Abstract
The contribution of apical Na+-H+ exchange to sodium reabsorption in the thick ascending limb of the loop of Henle (TALH) in vivo was examined in anaesthetized rats by perfusing loops of Henle of superficial nephrons with solutions containing the Na+-H+ exchange inhibitor, ethyl isopropyl amiloride (EIPA).
Using a standard perfusate, no statistically significant effect of EIPA on net sodium reabsorption (JNa) was detected. However, when sodium reabsorption in the pars recta of the proximal tubule was minimized by using a low-sodium perfusate, EIPA reduced JNa from 828 ± 41 to 726 ± 37 pmol min−1 (P < 0.05), indicating that apical Na+-H+ exchange can make a small contribution to net sodium reabsorption in the TALH in vivo. This contribution appears to be dependent on the bicarbonate load, since an increase in the latter led to an enhancement of EIPA-sensitive sodium transport.
Addition of the Na+-K+-2Cl− cotransport inhibitor, bumetanide, to the low-sodium perfusate reduced baseline JNa to 86 ± 27 pmol min−1. In this setting, EIPA reduced JNa further, to −24 ± 18 pmol min−1 (P < 0.05), an effect similar to that seen in the absence of bumetanide. This finding argues against previous suggestions (based on in vitro evidence) that inhibition of the Na+-K+-2Cl− cotransporter leads to an increase in apical Na+-H+ exchange in the TALH.
Reabsorption of sodium chloride in the thick ascending limb of the loop of Henle (TALH) provides the driving force for the operation of the countercurrent multiplier. Approximately half of the sodium reabsorption in the TALH is believed to be transcellular, the remainder being driven through the paracellular shunt as a consequence of the lumen-positive potential difference (Jamison & Gehrig, 1992).
The bulk of transcellular sodium reabsorption results from sodium entry on the apical Na+-K+-2Cl− cotransporter, coupled with expulsion across the basolateral membrane by the Na+-K+-ATPase. However, a Na+-H+ exchanger (NHE-3) has been identified in the apical membrane of the rat TALH (Amemiya et al. 1995), and in vitro evidence suggests that this exchanger mediates most of the bicarbonate reabsorption in this nephron segment (Good & Watts, 1996). Consequently, it is possible that Na+-H+ exchange might make a small but significant contribution to transcellular sodium reabsorption. However, no direct information is available concerning its magnitude. The present investigation has addressed this issue by using ethyl isopropyl amiloride (EIPA), a specific and potent inhibitor of Na+-H+ exchange (Geibel et al. 1989), to assess the extent of Na+-H+ exchange in loops of Henle perfused in vivo.
The loop of Henle is a heterogeneous nephron segment. In superficial nephrons it comprises, in addition to the TALH, the thin descending limb of Henle and the pars recta of the proximal tubule. Information on transport processes in the pars recta is limited, but it is thought that the reabsorptive mechanisms for sodium and water are similar to those in the late proximal convoluted tubule (Schafer & Barfuss, 1982). Bicarbonate reabsorption in the pars recta is believed to be mediated partly by an apical Na+-H+ exchanger and partly by an apical H+-ATPase (Mackovic-Basic & Kurtz, 1990), but it is thought likely that this segment plays only a minor role in bicarbonate transport under in vivo conditions (Good, 1993).
In the present study, we attempted to achieve some functional differentiation between the heterogeneous loop segments by using the model of Peterson et al. (1993) in which the loop is perfused with a low-sodium perfusate (sodium concentration, 110 mmol l−1) designed to suppress net reabsorption in the pars recta. A reduction in sodium concentration of this order results in enough passive sodium backflux to match active uptake (Giebisch et al. 1964).
In additional experiments, the role of the Na+-H+ exchanger was investigated during blockade of the Na+-K+-2Cl− cotransporter with bumetanide. The latter approach allowed us to test the hypothesis that, by lowering intracellular sodium, inhibition of the Na+-K+-2Cl− cotransporter leads to an increase in apical Na+-H+ exchange in the TALH (Hropot et al. 1985; Good, 1993).
