Abstract
A regenerative calcium wave is an increase in cytosolic free calcium concentration ([Ca2+]i) which extends beyond the stimulated cells without decrement of amplitude, kinetics of [Ca2+]i increase and speed of propagation.
The aim of the present study was to test the hypothesis that such a wave could be evoked by bradykinin stimulation and by scraping cultured endothelial cells from porcine coronary arteries.
Calcium imaging was performed using the calcium-sensitive dye fura-2. A wound or a delivery of bradykinin to two to three cells on growing clusters of ≈300 cells caused an increase in [Ca2+]i which was propagated throughout the cluster in a regenerative manner over distances up to 400 μm. This wave spread through gap junctions since it was inhibited by the cell uncoupler palmitoleic acid.
The same experiments performed in confluent cultures caused a rise in [Ca2+]i which failed to propagate in a regenerative way. The wave propagation probably failed because the confluent cells were less dye coupled than the growing cells. This was confirmed by immunohistology which detected a dramatic decrease in the number of connexin 40 gap junctions in the confluent cultures.
The regenerative propagation of the wave was blocked by inhibitors of calcium-induced calcium release (CICR) and phospholipase C (PLC), and by suppression of extracellular calcium, but not by clamping the membrane potential with high-potassium solution.
We conclude that regenerative intercellular calcium waves exist in cultured islets but not in confluent cultures of endothelial cells. An increase in [Ca2+]i is not sufficient to trigger a regenerative propagation. The PLC pathway, CICR and extracellular calcium are all necessary for a fully regenerated propagation.
A change in the cytosolic free calcium concentration ([Ca2+]i) is a major intracellular signalling pathway in many cell types.
Non-excitable cells can generate intracellular repetitive calcium spikes and waves in response to an increase in [Ca2+]i (Berridge, 1997). When the cells are coupled by gap junctions, these intracellular phenomena could potentially give rise to regenerative intercellular calcium waves.
Intercellular calcium waves have been studied in many different cell types in culture, including epithelial and endothelial cells, glial cells, glioma cells, osteoblastic cells and articular chondrocytes, as well as in co-cultured astrocytes and neurons (Xia & Ferrier, 1992; Charles et al. 1992, 1993; Boitano et al. 1992; Demer et al. 1993; Nedergaard, 1994; D'Andrea & Vittur, 1996). Calcium waves have also been shown in tissues, including blowfly salivary glands, liver, lung microvessels and central nervous system (Robb-Gaspers & Thomas, 1995; Ying et al. 1996; Newman & Zahs, 1997; Zimmermann & Walz, 1997).
Although these publications reported regenerative intercellular calcium waves, most of them describe, in fact, distinct spatio-temporal waves with different functional roles and possibly different underlying mechanisms. This could be caused by the cell type examined or whether the stimulus was mechanically, electrically or agonist induced. For instance, some of the reported waves seemed to depend more on diffusion of inositol 1,4,5-trisphosphate (InsP3) from the stimulated area than on a regenerative phenomenon of calcium increase, because in these cases the increased [Ca2+]i in the cells diminished rapidly with distance (Demer et al. 1993; Churchill et al. 1996).
Our concept of a regenerative intercellular calcium wave is that, once the wave has started, the maximal [Ca2+]i reached in the cells does not diminish with increasing distance from the origin of the wave, and the wave can regenerate itself from cell to cell thanks to local calcium mobilization phenomena produced in the stimulated cells, which are active beyond the range of simple diffusion of InsP3.
Two conditions are necessary for the existence of a regenerative intercellular calcium wave. Firstly, the cells should be able to present a positive and local feedback in the calcium cascade, as for example the calcium-induced calcium release (CICR) phenomenon, which amplifies the calcium response. This is possible when the cells possess endoplasmic reticulum calcium channels, such as ryanodine or InsP3 receptors, which can be gated by calcium ions themselves. Another possibility, which could be additive with the first one, is calcium entry via the plasmalemmal calcium-dependent calcium channels. Secondly, the self-generated second messengers, like InsP3 or calcium, should be able to diffuse from cell to cell through gap junctions.
Cultured vascular endothelial cells are coupled by gap junctions as demonstrated by dye and electrical coupling experiments (Larson et al. 1983). The response of these cells to bradykinin is caused by the activation of the phospholipase C (PLC) pathway, inducing an increase in [Ca2+]i. In addition, an increase in [Ca2+]i can be induced by wounding the cells (Drumheller & Hubbell, 1991). Furthermore, cultured endothelial cells from porcine coronary arteries possess calcium-gated plasmalemmal cationic channels which are permeable to calcium (Baron et al. 1996). Therefore, all the basic conditions required for a regenerative intercellular calcium wave are present in these cultured cells.
The goal of the present study was to test the hypothesis that an intercellular regenerative calcium wave could be evoked in such cultured endothelial cells of porcine coronary arteries firstly by bradykinin and secondly by wounding. We also examined the possible underlying mechanisms of the regeneration of these calcium waves.
Our results show the existence of an agonist-induced and a scrape-induced regenerative intercellular calcium wave in clusters of primary cultured endothelial cells before they reach confluence.
METHODS
Left anterior descending coronary artery branches of freshly killed domestic pigs Sus scrofa were obtained at the slaughterhouse. The endothelial cells were collected by gentle rubbing of the internal face of the vessel with a scalpel, and centrifuged at 800 g for 8 min in culture medium comprising M199 medium supplemented with 20 % fetal calf serum, 2 mm glutamine, non-essential amino acids, minimal essential medium (MEM) vitamin solution and 50 mg l−1 gentamicin (all from Gibco). The cell pellet was resuspended in M199 medium and plated on collagen-coated culture Petri dishes or glass coverslips. Cells were cultured at 37°C under 5 % CO2. The culture medium was changed 3 times a week. Cells were used after 2–7 days of primary culture. Endothelial cells were identified by their morphology, fusiform growing cells forming islets with a minimum radius of thirteen cells after 2–3 days (Fig. 1A), and a monolayer of polygonal cells (cobblestone-like) after 4 days in culture, as well as by their ability to take up labelled, low-density lipoproteins (Bény & Pacicca, 1994).
Figure 1. Bradykinin-evoked calcium wave in non-confluent endothelial cell culture.

A: top left, fusiform growing endothelial cells forming a cluster after 2 days of primary culture. X corresponds to the area in which the cells were directly stimulated by application of bradykinin (800 nm) and the coloured spots indicate the chosen regions of interest where the variations in [Ca2+]i shown in B were measured. The arrow indicates the direction of perfusion flow. Right, four coloured sequential ratiometric images of [Ca2+]i increase obtained 9, 18, 27 and 51 s following stimulation; a regenerative calcium wave started from the area directly stimulated by bradykinin. Scale bar, 200 μm. B, graph showing the calcium mobilization after bradykinin stimulation; the curves correspond to the variation of [Ca2+]i measured in the regions of interest shown in A. Distance from the stimulation area: 146 μm (^), 266 μm (▵), 412 μm (□), 545 μm (•). The mode of representation of the calcium wave in B was used in subsequent figures.
