Abstract
The effects of ventricular myosin heavy chain (MHC) composition on the kinetics of activation and relaxation were examined in both chemically skinned and intact myocardial preparations from adult rats. Thyroid deficiency was induced to alter ventricular MHC isoform expression from ∼80 %α-MHC/20 %β-MHC in euthyroid rats to 100 %β-MHC, without altering the expression of thin-filament-associated regulatory proteins.
In single skinned myocytes, increased expression of β-MHC did not significantly affect either maximal Ca2+-activated tension (P0) or the Ca2+ sensitivity of tension (pCa50). However, unloaded shortening velocity (V0) decreased by 80 % due to increased β-MHC expression.
The kinetics of activation and relaxation were examined in skinned multicellular preparations using the caged Ca2+ compound DM-nitrophen and caged Ca2+ chelator diazo-2, respectively. Myocardium expressing 100 %β-MHC exhibited apparent rates of submaximal and maximal tension development (kCa) that were 60 % lower than in control myocardium, and a 2-fold increase in the half-time for relaxation from steady-state submaximal force.
The time courses of cell shortening and intracellular Ca2+ transients were assessed in living, electrically paced myocytes, both with and without β-adrenergic stimulation (70 nm isoproterenol (isoprenaline)). Thyroid deficiency had no affect on either the extent of myocyte shortening or the resting or peak fura-2 fluorescence ratios. However, induction of β-MHC expression by thyroid deficiency was associated with increased half-times for myocyte shortening and relengthening and increased half-time for the decay of the fura-2 fluorescence ratio. Qualitatively similar results were obtained in both the absence and the presence of β-adrenergic stimulation although the β-agonist accelerated the kinetics of the twitch and the Ca2+ transient.
Collectively, these data provide evidence that increased β-MHC expression contributes significantly to the observed depression of contractile function in thyroid deficient myocardium by slowing the rates of both force development and force relaxation.
In striated muscle, Ca2+ binding to troponin C (TnC) initiates a series of events that permit strong interaction between myosin and actin (contraction), while dissociation of Ca2+ from TnC leads to reversal of these events and detachment of myosin from actin (relaxation). While Ca2+ binding to TnC initiates contraction, complete activation of the thin filament in terms of tension and the kinetics of tension development most probably involves the synergistic actions of Ca2+ and strong-binding myosin cross-bridges (Swartz & Moss, 1992; Geeves & Lehrer, 1994; Swartz et al. 1996). Furthermore, the kinetics of interaction of myosin with actin are thought to be determined at least in part by the myosin heavy chain (MHC) content of a given muscle. For example, the maximal rate of tension redevelopment is 8-fold faster in fast-twitch skeletal muscle fibres expressing type II B MHC than in slow-twitch fibres expressing type I MHC, i.e. cardiac β-MHC (Metzger & Moss, 1990). Also, muscle-to-muscle variations in maximal shortening velocity, an index of cross-bridge detachment rate, are thought to be determined by MHC content (Harris et al. 1994; VanBuren et al. 1995; Schiaffino & Reggiani, 1996). To date, little information is available as to whether there is MHC isoform-specific modulation of the kinetics of force development or relaxation, especially in heart muscle.
Two cardiac MHC isoforms, α and β, have been identified in the adult mammalian ventricle and are products of two closely related genes (Lompre et al. 1984). The phenotypic expression of cardiac MHC isoforms is dynamic and subject to a number of physiological influences, including regulation by hormones such as thyroid hormone (Lompre et al. 1984). Thyroid deficiency results in a complete remodelling of ventricular MHC distribution from ∼80 %α-MHC/20 %β-MHC to 100 %β-MHC (Morkin, 1993). It is interesting to note that while cardiac MHC expression varies with thyroid state, altered levels of triiodothyronine (T3) do not appear to alter the phenotypic expression of other myofibrillar protein isoforms, such as the thin filament proteins troponin I (TnI) and troponin T (TnT) (Averyhart-Fullard et al. 1994; Akella et al. 1997). However, it is well known that thyroid state markedly affects the Ca2+ handling properties of the sarcoplasmic reticulum (SR) (Kiss et al. 1994). Therefore, studies examining modulation of myocardial contraction subsequent to altered thyroid status must account for changes in Ca2+ handling as well as myofilament effects mediated by increased expression of β-MHC.
Here, we investigated the influence of thyroid status and altered MHC protein expression on the kinetics of myocardial contraction in both chemically skinned and intact myocardial preparations. The relative proportions of ventricular MHC isoforms in adult rat myocardium were transformed from predominantly α-MHC to exclusively β-MHC by inducing a thyroid deficient state. In skinned myocardium, activation and relaxation kinetics were examined using the photolabile caged Ca2+ compound DM-nitrophen (Kaplan & Ellis-Davies, 1988) and caged Ca2+ chelator diazo-2 (Adams et al. 1989), respectively. Electrically stimulated intact myocytes were used to examine possible interactions between thyroid deficiency-induced changes in MHC content and Ca2+ handling properties of the SR in determining twitch characteristics.