METHODS
Male Sprague-Dawley rats (n = 27; body weight, 200–240 g) were used in all experiments. They had been maintained on a standard diet with a sodium content of 140 mmol (kg dry weight)−1 and a potassium content of 180 mmol (kg dry weight)−1. The rats were anaesthetized intraperitoneally with 5-ethyl-5-(1′-methyl-butyl)-2-thiobarbiturate (Trapanal; 110 mg (kg body weight)−1; Byk Gulden, Konstanz, Germany) and prepared surgically for micropuncture as described previously (Unwin et al. 1994), the left kidney being exposed through a flank incision, freed of perirenal fat and immobilized in a Perspex dish with 3 % (w/v) agar in 0.9 % (w/v) NaCl solution. All animals were infused intravenously with 0.9 % NaCl solution at 2 ml h−1 throughout. The depth of anaesthesia was monitored by testing the limb withdrawal and pupillary reflexes at regular intervals; when necessary, supplementary doses of Trapanal were administered intravenously.
One hour after the completion of surgery, [3H] inulin (Amersham) was included in the intravenous infusion (2 μCi primer, 2 μCi h−1), and 1 h later loop microperfusions were begun. Superficial loops of Henle were perfused at 20 nl min−1 using methods described in detail in previous publications (Capasso et al. 1991; Unwin et al. 1994). The perfusion micropipette, connected to a thermally shielded microperfusion pump (Hampel, Neu-Isenburg, Germany), was positioned in a final surface proximal convolution and the collection micropipette in the first surface distal loop. Three perfusion solutions were used:
1. Standard perfusate
This was designed to mimic native late proximal tubular fluid and had a composition as described previously (Capasso et al. 1991), viz. (mmol l−1): 128 NaCl, 12 NaHCO3, 3.6 KCl, 1 MgCl2, 0.38 NaH2PO4 and 1.62 Na2HPO4, together with FD & C Blue dye (0.07 %, w/v) and [14C] inulin (12.5 μCi ml−1; Amersham).
2. Low-sodium perfusate
The solution proposed by Peterson et al. (1993) was used in order to minimize the contribution of the pars recta to sodium reabsorption. The perfusate sodium concentration is lowered in order to approximate the proximal tubular equilibrium concentration, while the total osmolality is maintained by addition of mannitol. The composition of the perfusate was as follows (mmol l−1): 93 NaCl, 10 NaHCO3, 3.8 KCl, 1 MgSO4, 1 NaH2PO4, 1 CaCl2, 4 urea, 6 sodium gluconate and 61 mannitol, together with FD & C Blue dye (0.07 %) and [14C] inulin (12.5 μCi ml−1). It has been shown by Peterson et al. (1993) and confirmed by us (see Results) that the use of this perfusate almost abolishes fluid reabsorption by the perfused loop. Peterson and colleagues also showed that the addition of frusemide to the perfusate (to block the Na+-K+-2Cl− cotransporter in the TALH) resulted in the abolition of chloride reabsorption. Both these findings are consistent with an absence of fluid reabsorption in the pars recta.
3. Low-sodium, high-bicarbonate perfusate
This was identical to the low-sodium perfusate described above, except that the NaHCO3 concentration was raised to 24 mmol l−1 and the NaCl concentration correspondingly lowered to 79 mmol l−1.
EIPA (2 × 10−4 mol l−1; Research Biochemicals) was included in some perfusates of all three types; bumetanide (10−6 mol l−1; Leo Pharmaceuticals, Copenhagen, Denmark) or a combination of bumetanide (10−6 mol l−1) and EIPA (2 × 10−4 mol l−1) was included in some ‘low-sodium’ perfusates. The maximum number of different perfusates used in a given rat was four. The combinations of perfusates were varied from rat to rat; in every animal, loops were perfused with at least one EIPA-containing solution and with its appropriate control perfusate.
Urine collections and femoral arterial blood samples (for measurement of plasma [3H] inulin) were taken at approximately hourly intervals. Arterial blood pressure was monitored using a Druck (Groby, Leics, UK) transducer. At the end of the experiment, the animal was killed by an overdose of anaesthetic.