Cytosolic Ca2+-variation measurements
Loading of endothelial cells with fura-2
Cultured endothelial cells were loaded with fura-2 AM (the acetoxymethyl ester form of fura-2; 10 μm) for 1 h in a Krebs Hepes-buffered solution containing (mm): NaCl, 145; KCl, 5; CaCl2, 1; MgSO4, 0.5; NaH2PO4, 1; Hepes, 20; and glucose, 10.1; pH 7.4 (37°C), in the presence of pluronic F-127 (25 %) and probenecid (1 mm) in order to improve fura-2 loading. Cells were rinsed for 30 min with the same Krebs-Hepes solution before the experiments.
Fura-2 fluorescence measurements
Endothelial cells were observed on an inverted microscope (Diaphot TMD; Nikon). Cells were continuously perfused with an oxygenated Krebs-buffered solution containing (mm): NaCl, 118.7; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 24.8; glucose, 10.1; pH 7.4, gassed with a mixture of 75 % N2, 20 % O2 and 5 % CO2, since high O2 concentrations greatly reduce the intensity of fura-2 fluorescence. Experiments were done at 32–33°C. Images were recorded through an Extended Isis Intensified CCD camera (Photonic Science Ltd, East Sussex, UK) installed on the inverted microscope and interfaced with a Macintosh II fx computer. The molecules of fura-2 were excited using a high pressure mercury lamp and dual excitation wavelengths of 340 and 380 nm, which were changed by rotating the filters mounted on an automatic filter wheel. The emitted light after excitation was collected at 510 nm. Two hundred and fifty-six grey scale images were stored, processed and pseudocoloured with the image analysis software IPLab Spectrum (Signal Analytics Corporation, Vienna, VA, USA). A pair of images was recorded every 3 s.
The process of ratioing was done on the pixels of the stored images, by dividing the fluorescence obtained at 340 nm excitation by that obtained at 380 nm excitation.
The intracellular free calcium concentration was calculated from the fluorescence ratio according to the following equation (Grynkiewicz et al. 1985):
where Rmin and Rmax are the minimum and maximum values of the fluorescence ratio R when all the fura-2 is in Ca2+-free form or saturated with Ca2+, respectively. The factor (Sf,2/Sb,2) is the fluorescence intensity at 380 nm when all the fura-2 is in the Ca2+-free form divided by the fluorescence intensity when the fura-2 is bound to Ca2+. Like Rmin and Rmax, this factor was determined from calibration experiments. In vivo calibration was done on endothelial cells. Cells were exposed to 4-bromo A23187 (1.7 μm) first in the presence of 2.5 mm extracellular Ca2+ (Rmax), and then in a Krebs solution containing no added Ca2+ and 2 mm EGTA (Rmin).
The Kd of fura-2 for Ca2+ was taken as 225 nm.
Local stimulation
To stimulate a selected region, 800 nm bradykinin was ejected during 100 ms from the tip of a pulled glass capillary connected to a pneumatic pump ejection system (PV 820; World Precision Instruments, Sarasota, FL, USA). A high concentration of bradykinin (800 nm) was used in order to compensate for the dilution that occurs before the agonist reaches the monolayer. The estimated final bradykinin concentration stimulating the cells was about 100 nm.
In order to avoid spontaneous diffusion of the peptide before injection, the pipette tip was waterproofed by being dipped in paraffin oil.
In preliminary experiments, the cone-shaped stream of the liquid ejected by the micropipette was visualized using black ink. Thanks to the oiled tip, the injection flux was carried only to a limited group of no more than two to three cells. The ejection of the solvent without bradykinin had no effect on the cytosolic free calcium concentration of the cells (3 observations).
Lucifer Yellow scrape loading
The culture was rinsed with phosphate buffer saline (PBS), then a solution of 45 mm Lucifer Yellow dipotassium in PBS was added and the cells immediately scrape loaded at room temperature (24–26°C) by incision of the monolayer with a scalpel blade. After 3 min in the solution containing the dye, the cells were washed several times with PBS and immediately examined on an inverted fluorescence microscope (Nikon Diaphot) with a × 10 fluor objective (Pepper et al. 1989). In order to quantify the gap junction permeability, the images were recorded with a CCD video camera (Photonic Science Ltd), digitized and analysed with the program IPLab Spectrum, running on a Power Macintosh G3 computer. To quantify the intercellular coupling, we counted the number of fluorescent cell ranks perpendicular to the scrape. A maximum of one scrape was done for every culture; for each scrape the count of the cell ranks was performed on a rectangle 101 μm× 420 μm in five different regions of interest and averaged. This approach was used on growing cells forming clusters (after 2–3 days of primary cell culture), on older confluent cultures (after 6–7 days of primary culture) and on clusters incubated with the gap junction uncoupler palmitoleic acid.
Immunohistology
The cultures were washed with PBS and fixed for 3 min with acetone at −20°C. The cells were then washed with PBS and pre-incubated for 30 min at room temperature in PBS containing 0.5 % bovine serum albumin, without azide. The primary monoclonal antibodies to connexin 40 and connexin 43 used in these experiments were characterized by Bruzzone et al. (1993) and by Haefliger et al. (1997), respectively, and were kindly provided by Professor P. Meda (Faculty of Medicine, Geneva, Switzerland). These antibodies (at 1 : 250 to 1 : 100 dilution) were incubated with the cells for 2 h at room temperature and the cells were then rinsed again with PBS. Cells were incubated for 1 h at room temperature in the fluorescein-labelled secondary antibody (at 1 : 500 dilution; Biosystem) in the dark and the preparation was counterstained with 0.03 % Evans Blue, for 3 min at room temperature. After being washed with PBS, the preparations were mounted in phenylenediamine glycerol and immediately observed on a fluorescence microscope (Nikon Diaphot) with an oil-immersion × 100 fluor objective and photographed.
Capacitance measurements
Whole-cell membrane currents were measured using ruptured patches (Hamill et al. 1981), with a patch-clamp amplifier (EPC7; List Medical, Darmstadt, Germany). Borosilicate glass patch pipettes were pulled with a BB-CH-PC puller (Mecanex SA, Nyon, Switzerland). Pipettes were filled with a solution containing (mm): KCl, 130; MgCl2, 1; Hepes, 10 (pH 7.4 with NaOH), and had a resistance of 4–6 MΩ. Currents were filtered with a low-pass filter at 1 kHz, digitized by an IT16 interface (Instrutech Corporation, Great Neck, NY, USA), sampled at 200 kHz and stored on a Macintosh II fx computer (Pulse; HEKA Electronik, Lambrecht, Germany).