METHODS
Experimental animals
Normal control and thyroidectomized female Sprague-Dawley rats initially weighing 200–224 g were purchased from Harlan Sprague-Dawley (Madison, WI, USA). All animals were housed in groups of two to three rats per cage in temperature- and light-controlled quarters and were provided with food and water ad libitum. Propylthiouracil (PTU; 12 mg kg−1) was administered daily by intraperitoneal injection for 5 weeks to all thryoidectomized rats. The combination of thyroidectomy and supplemental PTU administration has been shown to virtually eliminate circulating plasma T3 and thyroxine (T4) levels (Haddad et al. 1997). All control and thyroidectomized rats were inspected daily for signs of distress and discomfort throughout the time course of the study. We did not observe any signs of distress or discomfort in any animal, and thus no rats were excluded from use in this study. Animals were anaesthetized by placing them in a glass bell jar containing room air and 4 % methoxyflurane. Deep anaesthesia was confirmed by the loss of the pedal reflex and muscular tension of the limbs. All animals were killed by creating a pneumothorax following the establishment of deep anaesthesia by inhalation of methoxyflurane. All animal usage was conducted under the strict guidelines established by the University of Wisconsin Animal Care Committee.
Experimental solutions
Compositions of solutions used in the preparation of intact ventricular myocytes and relaxing and activating solutions used in the mechanical measurements were as follows. Ca2+ Ringer solution (mm): NaCl, 118; Hepes, 25; glucose, 11; pyruvate, 5; KCl, 4.8; NaH2PO4, 2.4; MgCl2, 1.2; and CaCl2, 1; pH 7.4 at 22°C. Relaxing solution (mm): KCl, 100; imidazole, 20; MgATP, 4; EGTA, 2; and free Mg2+, 1; pH 7.0 at 22°C. Activating solution (mm): KCl, 79.2; imidazole, 20; creatine phosphate, 14.5; EGTA, 7; MgCl2, 5.42; and ATP, 4.68; with free [Ca2+] ([Ca2+]free) ranging from 1 nm (i.e. pCa 9.0) to 32 μm (i.e. pCa 4.5), pH 7.0 at 15°C and an ionic strength of 180 mm. The composition of solutions used in flash photolysis experiments is shown in Table 1. The computer program of Fabiato (1988) was used to calculate the final concentration of each metal, ligand and metal-ligand complex based on stability constants described by Godt & Lindley (1982). Purified cardiac myofibrils were prepared using the following solutions. Homogenization buffer (mm): NaCl, 100; imidazole, 10; EDTA, 2; and dithiothreitol (DTT), 1; with 0.5 % Triton X-100, 1 mg l−1 leupeptin, pH 6.8 at 4°C. Rigor buffer (mm): KCl, 75; KH2PO4, 5; EGTA, 2; MgCl2, 2; and NaN3, 2; pH 7.2 at 4°C. Myofibril storage buffer (mm): NaCl, 100; imidazole, 10; EDTA, 2; and DTT, 1; with 1 mg ml−1 leupeptin, 50 % glycerol, pH 6.8 at 4°C.
Table 1.
Composition of experimental solutions used in flash photolysis experiments
| Solution | ATP (mm) | MgCl2 (mm) | EGTA (mm) | CaCl2(mm) |
|---|---|---|---|---|
| Relaxing (pCa 9.0) | 4.74 | 5.43 | 7.00 | 0.02 |
| Pre-activating | 4.74 | 5.28 | 0.07 | — |
| Activating (pCa 4.5) | 4.79 | 5.28 | 7.00 | 7.01 |
| Loading: | ||||
| DM-nitrophen | 4.77 | 5.97 | — | 0.40 |
| Diazo-2 | 4.74 | 5.28 | — | 0.90 |
All solutions contained 100 mm N,N-bis-(2-hydroxyethyl)-2-aminoethanesulfonic acid (Bes), 15 mm creatine phosphate and 5 mm DTT. Ionic strength was adjusted to 180 mm with potassium propionate, pH 7.0 at 15 °C. Loading solutions additionally contained 100 U ml−1 creatine kinase (CalBiochem).
Cellular preparations
Mechanical disruption
Single myocytes and multicellular preparations of myocardium were obtained following mechanical disruption of rat ventricles. Single myocytes were used for measurements of steady-state tension and unloaded shortening velocity, whereas multicellular preparations were used for studies of force development and relaxation following flash photolysis of caged Ca2+ compounds. Following death of the rat by inhalation of methoxyflurane, the heart was rapidly excised and trimmed free of atria and great vessels. The left ventricle plus septum (LV) and right ventricle (RV) were blotted dry and weighed. Approximately 300–400 mg of LV tissue was placed in a beaker of ice-cold relaxing solution and cut into 5–10 mm pieces. The RV and the remaining LV sample (∼200–400 mg) were placed in separate cryotubes and stored at −80°C until analysed for MHC distribution by SDS-PAGE. The minced LV sample was homogenized for 4 s in a Polytron homogenizer to yield multicellular bundles (dimensions, 600–900 μm× 100–180 μm) or for 8 s to yield single myocytes (dimensions, ∼100 μm× 20 μm). The cellular homogenate was centrifuged at 120 g for 2 min and resuspended twice in fresh relaxing solution. After the final spin, the pelleted myocardial preparations were rapidly and completely chemically skinned by resuspending the pellet for either 6 min (single myoyctes) or 15 min (multicellular bundles) in fresh relaxing solution containing 0.3 % Triton X-100. The preparations were washed twice in fresh relaxing solution and stored on ice until used.