Analyses
Urine sodium and potassium concentrations were measured by flame photometry (model 543, Instrumentation Laboratory, Warrington, UK). Fluid collections from perfused tubules were deposited under water-saturated paraffin oil prior to analysis. Their volumes were measured using calibrated constriction pipettes and duplicate samples were taken for measurement of [14C] inulin activity and sodium and potassium concentrations. [14C] inulin activities in perfusates and collectates, and [3H] inulin activities in plasma and urine, were measured by β emission spectroscopy (model 2000 CA, Canberra Packard, Pangbourne, Berks, UK); sodium and potassium concentrations in perfusates and collectates were measured by helium glow photometry (Aminco, Silver Spring, MD, USA) or by electrothermal atomic absorption spectroscopy (model 3110, Perkin-Elmer, Beaconsfield, Bucks, UK).
Calculations
Glomerular filtration rate (GFR) was calculated as the renal clearance of [3H] inulin. The microperfusion pump was calibrated by direct measurement of volumes delivered under oil over timed periods. When set at the nominal rate of 20 nl min−1, the actual pump rate was 19.9 ± 0.2 nl min−1 (mean ±s.e.m., n = 6). The in vivo perfusion rate during microperfusion was calculated as the rate of fluid collection at the early distal site multiplied by the collectate/perfusate concentration ratio for [14C] inulin ((C/P)In). Collected samples were accepted only if the calculated in vivo pump rate was in the range 90–110 % of the pre-determined rate.
Net fluxes of water (Jv), sodium (JNa) and potassium (JK) were calculated as the differences between perfusion and collection rates. Values were expressed per individual loop. Fractional reabsorption was calculated as absolute reabsorption per perfused load.
Statistics
Values are presented as means ±s.e.m. The effect of EIPA in each of the four different conditions was assessed using Student's unpaired t test; EIPA values were compared with those in the appropriate control group in each case.
RESULTS
Whole animal data
In the group of rats as a whole (n = 27), mean arterial pressure was 103 ± 2 mmHg, GFR was 1.12 ± 0.12 ml min−1, sodium excretion was 0.9 ± 0.2 μmol min−1 and potassium excretion was 1.1 ± 0.2 μmol min−1 (renal data apply to the left kidney only). These values fall within the normal ranges for Sprague-Dawley rats as documented in this laboratory (e.g. Shirley et al. 1990; Unwin et al. 1994).
Microperfusion with standard perfusate
Control values for net fluxes of water, sodium and potassium (Jv, JNa and JK) were comparable to those reported previously in perfused loops (Stanton, 1986; Capasso et al. 1991; Unwin et al. 1994). Inclusion of EIPA in the perfusate had no statistically significant effect on any of the variables measured, although a slight tendency for a reduced JNa and a corresponding increase in the sodium concentration of the collectate was noted (Table 1).
Table 1.
Effect of EIPA on water and ion transport in perfused loops of Henle
| n | Perfusion rate (nl min−1) | (C/P)In | Jv (nl min−1) | Collectate [Na7plus;] (mmol l−1) | JNa (pmol min−1) | Collectate [K+] (mmol l−1) | JK (pmol min−1) | |
|---|---|---|---|---|---|---|---|---|
| Standard perfusate | ||||||||
| Control | 34 | 19.7 ± 0.3 | 1.69 ± 0.06 | 7.7 ± 0.4 | 69 ± 3 | 1971 ± 71 | 2.5 ± 0.2 | 47 ± 2 |
| EIPA | 28 | 19.6 ± 0.3 | 1.61 ± 0.05 | 7.4 ± 0.4 | 76 ± 4 | 1851 ± 90 | 2.6 ± 0.1 | 44 ± 2 |
| Low-Na+ perfusate- | ||||||||
| Control | 46 | 19.9 ± 0.2 | 1.08 ± 0.01 | 1.5 ± 0.1 | 73 ± 2 | 828 ± 41 | 2.4 ± 0.1 | 28 ± 2 |
| EIPA | 40 | 20.2 ± 0.2 | 1.08 ± 0.01 | 1.4 ± 0.2 | 80 ± 2 * | 726 ± 37 * | 2.5 ± 0.1 | 27 ± 2 |
| Low-Na+ perfusate + bumetanide- | ||||||||
| Control | 32 | 19.8 ± 0.2 | 1.02 ± 0.01 | 0.4 ± 0.2 | 108 ± 1 | 86 ± 27 | 4.7 ± 0.2 | −20 ± 3 |
| EIPA | 30 | 20.3 ± 0.4 | 1.00 ± 0.01 | 0.1 ± 0.2 | 112 ± 2 | −24 ± 18 * | 4.5 ± 0.1 | −18 ± 2 |
| Low-Na+, high-bicarbonate perfusate- | ||||||||
| Control | 15 | 19.9 ± 0.3 | 1.04 ± 0.02 | 0.8 ± 0.2 | 68 ± 2 | 887 ± 42 | 2.6 ± 0.2 | 24 ± 3 |
| EIPA | 16 | 19.8 ± 0.3 | 1.06 ± 0.01 | 1.1 ± 0.2 | 80 ± 1† | 689 ± 24† | 2.6 ± 0.1 | 25 ± 3 |
Values are means ±s.e.m.n, number of tubules perfused; (C/P)In, collectate/perfusate [14C] inulin concentration ratio; Jv, net water flux; JNa, net sodium flux;JK, net potassium flux; EIPA, ethyl isopropyl amiloride.