In the cell-attached configuration, the capacitance of the pipette was compensated. In the whole-cell configuration, capacitive current transients were obtained by applying a 10 mV voltage step. After application of palmitoleic acid (50 μm), the capacitive current of the uncoupled cell was fitted with a single exponential (Pulsefit; HEKA Electronik). The capacitance of the cell membrane was calculated as described by de Roos et al. (1996).
Chemicals and drugs
Lucifer Yellow, Evans Blue, fura-2 AM, caffeine, EGTA, probenecid, 4-bromo A23187 and palmitoleic acid were purchased from Sigma. Bradykinin was purchased from Bachem Feinchemikalien AG (Bubendorf, Switzerland); pluronic F-127 from Calbiochem; and dantrolene sodium hydrate and U-73122 from Alexis Corporation (Läufelfingen, Switzerland).
Palmitoleic acid was directly dissolved at the final concentration in the Krebs-Hepes solution by sonication. Dantrolene sodium hydrate, caffeine, 4-bromo A23187 and U-73122 were directly dissolved at their final concentrations in Krebs-Hepes solution. Bradykinin was dissolved in Krebs-Hepes solution at the final concentration from 50 μl prepared samples (containing 1 mg bradykinin and 1 ml 0.25 % acetic acid; stored at −80°C).
Statistics
Experimental data are expressed as means ± standard error of the mean (s.e.m.); n refers to the number of measures. Student's t test was used to compare results, with P < 0.05 taken as the level of significance.
RESULTS
Bradykinin-evoked calcium waves
Non-confluent culture
Two- to 3-day-old cell cultures consisted of fusiform growing cells which formed clusters with a radius varying from thirteen to thirty cells. Stimulation by application of 800 nm bradykinin to a small group of cells (3 cells maximum) in such clusters of ∼300 cells caused an increase in [Ca2+]i, from a mean resting level of 49 ± 23 nm to 974 ± 117 nm (n = 12) in the stimulated cells. This [Ca2+]i increase propagated radially to the periphery of the cluster, including the entire surface and the entire cluster. The radius varied in this series of experiments from thirteen to twenty-one cells (n = 12). We did not observe a significant decrease in the maximal [Ca2+]i as the distance from the site of application increased. The maximal [Ca2+]i reached in the peripheral cells was 966 ± 106 nm (n = 12; P > 0.4; Fig. 1A). The kinetics of the [Ca2+]i increase are reproduced in the graph shown in Fig. 1B. The speed of the front propagation was constant and reached 11 ± 2 μm s−1 (n = 12).
The results clearly showed that the propagation of the wave was not related to the direction of superfusion flow.
A second stimulation of the same cells after a recovery period of 20 min caused the same effects, with the same qualitative and quantitative response (n = 12).
A concentration of 800 nm bradykinin was used in order to compensate for the rapid dilution that occurs before it reaches the monolayer.
Confluent cultures
The same delivery of 800 nm bradykinin to a localized region (2–3 cells) of a 6- to 7-day-old confluent culture caused a rise in [Ca2+]i in the stimulated cells. From a mean resting level of 46 ± 12 nm (n = 5), the [Ca2+]i reached a level of 477 ± 43 nm (n = 5). The increase in [Ca2+]i did not propagate in a regenerated manner (Fig. 2A), but diffused up to a radius of three to four cells (n = 5) with an important spatial decrease in the maximal calcium concentration.
Figure 2. Cytosolic free calcium mobilization in endothelial cells after bradykinin (800 nm) stimulation.
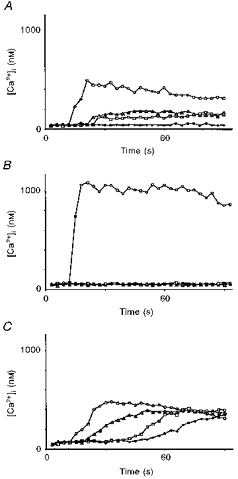
A, role of the age of the culture. The culture was confluent (6 days). ^, cell adjacent to the bradykinin stimulation area; ▵, 65 μm from the stimulation area; □, 83 μm from the stimulation area; •, 122 μm from the stimulation area. B, role of cell coupling. Three-day-old cell cluster in the presence of the gap junction uncoupler palmitoleic acid (50 μm). ^, cell directly stimulated by bradykinin; ▵, 20 μm from the stimulation area; □, 83 μm from the stimulation area; •, 300 μm from the stimulation area. C, role of extracellular calcium. Three-day-old cell cluster in the presence of Krebs solution containing low extracellular calcium (2 mm EGTA; 10−12 M Ca2+). ^, 143 μm from the stimulation area; ▵, 256 μm from the stimulation area; □, 363 μm from the stimulation area; •, 421 μm from the stimulation area.
Consequently, all the following experiments were performed on 2- to 3-day-old non-confluent cell cultures.
Role of the gap junctions
In order to prove that the calcium waves propagated from cell to cell through gap junctions and not by extracellular phenomena of agonist diffusion, bradykinin (800 nm) ejection experiments were done on clusters incubated in Krebs-Hepes solution supplemented with 50 μm of the gap junction uncoupler palmitoleic acid (Lavado et al. 1997).
This oleic acid inhibited the propagation, but not the increase in [Ca2+]i, in the few cells directly exposed to the stream of 800 nm bradykinin (Fig. 2B). In the stimulated cells, [Ca2+]i increased from a mean resting level of 59 ± 16 nm (n = 5) to 971 ± 102 nm (n = 5).
This experiment showed that the stimulated area corresponded to a maximal group of two to three cells (n = 5) directly exposed to the stream of applied peptide.
In order to explain why the cobblestone-like cell cultures did not exhibit the phenomena of regenerated intercellular calcium waves, cell-to-cell coupling was examined in non-confluent and confluent cultures.
Dye coupling
The spread of the intercellular diffusion of Lucifer Yellow perpendicular to the scrape was quantified by counting the number of fluorescent cell ranks. The spread showed a higher intraculture variability in the confluent cultures (s.e.m., 20 ± 2 % of the mean; n = 6) than in the clusters (s.e.m., 10 ± 1 % of the mean; n = 5).
The spread of Lucifer Yellow through the coupled endothelial cells on the monolayer was significantly greater in growing cell cultures (4.5 ± 0.2 cell ranks; n = 5) than in confluent cultures (2.7 ± 0.3 cell ranks; n = 6) (P < 0.05). Incubation of cell clusters in Krebs-Hepes solution containing 50 μm palmitoleic acid inhibited cell coupling. In this case only the first rank of cells adjacent to the scrape was labelled by the dye (Fig. 3).