Enzymatic digestion
Single living myocytes were isolated by enzymatic digestion of rat ventricles as described previously (Strang et al. 1994) and were used for measurements of the time courses of cell shortening and intracellular Ca2+ transients. Following enzymatic digestion, the resulting suspension of myocytes was filtered through 0.33 mm nylon mesh and centrifuged at 120 g for 90 s at room temperature. The myocytes were resuspended and washed twice in Ca2+ Ringer solution (0.5 mm CaCl2) and were then stored in Ca2+ Ringer solution (0.1 mm CaCl2) at room temperature until used.
Experimental protocols
During all experimental protocols, sarcomere length, cell length and cell width during activation and relaxation were recorded on videotape using a video camera (Panasonic, model WV-BL600; Secaucus, NJ, USA) and VHS recorder (JVC, model HR-S6600U; Elmwood Park, NJ, USA).
Tension-pCa relationship
An experimental apparatus similar to one described previously (Strang et al. 1994) was used for attachment of single skinned ventricular myocytes to record steady-state submaximal (P) and maximal Ca2+-activated tensions (P0), the Ca2+ sensitivity of tension (pCa50) and unloaded shortening velocity (V0). Myocytes were attached with silicone adhesive to steel pins (10 μm o.d.), which were fixed with paraffin wax to a piezoelectric translator (Physik Instrument, Waldbronn, Germany) and a force transducer (Cambridge Technology, model 403; Cambridge, MA, USA). P0 and pCa50 were determined at a sarcomere length of ∼2.25 μm. Apparent co-operativity of tension development was estimated from the steepness of the tension-pCa relationship for Ca2+-activated tensions less than 0.5P0 (i.e. n2, where n is the Hill coefficient), which was quantified using Hill plot transformation of tension-pCa data (Strang et al. 1994). We focused on this region of the curve because the tension-pCa relationship is biphasic, and most of the co-operative activation of the thin filament is evident at tensions less than 0.5P0 (Moss, 1992). Tension-pCa relationships were obtained by first maximally activating the myocyte in a solution of pCa 4.5 and then transferring the myocyte to a series of submaximal pCa solutions between pCa 6.0 and pCa 5.0. At each pCa, the amount of steady-state tension was measured by rapidly slackening the myocyte by 20 % of its total length. Ca2+-activated tension was calculated as the difference between total steady-state tension and the Ca2+-independent tension obtained by slackening the myocyte in a solution of pCa 9.0. To assess any decline in tension-generating capability, myocytes were maximally activated in a solution of pCa 4.5 at the end of each protocol and the reference P0 value for successive submaximal activations was interpolated between the initial and final measurements of maximal tension. Ca2+-activated tensions (P) obtained in solutions of submaximal pCa were expressed as a fraction of P0, i.e. P/P0. Tension-pCa data were fitted using the following equation:
where n is the Hill coefficient, and k corresponds to the [Ca2+] required for half-maximal activation.
Unloaded shortening velocity (V0)
V0 was measured during maximal activation of each myocyte using the slack test method (Edman, 1979; Strang et al. 1994). Once steady-state tension was reached, the myocyte was slackened by 16–20 % of the initial length, starting from a sarcomere length of ∼2.25 μm. The time between imposition of a slack step and the redevelopment of force was measured by fitting a horizontal line by eye through the tension baseline and determining its intersection with a straight line drawn through the initial portion of tension redevelopment. The maximum amount of slack imposed was such that the myocyte did not shorten below a sarcomere length of 1.80 μm, at which point distortion due to mechanical restoring forces within the myocytes is likely to occur (Strang et al. 1994). Length change (as percentage of initial length) was plotted versus duration of unloaded shortening (ms). V0 was determined from the slope of a line fitted to the data by linear regression analysis. Data from a given myocyte were discarded if the regression coefficient was < 0.95.
Rates of tension development and relaxation following flash photolysis of caged compounds
Multicellular skinned preparations were mounted in an experimental apparatus described previously for skeletal muscle fibres (Patel et al. 1996): one end of the preparation was attached to the arm of a torque motor (Cambridge Technology, model 350) and the other was attached to a force transducer (Cambridge Technology, model 403). All experiments were performed using the solutions listed in Table 1, with sarcomere length set at ∼2.30 μm in relaxing solution. The rates of tension development and tension relaxation were determined following flash photolysis of DM-nitrophen (CalBiochem) and diazo-2 (Molecular Probes), respectively. When exposed to a flash of UV light (λ, ∼360 nm), DM-nitrophen rapidly (< 2 ms) releases Ca2+ due to a decrease in Ca2+-binding affinity from 5 nm to 3 mm (Kaplan & Ellis-Davies, 1988), whereas diazo-2 rapidly (> 3000 s−1) chelates Ca2+ as a result of an increase in Ca2+-binding affinity from 2.2 μm to 73 nm (Adams et al. 1989). At the beginning of each experiment, P0 was assessed by bathing the myocardial preparation first in pre-activating solution and then in activating solution of pCa 4.5. Once steady-state force was reached, the preparation was slackened to achieve a force baseline and then returned to relaxing solution. The protocols used to determine the rate of tension development and the rate of tension relaxation consisted of four steps. The preparation was transferred from relaxing solution to the following sequence of solutions: (1) pre-activating solution (for 4 min with two solution changes); (2) loading solution containing either 0.4 mm CaCl2 and 1 mm DM-nitrophen or 0.9 mm CaCl2 and 2 mm diazo-2 for 5 min; (3) silicone oil (Dow Corning 200 fluid, viscosity = 10 cs) in an ∼80 μl quartz trough for recording changes in force following flash photolysis; and (4) relaxing solution. While the preparation was in silicone oil, different levels of activation were achieved by photolysing DM-nitophen with either a low or a high intensity UV flash from a flash lamp (λ, ∼360 nm; Optoelektronic, Hamburg, Germany), i.e. the power supply to the flash lamp was set at either 150 or 350 V. Relaxation of the preparation from steady-state force was achieved by photolysing diazo-2 with a high intensity UV flash. The amount of force (P) generated following flash photolysis of DM-nitrophen or prior to flash photolysis of diazo-2 was expressed relative to P0 (P/P0). Following flash photolysis of DM-nitrophen, the rate of force development (kCa) was determined by fitting the experimental data with a single exponential equation of the form:
where Ft is force at time t, F0 is maximum force and k is kCa.
