312043. 10.1023/A:1008839312043. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsuzsákiaacute;ki G, Jefferys JGR. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. The Journal of Physiology. 1996a;493:471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Stanford IM, Jefferys JGR. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996b;383:621–624. doi: 10.1038/383621a0. 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- Wang X-J, Buzsuzsákiaacute;ki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. Journal of Neuroscience. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Jefferys JGR, Traub RD. Effects of intravenous anaesthetic agents on fast inhibitory oscillations in the rat hippocampus in vitro. British Journal of Pharmacology. 1996;118:1977–1986. doi: 10.1111/j.1476-5381.1996.tb15633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JGR. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]