Figure 3. Electrical and chemical cell coupling in cultured endothelial cells.
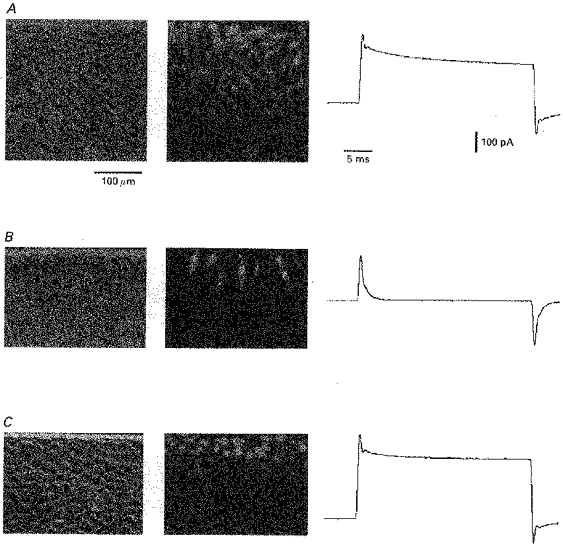
A-C: left, phase contrast micrographs of endothelial cell cultures. The horizontal line at the top of each micrograph is the mark of the scalpel blade used for Lucifer Yellow scrape loading. Middle, fluorescence images of the same areas showing the chemical cell coupling visualized by the diffusion of Lucifer Yellow from the wounded cells. Right, traces representing the current flowing through the syncytium in response to a 5 s, 10 mV change in membrane potential. A, 2- to 3-day-old fusiform growing cell cluster; B, 2- to 3-day-old fusiform growing cell cluster in the presence of the gap junction uncoupler palmitoleic acid; C, 6- to 7-day-old cobblestone-like confluent cell culture.
Electrical coupling
When the number of coupled cells increases in a syncytium, the capacitance that can be measured by the patch-clamp technique increases, since it is proportional to the total membrane surface area of the coupled cells. In our experiments, the capacitance of cell clusters of ∼300 cells and confluent cultures could not be calculated since the capacitance transient could not be fitted by a single exponential. The capacitance measured on clusters incubated in Krebs-Hepes solution containing 50 μm palmitoleic acid was 22.85 ± 3.1 pF (n = 6), which is not significantly different from the capacitance of an isolated cell, which is 26.35 ± 3.1 pF (n = 21; P > 0.4; Fig. 3).
Connexins
Growing cell monolayers were not stained with antibody to connexin 43, but the cell boundaries were strongly labelled with antibody to connexin 40. On confluent cells, labelling by antibody to connexin 40 was rarely observed (Fig. 4). Only a few cells were labelled and the labelling was not always on the cell boundaries. On the other hand a few cells were lightly labelled with antibody to connexin 43 (not shown).
Figure 4. Immunodetection of connexin 40 in cultured endothelial cells.
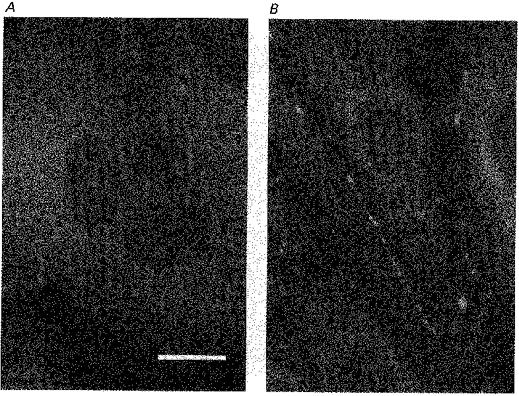
Staining by antibody to connexin 40 is shown by white spots. A, staining was rarely observed in a 6- to 7-day-old cobblestone-like confluent culture. B, staining of the intercellular boundaries in a 2- to 3-day-old fusiform growing cell culture. Scale bar, 1 μm.
Role of extracellular calcium
To examine the possible contribution of extracellular calcium and to test whether the plasmalemmal calcium-dependent cationic channels are implicated in the regeneration of the wave, bradykinin (800 nm) was applied during 100 ms in the absence of extracellular calcium, in Krebs solution supplemented with 2 mm EGTA with a [Ca2+] of 10−12 M. The cells were exposed to this solution for 30 to 40 min before the application of bradykinin. Under these conditions, the propagation of the calcium wave was decreased to ∼50 % of that reached in the presence of extracellular calcium. However, the amplitude did not decrease as the distance from the site of application increased. [Ca2+]i increased from a mean resting level of 48 ± 7 nm (n = 5) to 473 ± 65 nm (n = 5) in the excited cells and to 459 ± 54 nm (n = 5) in the peripheral cells. However, the kinetics of the [Ca2+]i increase changed in the cells with increasing distance from the application point (Fig. 2C), a phenomenon which was not observed in the presence of extracellular calcium (compare Fig. 2C with 1B). The speed of the front propagation was constant at 11 ± 3 μm s−1 (n = 5). Therefore, since the speed of the [Ca2+]i increase diminished with the distance, we consider that in this situation the wave was not fully regenerated.
Role of hyperpolarization
The increase in [Ca2+]i caused by application of bradykinin is accompanied by a transient hyperpolarization (Brunet & Bény, 1989). This hyperpolarization sustains the [Ca2+]i increase by enhancing the electrochemical gradient for extracellular calcium entry (Schilling, 1989).
To examine whether the hyperpolarization influences the regeneration of the calcium wave, bradykinin (800 nm) was applied during 100 ms in the presence of Krebs solution containing high-extracellular potassium ([K+]o = 120 mm). This [K+]o depolarizes the cells and therefore inhibits the bradykinin-induced hyperpolarization (Brunet & Bény, 1989). In this situation, the calcium wave propagation was not inhibited and the kinetics of the [Ca2+]i increase did not change (Fig. 5A). [Ca2+]i increased from a mean resting level of 57 ± 12 nm (n = 5) to 488 ± 25 nm (n = 5). This [Ca2+]i increase was only ∼50 % of that reached in the presence of a normal potassium concentration. It propagated radially up to the periphery of the cluster, covering its entire surface (radius of 18–20 cells) without significant decrease of the maximal [Ca2+]i, which was 475 ± 31 nm in the peripheral cells (n = 5; P > 0.2) before recovery. The speed of the front propagation was increased to 15 ± 1 μm s−1 (n = 5).
Figure 5. Cytosolic free calcium mobilization in endothelial cells after bradykinin (800 nm) stimulation.