Myocyte shortening and intracellular Ca2+ transients
Changes in cell length and cytosolic [Ca2+] were recorded simultaneously (Patel et al. 1997) in intact ventricular myocytes both prior to and during β-adrenergic stimulation. A video-edge detection system (Crystal Biotech, Northborough, MA, USA) was used to monitor myocyte shortening while the fluorescent Ca2+ indicator fura-2 AM (Molecular Probes) was used to monitor continuously intracellular Ca2+ during both rest and contraction. Briefly, intact ventricular myocytes were loaded with fura-2 by incubating them for 20 min in Ringer solution containing 0.1 mm CaCl2 and 0.03 mm fura-2 AM. Fura-2-loaded myocytes were then placed in a perfusion chamber mounted on the stage of an inverted microscope (Olympus). The perfusion chamber was equipped with platinum electrodes for field stimulation at 0.2 Hz (Beekman et al. 1988) with in-flow and out-flow tubes for continuous perfusion of myocytes with Ringer solution containing 1 mm CaCl2. Intracellular fura-2 was excited at 340 and 380 nm and the emitted fluorescence was detected at 510 nm. The 340 nm/380 nm fluorescence ratio (F340/F380) was used to assess changes in intracellular Ca2+ concentration due to electrical stimulation of the myocytes. β-Adrenergic stimulation was achieved by continuous perfusion with Ringer solution containing 1 mm CaCl2 and 70 nm isoproterenol (isoprenaline).
Myofibril purification and electrophoretic analysis of ventricular MHC isoforms
A polytron tissue homogenizer (3 × 5 s pulses) was used to homogenize 200–300 mg of left ventricular tissue in twenty volumes of homogenization buffer. The homogenate was centrifuged at 1500 g for 10 min at 4°C. The resulting pellet was washed twice in the same buffer without Triton X-100. The purified myofibrils were resuspended in rigor buffer and were then used to determine myofibrillar protein concentration (BCA protein assay; Sigma). Following centrifugation, cardiac myofibrils were resuspended to a final concentration of 1 mg protein ml−1 in storage buffer and were then stored at −20°C until analysed by SDS-PAGE. Left ventricular MHC content was determined using 12 % SDS-PAGE and ultrasensitive silver staining (Sweitzer & Moss, 1993). The relative proportions of ventricular MHC isoforms were determined by densitometric analysis of silver-stained gels using a GS-670 imaging densitometer and Molecular Analyst software (BioRad Laboratories, Hercules, CA, USA). The relative proportions of α- and β-MHC isoforms were estimated by expressing the area under the peak for each isoform as a fraction of the sum of the areas for both isoforms.
Statistics
All data are expressed as means ±s.e.m. Data were examined for homogeneity of variance using Bartlett's test (Zar, 1984). Student's two-tailed t test for independent samples was used as a post hoc test, with significance set at P < 0.05.
RESULTS
The effects of thyroid deficiency on ventricular morphology and left ventricular MHC expression
Treatment with surgical thyroidectomy and daily supplemental injections of PTU markedly altered cardiac morphology and ventricular MHC expression. Significant reductions in absolute ventricular weight, left ventricular weight and ventricular weight to body weight ratio were seen as a consequence of thyroid deficiency (Table 2), consistent with previous results (Fitzsimons et al. 1990; Haddad et al. 1997). In addition to its impact on myocardial mass, thyroid hormone is also known to be a potent regulator of ventricular MHC expression (Lompre et al. 1984). In the present study, thyroid deficiency altered the distribution of left ventricular MHC isoforms in adult rats from predominantly α-MHC (i.e. ∼80 %α-MHC/20 %β-MHC) to exclusively β-MHC (Fig. 1). In contrast to its dramatic effects on ventricular MHC expression, thyroid deficiency did not alter the expression of ventricular myosin light chains (i.e. MLC1v and MLC2v) or thin filament proteins (Fig. 1A), confirming previously published findings (Averyhart-Fullard et al. 1994; Schiaffino & Reggiani, 1996; Akella et al. 1997). Since the cardiac myofibrillar effects of thyroid deficiency are limited to changes in the expression of MHC isoforms, any alterations in contractile properties in skinned myocardium may be attributed to altered MHC expression.
Table 2.