P < 0.05
P < 0.001 compared with corresponding value in the absence of EIPA.
Microperfusion with low-sodium perfusate
When using the low-sodium perfusate, the control value for JNa was reduced to approximately half that found with the standard perfusate (Table 1). Jv was also reduced markedly, although remaining slightly greater than zero, as reflected by a (C/P)In ratio exceeding unity. JK was also lower than that seen with the standard perfusate.
In this setting, when EIPA was included in the perfusate, small inhibitory effects on JNa and fractional sodium reabsorption, associated with an increase in the sodium concentration of the collectate, were discernible (Table 1, Fig. 1). There were no significant changes in the other variables monitored.
Figure 1. Effect of EIPA on fractional sodium reabsorption in perfused loops of Henle.
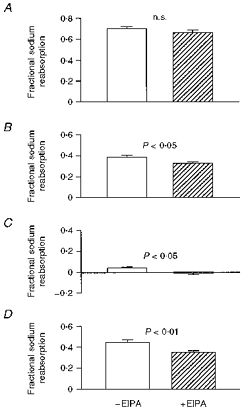
A, standard perfusate; B, low-sodium perfusate; C, low-sodium perfusate with bumetanide; D, low-sodium, high-bicarbonate perfusate. Values are means and s.e.m. For number of perfusions, see Table 1.
Inclusion of bumetanide in the low-sodium perfusate severely reduced JNa to a value that only just exceeded zero (P < 0.05, 95 % confidence limits), and markedly increased the sodium concentration of the collectate (Table 1). Somewhat surprisingly, JV and (C/P)In were also reduced significantly, compared with control values, while the direction of net potassium transport was reversed, reabsorption being replaced by net secretion.
When EIPA and bumetanide were both included in the low-sodium perfusate, JNa fell to a value that was significantly lower than with bumetanide alone, and which was not significantly different from zero (Table 1). The same comment applies to the fractional reabsorption of sodium (Fig. 1). Values for other variables were indistinguishable from those seen with bumetanide alone.
Microperfusion with low-sodium, high-bicarbonate perfusate
These experiments were performed to assess the effect of EIPA in the presence of a raised bicarbonate load. EIPA was found to cause greater reductions in absolute and fractional sodium reabsorption than those seen with the normal bicarbonate load (Table 1, Fig. 1). This effect was associated with an increase in the sodium concentration of the collectate, but no changes in Jv or JK were observed (Table 1).
DISCUSSION
Previous studies have established the existence of a Na+-H+ exchanger in the apical membrane of the rat TALH (Amemiya et al. 1995), and functional evidence for Na+-H+ exchange at this site has come from in vitro microperfusion of rat medullary TALH segments (e.g. Good & Watts, 1996). However, the quantitative significance of any such mechanism in vivo, in circumstances in which the loop of Henle is operating within its normal surroundings in the intact animal, has not previously been assessed. In order to do this, we elected to use in vivo microperfusion of loops of Henle, rather than free-flow micropuncture, for two reasons. First, microperfusion allows precise quantitative assessment of net water and ionic fluxes, as the quantities entering and emerging from a given loop can be compared directly. Secondly, the Na+-H+ exchange inhibitor (EIPA) can be included directly in the perfusate, thereby avoiding unwanted systemic effects. The concentration of EIPA used in the present study has been shown previously to be effective in inhibiting bicarbonate reabsorption in perfused loops in vivo (Capasso et al. 1991).