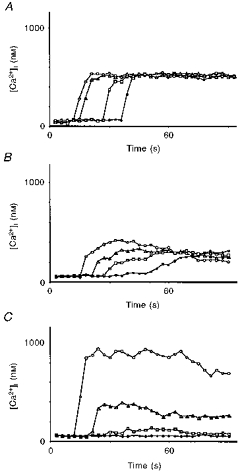
A, role of membrane potential. Three-day-old cell cluster in an extracellular solution containing high potassium (120 mm). Distance from the stimulation area: 182 μm (^), 235 μm (▵), 403 μm (□), 541 μm (•). B, role of membrane potential plus extracellular calcium. Two-day-old cell cluster in solution containing high potassium (120 mm) and low calcium (2 mm EGTA; 10−12 M Ca2+). Distance from the stimulation area: 203 μm (^), 294 μm (▵), 368 μm (□), 569 μm (•). C, role of CICR. Three-day-old cell culture in solution containing dantrolene (10 μm), an inhibitor of ryanodine receptor-dependent CICR. ^, cell directly stimulated by bradykinin; ▵, 122 μm from the stimulation area; □, 167 μm from the stimulation area; •, 250 μm from the stimulation area.
Similar experiments were done in Krebs solution containing high [K+]o, without extracellular calcium. The results showed that [Ca2+]i increased from a mean resting level of 51 ± 14 nm (n = 4) to 463 ± 34 nm (n = 4). The kinetics of the [Ca2+]i increase changed in the cells with increasing distance from the application point (Fig. 5B). The speed of the front propagation reached 14 ± 2 μm s−1 (n = 4).
Role of CICR
The cells were stimulated with 800 nm bradykinin during 100 ms in Krebs perfusion solution supplemented with dantrolene sodium hydrate (10 μm). Dantrolene has been found to inhibit CICR without affecting the calcium release mediated by InsP3 (Palade et al. 1989).
In the present experiments, [Ca2+]i increased from a mean resting level of 58 ± 15 nm (n = 5) to 946 ± 83 nm (n = 5) in the stimulated cells. The [Ca2+]i increase and spreading did not propagate in a regenerated manner to the whole islet, but diffused to the surrounding cells up to a radius of six to eight cells (n = 5), with a significant spatial decrease of maximal calcium concentration (Fig. 5C).
Calcium ionophore-evoked calcium wave
To test whether only the increase in [Ca2+]i is sufficient to trigger the wave, the ejection pipette was loaded with Krebs solution supplemented with the calcium ionophore 4-bromo A23187 (1.7 μm). An ejection time of 5 s was necessary to produce an increase in [Ca2+]i in the stimulated cells. The kinetics of the [Ca2+]i increase are shown in Fig. 6A. [Ca2+]i increased from a mean resting level of 49 ± 10 nm (n = 5) to 963 ± 28 nm (n = 5) in the stimulated cells. No calcium wave was propagated from the stimulated area. The experiments were done in normal Krebs perfusion solution.
Figure 6. Cytosolic free calcium mobilization in endothelial cells caused by an ionophore or wounding.
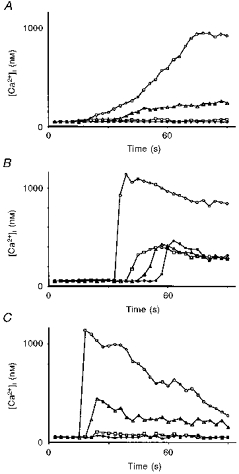
A, mobilization after an increase in [Ca2+]i caused by the calcium ionophore 4-bromo A23187 (1.7 μm) in a 2-day-old cluster. ^, cell directly stimulated by the ionophore; ▵, 60 μm from the site of stimulation; □, 125 μm from the site of stimulation; •, 300 μm from the site of stimulation. B, mobilization after an increase in [Ca2+]i caused by a small scrape in a 3-day-old cluster. ^, cell adjacent to the injury; ▵, 126 μm from the site of the injury; □, 262 μm from the site of the injury; •, 498 μm from the site of the injury. C, mobilization after a small scrape in a 3-day-old cluster in the presence of solution containing the aminosteroid U-73122 (10 μm), an inhibitor of PLC. ^, cell adjacent to the wound; ▵, 20 μm from the site of the injury; □, 40 μm from the site of the injury; •, 62 μm from the site of the injury.
Scrape-induced calcium wave
A fast increase in [Ca2+]i was produced by wounding a group of two to three cells in a cultured monolayer with an empty pipette. This caused an increase in [Ca2+]i from a resting level of 48 ± 12 nm to 1210 ± 117 nm (n = 5) in the first line of cells directly in contact with the damaged cells. In this case a calcium wave started from these neighbouring cells. Although the amplitude of the wave reached only 377 ± 35 nm (n = 5), it radiated from the cells surrounding the wounded region, up to the periphery of the cluster, without significant decrement in [Ca2+]i (P > 0.3). The speed of propagation increased to 21 ± 2 μm s−1 (n = 5).
The kinetics of the [Ca2+]i increase in scrape-induced calcium waves are shown in Fig. 6B.
The regenerative scrape-induced calcium wave was inhibited when the cell clusters were incubated in Krebs-Hepes solution supplemented with the uncoupler palmitoleic acid (50 μm; 7 observations). However, in four cases out of seven, an increase in [Ca2+]i was observed in some cells downstream of the superfusion flow.
Role of the PLC pathway
InsP3 is a plausible second messenger implicated in the triggering and spreading of intercellular calcium waves.
InsP3 is produced through the activation of PLC and, therefore, an inhibition of the latter should block a wave that is dependent only on the presence and production of InsP3. It should be pointed out that the effect of the PLC inhibitor U-73122 on the bradykinin-induced calcium wave could not be tested in our system because the aminosteroid inhibits the bradykinin-induced increase in [Ca2+]i, and consequently the initiation of the wave. Therefore the effect of the inhibitor was investigated only in scrape-induced calcium wave experiments.
We repeated the experiments of scrape-induced calcium waves on clusters previously perfused in Krebs solution supplemented with the aminosteroid U-73122 (10 μm), an inhibitor of PLC.
The results showed an increase in [Ca2+]i from a resting level of 54 ± 7 nm to 1170 ± 63 nm (n = 5) in the first ring of cells surrounding the damaged area. No long-range regenerated calcium wave was propagated from these cells; only an increase in [Ca2+]i which involved a radius of two to three cells (n = 5) was observed (Fig. 6C).
Similar to the experiments done in the presence of palmitoleic acid, in two cases out of five, an increase in [Ca2+]i was observed in more distant cells downstream of the stimulated area, in clear relation with the direction of the superfusion flow.