Myocardial morphological characteristics as a function of thyroid state
| Group | BW (g) | VW (mg) | LV (mg) | VW/BW | α-MHC (%) | β-MHC (%) |
|---|---|---|---|---|---|---|
| Euthyroid | 268 ± 8 | 769 ± 29 | 635 ± 29 | 2.87 ± 0.05 | 83.2 ± 2.6 | 16.8 ± 2.6 |
| Thyroid deficient | 232 ± 2* | 538 ± 11* | 441 ± 14* | 2.35 ± 0.06* | n.d. | 100.0* |
Data are presented as means ±s.e.m., with n = 5 animals per group. BW, body weight; VW, left and right ventricular weight; LV, left ventricle plus septum weight; VW/BW, ventricular weight to body weight ratio; percentage α-MHC and β-MHC are expressed as a fraction of the total MHC pool (n.d., not detectable).
Significantly different from euthyroid (P < 0.05).
Figure 1. Myofibrillar protein composition of left ventricular myocardium from euthyroid and hypothyroid rat.
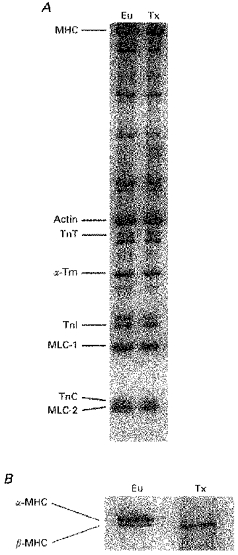
Representative 12 % SDS-PAGE of euthyroid (Eu) and thyroid deficient (Tx) left ventricular myocardium. Myofibrillar contractile proteins (A) and MHC isoforms (B) are identified in order of increasing mobility. Abbreviations: MHC, myosin heavy chain; TnT, troponin T; α-Tm, α-tropomyosin; TnI, troponin I; TnC, troponin C; MLC, myosin light chain.
Effects of altered MHC expression on steady-state mechanical properties (P0, pCa50 and V0)
Table 3 summarizes maximal Ca2+-activated tensions, Ca2+ sensitivities of tension, Hill coefficients and unloaded shortening velocities from single skinned myocytes expressing either ∼80 %α-MHC/20 %β-MHC (euthyroid) or 100 %β-MHC (thyroid deficient). Altered ventricular MHC expression had no significant effect on either maximal Ca2+-activated tension or the Ca2+ sensitivity of tension (Fig. 2). Recent work suggests that the β-MHC produces approximately twice the force per cross-bridge as the α-MHC, possibly due to a prolonged duty cycle of the β-MHC (VanBuren et al. 1995). If this is the case, our observation of no significant change in maximum force with increased expression of β-MHC might indicate that fewer cross-bridges are bound during activation of thyroid deficient myocardium. The lack of change in pCa50 provides corroborating evidence that thyroid deficiency did not alter the relative expression of the thin-filament-associated regulatory proteins, since variations in expression of TnT (Nasser et al. 1991; Reiser et al. 1992; Hofmann et al. 1995) have previously been shown to alter significantly the Ca2+ sensitivity of tension. Increased β-MHC expression did not significantly alter the steepness of the tension-pCa relationship (n2) at least within the variability of the data (Table 3), suggesting that MHC content has no effect on the apparent co-operativity of tension development. However, as a consequence of increased β-MHC expression, V0, a measure of cross-bridge detachment rate (Huxley, 1957), was reduced more than 5-fold relative to myocardium expressing predominantly α-MHC (Table 3). Down-regulation of α-MHC expression and upregulation of β-MHC expression were previously shown to reduce dramatically unloaded shortening velocity in skinned multicellular myocardial preparations (Pagani & Julian, 1984; VanBuren et al. 1995).
Table 3.
Data from dynamic mechanical measurements in skinned left ventricular myocardium
| A.Steady-state mechanical measurements | ||||
|---|---|---|---|---|
| Group | Po (μN) | pCa50 | n2 | Vo (% length change s−1) |
| Euthyroid (8) | 7.5 ± 0.4 | 5.42 ± 0.02 | 3.57 ± 0.21 | 4.72 ± 0.31 |
| Thyroid deficient (8) | 8.9 ± 1.5 | 5.45 ± 0.04 | 2.93 ± 0.19 | 0.92 ± 0.09* |
| B.Actvation and relaxation kinetics | ||||
|---|---|---|---|---|
| Activation | Relaxation | |||
| Group | P/Po (%) | kCa (s−1) | P/Po (%) | Rt1/2 (ms) |
| Euthyroid (4) | 53.6 ± 5.0 | 5.95 ± 0.18 | 52.3 ± 1.8 | 64.5 ± 5.3 |
| 90.1 ± 3.5 | 8.88 ± 0.53 | |||
| Thyroid deficient (8) | 47.4 ± 4.9 | 2.63 ± 0.06* | 56.7 ± 4.0 | 139.1 ± 5.6* |
| 79.7 ± 3.0 | 3.47 ± 0.29* | |||
All values are expressed as means ±s.e.m., with number of cells given in parentheses. Po, maximal Ca2+- activated tension; pCa50, pCa required for half-maximal activation; n2, Hill coefficient for Ca2+-activated tensions less than 0.50P0; Vo, unloaded shortening velocity; kCa, rate of tension development; Rt1/, halftime for tension relaxation; percentage P/Po corresponds to the relative forces generated following flash photolysis of DM-nitrophen (activation) with a low (upper values) and high (lower values) intensity UV flash, and diazo-2 (relaxation) with a high intensity UV flash.