It should be emphasized at the outset that the potential contribution of Na+-H+ exchange to net sodium transport is likely to be limited by the availability of luminal bicarbonate (in the absence of substantial acidification of the luminal contents of the TALH). The sodium bicarbonate concentration of the standard perfusate, designed to mimic native end-proximal convoluted tubular fluid, was 12 mmol l−1, i.e. < 10 % of the total sodium load. It may not seem surprising, therefore, that when EIPA was included in the standard perfusate, no evidence was found for a significant inhibitory effect on overall absolute or fractional sodium reabsorption in the loop segments. On this basis, apical Na+-H+ exchange is unlikely to make a major contribution to sodium reabsorption in the TALH. However, a relatively small contribution, beyond the resolution of our methodology, could not be ruled out. Moreover, a small effect on the TALH might be masked by other mechanisms operating in the loop segments as a whole. In this context, it should be recalled that the loops of Henle of superficial nephrons comprise three nephron segments: the pars recta, the thin descending limb and the TALH. Any sodium reabsorption in the thin descending limb of superficial (short-looped) nephrons is likely to be trivial (Imai et al. 1987), but a significant quantity of sodium is reabsorbed in the pars recta. We therefore re-assessed the effect of EIPA using the model of Peterson et al. (1993), in which the contribution of the pars recta to net sodium reabsorption is minimized by perfusing the loop with a low-sodium perfusate to which mannitol has been added to maintain approximate isotonicity.
Peterson and colleagues have reported that with their perfusate Jv was only slightly greater than zero (McKay & Peterson, 1993). Furthermore, when frusemide was included, chloride reabsorption fell to zero (Peterson et al. 1993). However, in those studies JNa was not measured; moreover, the criteria for acceptance of perfusion results (collectate/ perfusate inulin concentration ratios between 0.95 and 1.15) excluded the possibility of substantial water reabsorption. The present study included all perfusions for which the perfusion rate was in the acceptable range. In agreement with Peterson and colleagues, we found that the low-sodium perfusate greatly reduced water reabsorption (to a value consistent with that presumed to occur in the thin descending limb; Jamison & Gehrig, 1992) and halved JNa. Addition of bumetanide, to block the Na+-K+-2Cl− cotransporter in the TALH, reduced JNa almost (but not quite) to zero. These results indicate that the contribution of pars recta segments to overall sodium reabsorption in the loop was greatly reduced if not abolished.
When using the low-sodium perfusate, inclusion of EIPA caused a small but significant reduction in sodium reabsorption and a small increase in the sodium concentration of the collectate; Jv was unaffected. These observations strongly suggest that under the conditions of our study the apical Na+-H+ exchanger does make a small contribution to sodium transport in the TALH.
The fact that EIPA-sensitive sodium transport was detectable with the low-sodium perfusate (when sodium reabsorption in the pars recta was absent or minimal), but not with the standard perfusate, is probably a consequence of the lower variability of overall JNa in the former situation; there is no reason to believe that Na+-H+ exchange should be enhanced when using the low-sodium perfusate. Indeed, the mean EIPA-induced reduction in JNa was very similar with the two perfusates. At the same time, in our view it is unsurprising that there was no diminution of Na+-H+ exchange when using the low-sodium perfusate. Immunohistochemical studies and Western blot analyses using polyclonal antibodies have demonstrated intense staining for NHE-3 in the apical membrane of rat medullary and cortical TALH (Amemiya et al. 1995; Attmane-Elakeb et al. 1996), whereas staining in the pars recta was relatively meagre in the S2 segment and completely absent from the S3 segment (Amemiya et al. 1995). Furthermore, apical carbonic anhydrase (type IV) is present in the TALH and the S2 segment of the pars recta, but not in the S3 segment (Brown et al. 1990). These observations, together with our experimental findings, suggest that the bulk of the Na+-H+ exchange in the loop as a whole is located in the TALH.