Role of CICR
In order to test the importance of CICR on mechanically induced calcium waves, we repeated the scrape-induced calcium wave experiments on clusters previously perfused with Krebs solution supplemented with dantrolene sodium hydrate salt (10 μm).
The results showed an increase in [Ca2+]i from a resting level of 59 ± 6 nm to 1148 ± 128 nm (n = 8) in the first line of cells surrounding the damaged area. No long-range regenerated calcium wave was propagated from these cells, but only an increase in [Ca2+]i which involved a radius of four to seven cells (n = 8; Fig. 7A).
Figure 7. Cytosolic free calcium mobilization in endothelial cells.
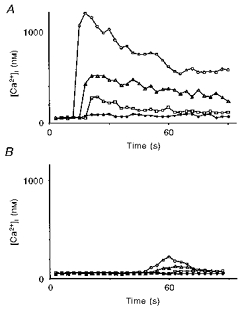
A, mobilization after an increase in [Ca2+]i caused by a small scrape in a 3-day-old cluster in the presence of dantrolene (10 μm), an inhibitor of ryanodine receptor-dependent CICR. ^, cell adjacent to the injury; ▵, 60 μm from the site of the injury; □, 112 μm from the site of the injury; •, 200 μm from the site of the injury. B, mobilization after an increase in [Ca2+]i caused by caffeine (20 mm) in a 3-day-old cluster. ^, cell directly stimulated by caffeine; ▵, adjacent cell; □, 80 μm from the stimulation area; •, 120 μm from the stimulation area.
Again, in three cases out of eight, an increase in [Ca2+]i was observed in more distant cells downstream of the superfusion flow.
Role of ryanodine receptors and dependent calcium pools
Caffeine is a potent activator of ryanodine receptor stores and allows calcium release from these intracellular calcium pools. This effect is opposite to that of bradykinin, which indirectly activates the pools controlled by the InsP3 receptors. When 20 mm caffeine in Krebs solution was applied, no calcium wave was propagated from the stimulated cells. An application period of 3 s was necessary to reach the maximal increase in [Ca2+]i in the stimulated cells, which was 212 ± 11 nm (n = 4; Fig. 7B).
DISCUSSION
Regenerative intercellular calcium waves
The observation that a bradykinin- or a scrape-induced [Ca2+]i increase extends from cell to cell beyond the cells directly stimulated without decrement of the amplitude, the kinetics and the speed of propagation over distances up to 400 μm, shows that these waves of cytosolic calcium are propagated in a regenerated manner.
This result is in contradiction with that of Honda et al. (1996), who did not observe such regenerative calcium waves in cultured endothelial cells. However, our observation that a regenerative calcium wave does not occur in older confluent cobblestone-like cultures is in agreement with these authors, since they used confluent cell cultures. This emphasizes that a regenerative wave only occurs in non-confluent growing endothelial cell cultures.
The propagation of the observed waves probably occurs through gap junctions, since the present study demonstrates that the calcium waves can be inhibited by palmitoleic acid. The fact that the waves can be observed in growing cell clusters, but not in older confluent cultures, can be explained by the fact that gap junctional permeability is higher in growing monolayers than in confluent cultures (Pepper et al. 1989).
This hypothesis was examined in the present work by dye and electrical coupling experiments, as well as by immunohistological detection of connexins.
Intercellular cell coupling
Dye coupling experiments and capacitance measurements showed that an electrical and chemical cell coupling exists in growing and confluent cultures. Both approaches also allowed detection of changes in cell coupling, since both methods proved that palmitoleic acid suppresses the cell-to-cell coupling. However, only the dye coupling experiments showed that the confluent cells were less chemically coupled than the cells which formed growing clusters. Dye coupling results from the speed of the dye diffusion and the buffering capability of the cytoplasm, hence dye coupling experiments accurately reflect the permeability of cell coupling.
These observations show that the gap junctions are less permeable in confluent cultures than in growing cell clusters.
These results were confirmed by the immunohistological analysis of connexins, which directly demonstrates gap junctions. Gap junctions were abundant in growing cells and were composed of connexin 40, which also characterizes the gap junctions in the intact endothelium in vivo. In confluent cells the number of gap junctions markedly diminished. Such a dramatic change in the number of gap junctions could explain the absence of a regenerative wave in confluent cultures, although these cultures have good electrical coupling. This may be because diffusion of monovalent ions is less restricted than that of calcium ions (Allbritton et al. 1992). However, this quantitative and qualitative change in gap junctions does not prove that it is the only cause of the lack of regenerative waves in confluent cultures. A qualitative or quantitative change in calcium-gated channels and a potentially different subcellular location of calcium pools could be an alternative explanation of the lack of the described waves in confluent cultures (Holliday et al. 1991; Young et al. 1996).
Existence of an extracellular factor
During our experiments of scrape-induced calcium waves, we observed that inhibition of cell coupling by palmitoleic acid occasionally failed to inhibit an increase in the [Ca2+]i in cells located downstream of the perfusion flow. This phenomenon, which has already been observed by others (Churchill et al. 1996), could possibly be caused by a cytosolic factor released into the extracellular medium by the wounded cells, provoking an increase in [Ca2+]i in neighbouring cells as it comes in contact with their membranes.
However, the observation that our agonist- and scrape-induced calcium waves can propagate in a multidirectional and upstream manner reveals that such an alternative extracellular mechanism, dependent on the perfusion flow, is not responsible for the observed phenomena of regenerative intercellular calcium waves.
Is the increase in [Ca2+]i sufficient to initiate a wave?
The fact that a wave did not start when the increase in [Ca2+]i was induced by a calcium ionophore suggests that a simple increase in [Ca2+]i is not sufficient to trigger a regenerative propagation. A possible explanation is that the initiation of the calcium wave requires a rapid increase in [Ca2+]i, since a regenerative wave is induced by wounding, where the increase in [Ca2+]i occurs rapidly through the lacerated cells.
The observation that a scrape made in the presence of a PLC inhibitor was not accompanied by an intercellular regenerative calcium wave would, on the other hand, suggest that activation of the PLC pathway, which has been previously described (Hansen et al. 1995), appears necessary to cause the regenerative amplification of the [Ca2+]i increase.
Role of extracellular calcium in the regeneration of the waves
Our previous observations of calcium-dependent cationic channels (Baron et al. 1996) led us to suppose that these plasmalemmal channels could be responsible for the regeneration of a calcium wave in the porcine endothelial monolayer. The present work showed that a regenerative wave, as we define it, cannot propagate in a very low extracellular calcium concentration, since the kinetics of the [Ca2+]i increase changed in the cells with increasing distance from the application point. Therefore, the extracellular calcium is necessary for the maintenance of the kinetics of the [Ca2+]i increase and hence for a complete regeneration of the calcium wave.