Significantly different from euthyroid (P < 0.05).
Figure 2. Tension-pCa relationship of skinned single myocytes with differing MHC contents.
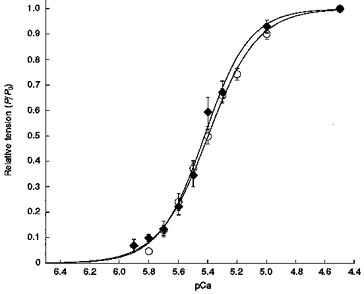
Tension-pCa relationship was determined using single skinned myocytes (n = 8) from left ventricles of the hearts expressing α-MHC (○) or β-MHC (♦). Data points are means and the error bars represent the s.e.m. Smooth lines were fitted using the Hill equation: P/P0 =[Ca2+]n/(kn+[Ca2+]n), where n is the Hill coefficient, and k is the [Ca2+] required for half-maximal activation (i.e. pCa50).
Kinetics of cross-bridge interaction probed by flash photolysis of caged Ca2+ chelators
A summary of the mean rates of tension development for a given level of activation in myocardium expressing either predominantly α-MHC or exclusively β-MHC is presented in Table 3. Figure 3A and B shows the time courses of changes in force recorded from skinned myocardial preparations following flash photolysis of the caged Ca2+ chelator DM-nitrophen. In Fig. 3A, a skinned preparation expressing ∼80 %α-MHC/20 %β-MHC was pre-equilibrated for 5 min in loading solution containing 0.4 mm CaCl2 and 1 mm DM-nitrophen and then exposed to flashes of low (curve a) and high (curve b) intensity UV light. Prior to the flash, the preparation generated negligible active force since the calculated [Ca2+]free (∼pCa 7.0) was well below the threshold for activation of the myofilaments (see Fig. 2). Following photolysis with a low intensity UV flash, tension increased to a steady level of 0.46P0 with an apparent rate constant (kCa) of 6.03 s−1. When the same preparation was exposed to a high intensity UV flash, force increased to a steady level of 0.95P0 with a kCa of 9.90 s−1. Under identical experimental conditions, a myocardial preparation expressing 100 %β-MHC developed a steady tension of 0.52P0 (kCa = 2.75 s−1) following a low intensity flash (curve c, Fig. 3B), and 0.82P0 (kCa = 3.77 s−1) following a high intensity flash (curve d, Fig. 3B). Although the rate of tension development varied with activation level in all myocardial preparations regardless of MHC content, the rate at any given activation was always significantly slower in myocardium expressing 100 %β-MHC (inset Fig. 3A and B).
Figure 3. Force-dependent changes in rate of force development in myocardium expressing α-MHC and β-MHC.
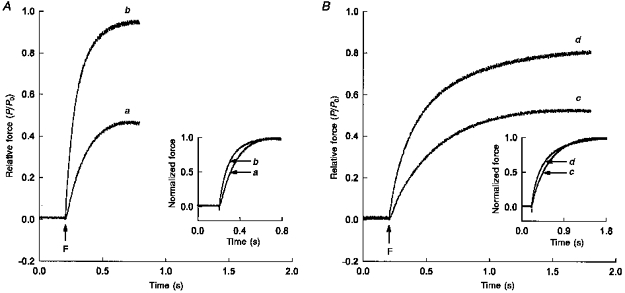
A, a skinned multicellular preparation expressing predominantly α-MHC was incubated for 5 min in loading solution containing 0.4 mm CaCl2 and 1 mm DM-nitrophen ([Ca2+]free, pCa > 7.0). The preparation was then transferred to a quartz trough filled with silicone oil and exposed to a flash (F) of low (a) or high (b) intensity UV light. B, force changes recorded following flash photolysis of DM-nitrophen with a flash of low (c) or high (d) intensity UV light in a skinned multicellular preparation expressing β-MHC; conditions were identical to those in A. Change in force is expressed relative to maximum force (P0) generated by the same preparation when exposed to a pCa 4.5 solution. To illustrate differences in the rate of tension rise, changes in force are expressed relative to respective peak forces generated following flash photolysis of DM-nitrophen and replotted as a function of time (inset).
Figure 4 shows relaxations of force recorded in a skinned multicellular preparation expressing either predominantly α-MHC or exclusively β-MHC, following flash photolysis of diazo-2 (Adams et al. 1989). The conditions used to determine the rate of tension relaxation were designed such that all preparations would relax greater than 90 % from pre-photolysis steady-state force. When these preparations were incubated in loading solution containing 0.9 mm CaCl2 and 2 mm diazo-2 (calculated [Ca2+]free, ∼pCa 5.5), the pre-flash force generated by each preparation was similar, i.e. 0.48P0 in the preparation expressing ∼80 %α-MHC/20 %β-MHC and 0.50P0 in the preparation expressing 100 %β-MHC. In fact, the mean level of steady-state submaximal force generated in the diazo-2 loading solution was unaffected by ventricular MHC isoform content (Table 3). Following flash photolysis of diazo-2, the mean time taken for steady-state force to decrease by half (i.e. t½) was significantly greater in preparations expressing 100 %β-MHC (139.14 ± 5.64 ms) than in myocardium expressing ∼80 %α-MHC/20 % -MHC (64.50 ± 5.31 ms).