Although, as discussed above, the residual Jv observed when using the low-sodium perfusate (∼1 nl min−1) is most likely to be attributable to water reabsorption in the thin descending limb, it could be argued that there is normally no water reabsorption in the thin descending limb of superficial nephrons and that only a Jv of zero would guarantee complete suppression of fluid reabsorption in the pars recta. If so, it could be claimed that the EIPA-sensitive sodium reabsorption we observed with the low-sodium perfusate resulted at least partly from residual Na+-H+ exchange in the pars recta. To investigate this, we performed an extra series of microperfusion studies in five Sprague-Dawley rats using a perfusate with a lower sodium concentration (104 vs. 110 mmol l−1), and re-assessed the effect of EIPA (2 × 10−4 mol l−1). The results were as follows (figures for control perfusions given first): number of perfusions, 19 vs. 20; perfusion rate, 19.6 ± 0.4 vs. 19.4 ± 0.4 nl min−1; Jv, 0.1 ± 0.4 vs.−0.1 ± 0.4 nl min−1; JNa, 754 ± 69 vs. 585 ± 44 pmol min−1, P < 0.05. Thus although it would be unwise to rule out some contribution to Na+-H+ exchange by the pars recta under normal conditions, this reduction in JNa in the complete absence of water reabsorption by the loop as a whole provides strong support for EIPA-sensitive sodium reabsorption in the TALH.
Although the contribution of Na+-H+ exchange to overall sodium reabsorption in the loop of Henle is relatively minor under normal circumstances, our results indicate that it becomes more significant if the bicarbonate load presented to the loop is increased. Thus raising the bicarbonate concentration of the low-sodium perfusate from 10 to 24 mmol l−1 resulted in a 2-fold increase in EIPA-sensitive sodium reabsorption, so that the proportion of total sodium reabsorption in the TALH attributable to Na+-H+ exchange increased from ∼12 to ∼23 %.
As already noted, when bumetanide was included in the low-sodium (normal bicarbonate) perfusate, sodium reabsorption in the loop was reduced to a very low value. This residual sodium reabsorption was abolished by EIPA. These findings confirm that the major route for apical sodium entry in the TALH is the Na+-K+-2Cl− cotransporter and in addition indicate that all the sodium reabsorption in the TALH can be accounted for by a combination of Na+-H+ exchange-dependent and Na+-K+-2Cl− cotransporter-dependent mechanisms.
It is well known that loop diuretics increase urinary acidification, and micropuncture studies have shown that frusemide lowers tubular fluid pH within the loop itself (Hropot et al. 1985). In the amphibian early distal tubule (diluting segment), which is believed to be functionally analogous to the TALH (Oberleithner et al. 1982a), inhibition of the Na+-K+-2Cl− cotransporter with frusemide leads to a fall in intracellular sodium activity (Oberleithner et al. 1982b), and it has been proposed that this increased sodium gradient might be responsible for enhanced tubular acidification, through stimulation of Na+-H+ exchange (Hropot et al. 1985; Good, 1993). However, no support for this proposal can be found in the present in vivo investigation: the magnitude of EIPA-sensitive sodium reabsorption was virtually identical in the presence and absence of bumetanide. An alternative explanation for increased acidification of tubular fluid in the loop during treatment with loop diuretics might be reduced paracellular reabsorption of H+ ions as a result of the abolition of the lumen-positive potential difference in the TALH. In addition, there is evidence for an apical H+-ATPase in the TALH (e.g. Brown et al. 1988), the activity of which might be enhanced in this situation.
Potassium transport
When using the standard perfusate, there was net reabsorption of potassium in perfused loops, amounting to ∼65 % of the delivered load. With the low-sodium perfusate, potassium reabsorption was only ∼40 % of the delivered load, suggesting that the net contribution of the pars recta to potassium transport is in the reabsorptive direction. When bumetanide was added to the low-sodium perfusate, net potassium reabsorption was converted to net secretion. The source of this potassium secretion is unknown. However, recent experiments in our laboratory have shown that approximately half of it can be prevented by the addition of the potassium channel blocker, barium, to the perfusate (S. J. Walter, R. J. Unwin & D. G. Shirley, unpublished observations), suggesting that the secretion occurs partly via the apical potassium channels present in the TALH, through which potassium ions are normally ‘recycled’ (Wang et al. 1997).