Alternatively, one may hypothesize that the observed decrease in the kinetics is the consequence of a possible partial depletion of calcium from the endoplasmic reticulum, induced by the absence of extracellular calcium.
Role of hyperpolarization
The [Ca2+]i increase caused by the action of bradykinin is coupled to a transient hyperpolarization in cultured pig coronary endothelial cells (Brunet & Bény, 1989). The results reported in the present paper support the idea that this hyperpolarization is not necessary for either the start or the regeneration of the waves.
However, a lack of hyperpolarization reduced the maximal [Ca2+]i by half, as in the absence of extracellular calcium (Fig. 8). This is compatible with the concept that the hyperpolarization is necessary to maintain the electrochemical gradient that ensures the entry of extracellular calcium (Schilling, 1989).
Figure 8. Maximal [Ca2+]i reached in the stimulated cells in the different experiments.
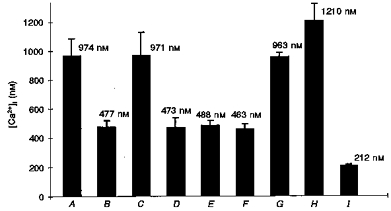
A, after bradykinin stimulation in a 3-day-old cell cluster; B, after bradykinin stimulation in a 7-day-old confluent culture; C, after bradykinin stimulation in the presence of the gap junction uncoupler palmitoleic acid; D, after bradykinin stimulation in a 3-day-old cell cluster in solution containing low calcium (2 mm EGTA; 10−12 M Ca2+); E, after bradykinin stimulation in a 3-day-old cell cluster in solution containing high potassium (120 mm); F, after bradykinin stimulation in a 3-day-old cell cluster in solution containing high potassium (120 mm) and low calcium (2 mm EGTA; 10−12 M Ca2+); G, after stimulation in a 3-day-old cell cluster caused by the calcium ionophore 4-bromo A23187; H, after a small scrape in a 3-day-old cell cluster; I, after caffeine stimulation in a 3-day-old cell cluster.
Role of the intracellular calcium stores
The calcium stores in the endoplasmic reticulum are organized into small, spatially and functionally distinct compartments that are dependent either on InsP3 receptors or ryanodine receptors (Berridge, 1997; Golovina & Blaustein, 1997). Caffeine mobilizes calcium by the activation of ryanodine receptor-dependent calcium pools, through a mechanism independent of the InsP3 receptors and PLC pathway.
CICR is observed for both stores, therefore they could both be responsible for the regeneration of the described calcium waves. The InsP3-dependent calcium pools are essential for the existence of an intercellular regenerative calcium wave, since we demonstrated that the presence of the PLC inhibitor aminosteroid U-73122 blocked the spread of scrape-induced calcium waves. This observation implies that a scrape activates PLC and it also suggests that InsP3 and the calcium pools directly activated by the latter are essential for the initiation and the regeneration of the waves.
In addition, the absence of regenerative bradykinin- or scrape-induced intercellular calcium waves in the presence of dantrolene suggests that CICR dependent upon ryanodine receptor calcium pools is also necessary for the regeneration of the calcium waves.
Taken together, these observations indicate that an increase in [Ca2+]i alone, with CICR intact, is not sufficient to create a regenerative propagation, but that the increase in [Ca2+]i due to the activation of PLC, where CICR is functional, triggers and regenerates a calcium wave. In addition, extracellular calcium, entering into the cells probably through calcium-gated cationic channels (Baron et al. 1996), is necessary for the maintenance of the kinetics of the calcium wave and hence for a complete regeneration of the latter, where the amplitude, the speed of propagation, and the kinetics of the [Ca2+]i increase remain constant with increasing distance from the initiation point.
The activation of these pathways has been described previously using other models, but different roles and importance were attributed to the second messengers or phenomena possibly involved (Allbritton & Meyer, 1993; Kim et al. 1994; Churchill et al. 1996; Sanderson, 1996).
Physiological relevance of the calcium waves
The present work does not allow us to know whether the described regenerative intercellular propagation exists in intact tissues. The speeds of the described waves are too low to carry long distance signals, since at 20 μm s−1 the wave would only cover a distance of 1 cm in 8 min. However, regenerated calcium waves such as the ones described in the present work could be important for local co-ordination, promoting upstream and downstream vasodilatation in the microcirculation. In addition, the increase in [Ca2+]i in pig coronary endothelial cells is an important signal, because it triggers the synthesis and release of many mediators, including von Willebrand factor, prostacyclin, endothelium-derived hyperpolarizing factor and nitric oxide.
The finding that the regenerative intercellular calcium waves occur in islets of growing endothelial cells, but are lacking in older confluent cultures, suggests that these calcium waves could play a role in the process of endothelium regeneration which occurs after a lesion or a ballooning injury. Moreover, the calcium waves observed in our work may play a role in angio- and vasculogenesis, since calcium signalling plays an important role in regulating and promoting cell proliferation (Berridge, 1995).
In conclusion, our results demonstrate for the first time that regenerative intercellular calcium waves exist in clusters of endothelial cells in primary culture but not in confluent cultures. An increase in [Ca2+]i alone is not a sufficient condition to trigger a regenerative propagation. The activation of PLC and CICR of the ryanodine calcium pools, and probably the InsP3 calcium pools as well, are necessary to cause a fully regenerated intercellular wave. In addition, extracellular calcium is necessary for the maintenance of the kinetics of the calcium wave and hence for a complete regeneration of the latter, where the amplitude, the speed of propagation, as well as the kinetics of the calcium increase, remain constant with increasing distance from the initiation point.
Acknowledgments
This work was supported by the Swiss National Science Foundation, grant 31-49163.96. We thank D. Solomos for performing the cell cultures and F. Gribi for performing the immunohistology. We are grateful to Professor P. Meda for provision of the antibodies used in this study and to Professor B. Peck for helpful comments and reviewing of the manuscript. Our thanks are due to the Roche Research Foundation and the Sandoz-Stiftung zur Forderung des medizinisch-biologischen Wissenschaften for calcium measurement set-up.