Figure 4. Relaxation from steady-state force initiated following flash photolysis of diazo-2 in skinned myocardial preparations with differing MHC contents.
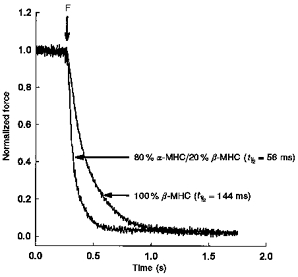
Skinned myocardial preparations, one from a heart expressing predominantly α-MHC and the other from a heart expressing exclusively β-MHC, were incubated for 2 min in loading solution containing 0.8 mm CaCl2 and 2 mm diazo-2. The preparations expressing predominantly α-MHC and exclusively β-MHC generated 0.48P0 and 0.50P0, respectively. At the end of the incubation period, the preparation was transferred to a quartz trough and exposed to a flash (F) of high intensity UV light. To illustrate differences in the rate of relaxation from steady-state force between the two preparations, the changes in force are expressed relative to the peak steady-state force prior to flash photolysis of diazo-2.
Twitch characteristics and Ca2+ transients recorded in intact ventricular myocytes
Since thyroid deficiency increases the expression of β-MHC and concomitantly alters the kinetics of Ca2+ handling by the SR, we assessed the relative contributions of myofibrillar and SR changes to twitch kinetics in living ventricular myocytes. β-Adrenergic stimulation was used in both euthyroid and thyroid deficient myocytes in an attempt to stimulate fully the SR Ca2+-ATPase pump via phosphorylation of phospholamban, and thereby allow direct assessment of the roles of predominantly α-MHC or exclusively β-MHC preparations in determining twitch kinetics. The extent of cell shortening, half-times for cell shortening and relengthening, resting and peak fura-2 fluorescence ratios, and half-times for the rise and decay of the fura-2 fluorescence ratios are summarized in Table 4. Representative time courses of cell shortening and fura-2 fluorescence ratios recorded during electrical stimulation in the absence and presence of 70 nm isoproterenol are presented for myocytes expressing either ∼80 %α-MHC (Fig. 5A–C) or 100 %β-MHC (Fig. 6A–C). While the extent of myocyte shortening and the fura-2 fluorescence ratios did not differ with ventricular MHC content either prior to or during β-adrenergic stimulation (Figs 5B and C, and 6B and C), the time courses of myocyte shortening and relengthening were significantly longer in myocytes exclusively expressing β-MHC. Under control conditions, myocytes expressing 100 %β-MHC exhibited a 2-fold longer half-time to peak cell shortening (201.7 ± 17.0 versus 94.5 ± 7.1 ms) and a 48 % greater half-time for cell relengthening (559.4 ± 61.5 versus 377.0 ± 54.0 ms) relative to myocytes expressing ∼80 %α-MHC. As anticipated from earlier work, β-adrenergic stimulation shortened the time course for contraction and relaxation in both groups of myocytes (Beekman et al. 1988); however, myocytes expressing 100 %β-MHC still exhibited significantly slower half-times for cell shortening (59.4 ± 4.4 versus 38.0 ± 2.5 ms) and relengthening (200.0 ± 8.0 versus 101.5 ± 7.3 ms). Both under control conditions and during β-adrenergic stimulation, thyroid deficiency did not change the half-time for the increase of intracellular Ca2+, the amplitude of the Ca2+ signal during contraction (peak fura-2 fluorescence ratio), or the level of free Ca2+ during diastole (resting fura-2 fluorescence ratio). Collectively, these data suggest that neither SR Ca2+ content nor SR Ca2+ release was significantly affected by thyroid deficiency. However, the rate of SR Ca2+ uptake was slowed, since the half-time for decay in the fura-2 fluorescence ratio in myocytes from thyroid deficient rats was significantly greater both under control conditions (576.0 ± 67.8 versus 374.4 ± 27.2 ms) and during β-adrenergic stimulation (232.0 ± 14.5 versus 123.0 ± 11.4 ms) compared with myocytes from euthyroid rats.
Table 4.
Twitch and Ca2+ transient characteristics of intact myocytes in the absence (Control) or presence of β-adrenergic stimulation by 70 nm isoproterenol
| A.Twith characteristics | ||||
|---|---|---|---|---|
| Group | Condition | Myocyte shortening (%) | t1/2 myocyte shortening (ms) | t1/2 myocyte relengthening (ms) |
| Euthyroid (9) | Control | 3.24 ± 0.37 | 94.5 ± 7.1 | 377.0 ± 54.0 |
| Thyroid deficient (8) | Control | 3.94 ± 0.32 | 201.7 ± 17.0* | 559.4 ± 61.5* |
| Euthyroid (9) | Iso | 19.30 ± 1.07 | 38.0 ± 2.5 | 101.5 ± 7.3 |
| Thyroid deficient (8) | Iso | 18.46 ± 0.63 | 59.4 ± 4.4* | 200.0 ± 8.0* |
| B.Ca2+ | |||||
|---|---|---|---|---|---|
| Group | Condition | Resting F340/F380 | Peak F340/F380 | t fluorescence increase (ms) | t fluorescence decay(ms) |
| Euthyroid (9) | Control | 0.125 ± 0.003 | 0.228 ± 0.007 | 30.0 ± 3.8 | 374.4 ± 27.2 |
| Thyroid deficient (8) | Control | 0.128 ± 0.005 | 0.237 ± 0.013 | 42.0 ± 4.4 | 576.0 ± 67.8* |
| Euthyroid (9) | Iso | 0.137 ± 0.006 | 0.430 ± 0.001 | 33.0 ± 1.6 | 123.0 ± 11.4 |
| Thyroid deficient (8) | Iso | 0.137 ± 0.008 | 0.455 ± 0.028 | 34.7 ± 3.0 | 232.0 ± 14.5* |
All values are expressed as means ±s.e.m., with number of myocytes given in parentheses. Iso, isoproterenol. The ratio of fura-2 fluorescence at 340 and 380 nm was used to measure changes in Ca2+ levels.