In vitro patch-clamp studies of rat TALH and amphibian diluting segment have indicated that the apical potassium channels are pH sensitive, channel activity decreasing when intracellular pH is lowered (Greger et al. 1990; Hurst & Hunter, 1990; Schlatter et al. 1993). Since blockade of apical Na+-H+ exchange in the TALH will cause intracellular acidification, the observation in the present study that potassium secretion was unaffected by EIPA might be taken as evidence that the channels responsible for potassium recycling are insensitive to changes in intracellular pH in vivo. However, without additional information, such a conclusion would be unwarranted for at least two reasons. First, although the apical Na+-H+ exchanger has been shown to be a major determinant of intracellular pH (Good & Watts, 1996), it is likely that the basolateral Na+-H+ exchanger (Good, 1993) also contributes to the control of cell pH. (The in vivo nature of the present experiments precluded direct measurement of the pH of TALH cells.) Secondly, it is possible that cellular acidification could result in both a reduction in potassium channel activity and depolarization of the apical membrane, the latter increasing the driving force for potassium exit. If so, these two effects could offset one another and explain why no change in potassium efflux was seen in vivo.
In summary, the present study in loops of Henle perfused in vivo suggests that a small component of sodium reabsorption in the TALH is mediated by apical Na+-H+ exchange. No evidence was found for upregulation of Na+-H+ exchange during blockade of the Na+-K+-2Cl− cotransporter or for pH sensitivity of potassium efflux across the apical membrane of the TALH.
Acknowledgments
This study was supported by The Wellcome Trust. We also thank J. Skinner and E. J. Folkerd for technical assistance.
References
- Amemiya M, Loffing J, Lötscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney International. 1995;48:1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- Attmane-Elakeb A, Chambrey R, Tsimaratos M, Leviel F, Blanchard A, Warnock DG, Paillard M, Podevin R-A. Isolation and characterization of luminal and basolateral plasma membrane vesicles from the medullary thick ascending loop of Henle. Kidney International. 1996;50:1051–1057. doi: 10.1038/ki.1996.408. [DOI] [PubMed] [Google Scholar]
- Brown D, Hirsch S, Gluck S. Localization of a proton-pumping ATPase in rat kidney. Journal of Clinical Investigation. 1988;82:2114–2126. doi: 10.1172/JCI113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D, Zhu XL, Sly WS. Localization of membrane-associated carbonic anhydrase type IV in kidney epithelial cells. Proceedings of the National Academy of Sciences of the USA. 1990;87:7457–7461. doi: 10.1073/pnas.87.19.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso G, Unwin R, Agulian S, Giebisch G. Bicarbonate transport along the loop of Henle. 1. Microperfusion studies of load and inhibitor sensitivity. Journal of Clinical Investigation. 1991;88:430–437. doi: 10.1172/JCI115322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geibel J, Giebisch G, Boron WF. Basolateral sodium-coupled acid-base transport mechanisms of the rabbit proximal tubule. American Journal of Physiology. 1989;257:F790–797. doi: 10.1152/ajprenal.1989.257.5.F790. [DOI] [PubMed] [Google Scholar]
- Giebisch G, Klose RM, Malnic G, Sullivan WJ, Windhager EE. Sodium movement across single perfused proximal tubules of rat kidneys. Journal of General Physiology. 1964;47:1175–1194. doi: 10.1085/jgp.47.6.1175. 10.1085/jgp.47.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good DW. The thick ascending limb as a site of renal bicarbonate reabsorption. Seminars in Nephrology. 1993;13:225–235. [PubMed] [Google Scholar]