References
- Allbritton NL, Meyer T. Localized calcium spikes and propagating calcium waves. Cell Calcium. 1993;14:691–697. doi: 10.1016/0143-4160(93)90095-n. 10.1016/0143-4160(93)90095-N. [DOI] [PubMed] [Google Scholar]
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Baron A, Frieden M, Chabaud F, Bény J-L. Ca2+-dependent non-selective cation and potassium channels activated by bradykinin in pig coronary artery endothelial cells. The Journal of Physiology. 1996;493:691–706. doi: 10.1113/jphysiol.1996.sp021415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bény J-L, Pacicca C. Bi-directional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. American Journal of Physiology. 1994;266:H1465–1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium signaling and cell proliferation. BioEssays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Elementary and global aspects of calcium signalling. The Journal of Physiology. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- Brunet PC, Bény J-L. Substance P and bradykinin hyperpolarize pig coronary artery endothelial cells in primary culture. Blood Vessels. 1989;26:228–234. doi: 10.1159/000158770. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Haefliger J-A, Gimlich RL, Paul DL. Connexin 40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Molecular Biology of the Cell. 1993;4:7–20. doi: 10.1091/mbc.4.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Dirksen ER, Merrill JE, Sanderson MJ. Mechanisms of intercellular calcium signaling in glial cells studied with dantrolene and thapsigargin. Glia. 1993;7:134–145. doi: 10.1002/glia.440070203. [DOI] [PubMed] [Google Scholar]
- Charles AC, Naus CCG, Zhu D, Kidder GM, Dirksen ER, Sanderson MJ. Intercellular calcium signaling via gap junctions in glioma cells. Journal of Cell Biology. 1992;118:195–201. doi: 10.1083/jcb.118.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GC, Atkinson MM, Louis CF. Mechanical stimulation initiates cell-to-cell calcium signaling in ovine lens epithelial cells. Journal of Cell Science. 1996;109:355–365. doi: 10.1242/jcs.109.2.355. [DOI] [PubMed] [Google Scholar]
- D'Andrea P, Vittur F. Gap junctions mediate intercellular calcium signaling in cultured articular chondrocytes. Cell Calcium. 1996;20:389–397. doi: 10.1016/s0143-4160(96)90001-9. 10.1016/S0143-4160(96)90001-9. [DOI] [PubMed] [Google Scholar]
- Demer LL, Wortham CM, Dirksen ER, Sanderson MJ. Mechanical stimulation induces intercellular calcium signaling in bovine aortic endothelial cells. American Journal of Physiology. 1993;264:H2094–2102. doi: 10.1152/ajpheart.1993.264.6.H2094. [DOI] [PubMed] [Google Scholar]
- de Roos ADG, van Zoelen EJJ, Theuvenet APR. Determination of gap junctional intercellular communication by capacitance measurements. Pflügers Archiv. 1996;431:556–563. doi: 10.1007/BF02191903. [DOI] [PubMed] [Google Scholar]
- Drumheller PD, Hubbell JA. Local modulation of intracellular calcium levels near a single-cell wound in human endothelial monolayers. Arteriosclerosis, Thrombosis and Vascular Biology. 1991;11:1258–1265. doi: 10.1161/01.atv.11.5.1258. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Blaustein MP. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Haefliger J-A, Castillo E, Waeber G, Bergonzelli GE, Aubert J-F, Sutter E, Nicod P, Waeber B, Meda P. Hypertension increases connexin 43 in a tissue-specific manner. Circulation. 1997;95:1007–1014. doi: 10.1161/01.cir.95.4.1007. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hansen M, Boitano S, Dirksen ER, Sanderson MJ. A role for phospholipase C but not ryanodine receptors in the initiation and propagation of intercellular calcium waves. Journal of Cell Science. 1995;108:2583–2590. doi: 10.1242/jcs.108.7.2583. [DOI] [PubMed] [Google Scholar]
- Holliday J, Adams RJ, Sejnowski TJ, Spitzer NC. Calcium induced release of calcium regulates differentiation of cultured spinal neurons. Neuron. 1991;7:787–796. doi: 10.1016/0896-6273(91)90281-4. [DOI] [PubMed] [Google Scholar]
- Honda HM, Goldhaber JI, Demer LL, Weiss JN. Endothelium-dependent vasodilators do not cause propagated intercellular Ca2+ waves in vascular endothelial monolayers. Cell Calcium. 1996;19:97–104. doi: 10.1016/s0143-4160(96)90078-0. [DOI] [PubMed] [Google Scholar]
- Kim WT, Rioult MG, Cornell-Bell AH. Glutamate-induced calcium signaling in astrocytes. Glia. 1994;11:173–184. doi: 10.1002/glia.440110211. [DOI] [PubMed] [Google Scholar]
- Larson DM, Kam EY, Sheridan JD. Junctional transfer in cultured vascular endothelium: I. Electrical coupling. Journal of Membrane Biology. 1983;74:103–113. doi: 10.1007/BF01870499. [DOI] [PubMed] [Google Scholar]
- Lavado E, Sanchez-Abarca LI, Tabernero A, Bolanos JP, Medina JM. Oleic acid inhibits gap junction permeability and increases glucose uptake in cultured rat astrocytes. Journal of Neurochemistry. 1997;69:721–728. doi: 10.1046/j.1471-4159.1997.69020721.x. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275:844–847. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P, Dettbarn C, Alderson B, Volpe P. Pharmacologic differentiation between inositol-1,4,5-trisphosphate-induced Ca2+ release and Ca2+- or caffeine-induced Ca2+ release from intracellular membrane systems. Molecular Pharmacology. 1989;36:673–680. [PubMed] [Google Scholar]
- Pepper MS, Spray DC, Chanson M, Montesano M, Orci L, Meda P. Junctional communication is induced in migrating capillary endothelial cells. Journal of Cell Biology. 1989;109:3027–3038. doi: 10.1083/jcb.109.6.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb-Gaspers LD, Thomas AP. Coordination of Ca2+ signaling by intercellular propagation of Ca2+ waves in the intact liver. Journal of Biological Chemistry. 1995;270:8102–8107. doi: 10.1074/jbc.270.14.8102. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ. Intercellular waves of communication. News in Physiological Sciences. 1996;11:262–269. [Google Scholar]
- Schilling WP. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. American Journal of Physiology. 1989;257:H778–784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- Xia S-L, Ferrier J. Propagation of calcium pulse between osteoblastic cells. Biochemical and Biophysical Research Communications. 1992;186:1212–1219. doi: 10.1016/s0006-291x(05)81535-9. [DOI] [PubMed] [Google Scholar]
- Ying X, Minamiya Y, Fu C, Bhattacharya J. Ca2+ waves in lung capillary endothelium. Circulation Research. 1996;79:898–908. doi: 10.1161/01.res.79.4.898. [DOI] [PubMed] [Google Scholar]
- Young SH, Ennes HS, Mayer EA. Propagation of calcium waves between colonic smooth muscle cells in culture. Cell Calcium. 1996;20:257–271. doi: 10.1016/s0143-4160(96)90031-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann B, Walz B. Serotonin-induced intercellular calcium waves in salivary glands of the blowfly Calliphora erythrocephala. The Journal of Physiology. 1997;500:17–28. doi: 10.1113/jphysiol.1997.sp021995. [DOI] [PMC free article] [PubMed] [Google Scholar]