- Good DW, Watts BA. Functional roles of apical membrane Na+-H+ exchange in rat medullary thick ascending limb. American Journal of Physiology. 1996;270:F691–699. doi: 10.1152/ajprenal.1996.270.4.F691. [DOI] [PubMed] [Google Scholar]
- Greger R, Bleich M, Schlatter E. Ion channels in the thick ascending limb of Henle's loop. Renal Physiology and Biochemistry. 1990;13:37–50. doi: 10.1159/000173346. [DOI] [PubMed] [Google Scholar]
- Hropot M, Fowler N, Karlmark B, Giebisch G. Tubular action of diuretics: distal effects on electrolyte transport and acidification. Kidney International. 1985;28:477–489. doi: 10.1038/ki.1985.154. [DOI] [PubMed] [Google Scholar]
- Hurst AM, Hunter M. Acute changes in channel density of amphibian diluting segment. American Journal of Physiology. 1990;259:C1005–1009. doi: 10.1152/ajpcell.1990.259.6.C1005. [DOI] [PubMed] [Google Scholar]
- Imai M, Taniguchi J, Tabei K. Function of thin loops of Henle. Kidney International. 1987;31:565–579. doi: 10.1038/ki.1987.37. [DOI] [PubMed] [Google Scholar]
- Jamison RL, Gehrig JJ., Jr . Urinary concentration and dilution: physiology. In: Windhager EE, editor. Handbook of Physiology, section 8, Renal Physiology. New York: Oxford University Press; 1992. pp. 1219–1279. [Google Scholar]
- McKay AJ, Peterson LN. K infusion corrects thick ascending limb Cl reabsorption in K-depleted rats by an aldosterone-independent mechanism. American Journal of Physiology. 1993;264:F792–799. doi: 10.1152/ajprenal.1993.264.5.F792. [DOI] [PubMed] [Google Scholar]
- Mackovic-Basic M, Kurtz I. H+/base transport in the proximal straight tubule and thin descending limb of Henle. Seminars in Nephrology. 1990;10:122–131. [PubMed] [Google Scholar]
- Oberleithner H, Giebisch G, Lang F, Wang W. Cellular mechanism of the furosemide sensitive transport system in the kidney. Klinische Wochenschrift. 1982a;60:1173–1179. doi: 10.1007/BF01716719. [DOI] [PubMed] [Google Scholar]
- Oberleithner H, Lang F, Wang W, Giebisch G. Effects of inhibition of chloride transport on intracellular sodium activity in distal amphibian nephron. Pflügers Archiv. 1982b;394:55–60. doi: 10.1007/BF01108308. [DOI] [PubMed] [Google Scholar]
- Peterson LN, McKay AJ, Borzecki JS. Endogenous prostaglandin E2 mediates inhibition of rat thick ascending limb Cl reabsorption in chronic hypercalcemia. Journal of Clinical Investigation. 1993;91:2399–2407. doi: 10.1172/JCI116473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JA, Barfuss DW. The study of pars recta function by the perfusion of isolated tubule segments. Kidney International. 1982;22:434–448. doi: 10.1038/ki.1982.196. [DOI] [PubMed] [Google Scholar]
- Schlatter E, Bleich M, Hirsch J, Greger R. pH-sensitive K+ channels in the distal nephron. Nephrology, Dialysis & Transplantation. 1993;8:488–490. doi: 10.1093/ndt/8.6.488. [DOI] [PubMed] [Google Scholar]
- Shirley DG, Zewde T, Walter SJ. Renal function in normal and potassium-depleted rats before and after preparation for micropuncture experimentation. Pflügers Archiv. 1990;416:74–79. doi: 10.1007/BF00370225. [DOI] [PubMed] [Google Scholar]
- Stanton BA. Regulation by adrenal corticosteroids of sodium and potassium transport in loop of Henle and distal tubule of rat kidney. Journal of Clinical Investigation. 1986;78:1612–1620. doi: 10.1172/JCI112754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin RJ, Walter SJ, Shirley DG. Lithium reabsorption in perfused loops of Henle: effects of perfusion rate and bumetanide. American Journal of Physiology. 1994;266:F806–812. doi: 10.1152/ajprenal.1994.266.5.F806. [DOI] [PubMed] [Google Scholar]
- Wang W, Hebert SC, Giebisch G. Renal K+ channels: structure and function. Annual Review of Physiology. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]