Significantly different from euthyroid (P < 0.05).
Figure 5. Myocyte shortening and Ca2+ transients recorded in single intact myocytes isolated from left ventricular myocardium predominantly expressing α-MHC.
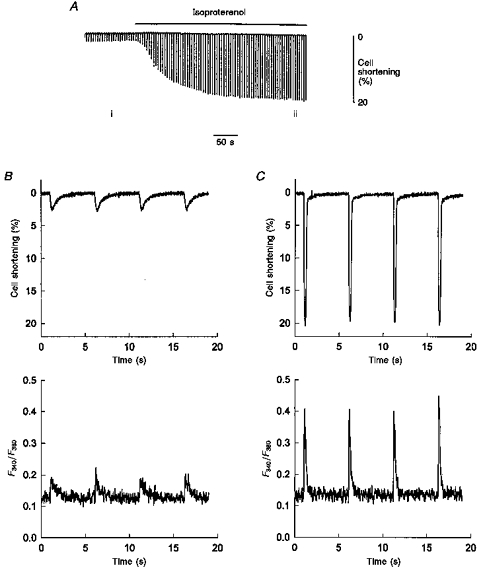
A, the extent of myocyte (length, 132 μm) shortening recorded on a slow time base during electrical stimulation in the absence and presence of 70 nm isoproterenol. B, simultaneous recording (fast time base) of myocyte shortening (top panel) and fura-2 fluorescence ratio (bottom panel) during four twitches from the area designated i in A. C, simultaneous recording (fast time base) of myocyte shortening (top panel) and fura-2 fluorescence ratio (bottom panel) of four twitches during isoproterenol administration, from the area designated ii in A.
Figure 6. Myocyte shortening and Ca2+ transients recorded in a single intact myocyte isolated from left ventricular myocardium expressing 100 %β-MHC.
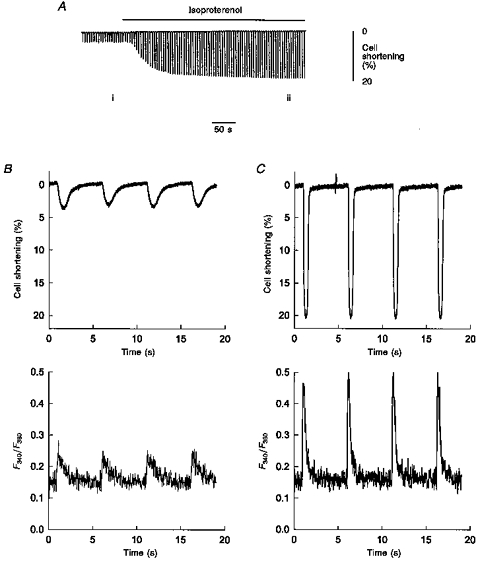
A, the extent of myocyte (length, 108 μm) shortening recorded on a slow time base during electrical stimulation in the absence and presence of 70 nm isoproterenol. B, simultaneous recording (fast time base) of myocyte shortening (top panel) and fura-2 fluorescence ratio (bottom panel) during four twitches from the area designated i in A. C, simultaneous recording (fast time base) of myocyte shortening (top panel) and fura-2 fluorescence ratio (bottom panel) of four twitches during isoproterenol administration, from the area designated ii in A.
DISCUSSION
The results of these experiments demonstrate that increased β-MHC expression (1) significantly depresses the rate of tension development and tension relaxation in skinned myocardium, and (2) markedly prolongs the time courses of myocyte shortening, myocyte relengthening and the decline in intracellullar [Ca2+] during twitch contractions of both control and β-adrenergic receptor-stimulated preparations.
Kinetics of cross-bridge interaction in skinned myocardium
Photolabile Ca2+ chelators, such as DM-nitrophen, nitr-5 and nitr-7, have commonly been used to assess the kinetics of cross-bridge interaction in striated muscle. For example, the Ca2+ dependence of the rate of tension development has been established in skinned preparations from frog skeletal muscle (Ashley et al. 1991; Lenart et al. 1993), rabbit psoas muscle (Araujo & Walker, 1994; Patel et al. 1996) and rat myocardium (Araujo & Walker, 1994). Similar results were obtained in the present study, as the rate of tension development in skinned myocardium varied with the level of Ca2+ activation regardless of MHC content (Fig. 3A and B). Although Ca2+ dependence of the rate of tension development was observed in all preparations, tension developed at significantly slower rates in myocardium expressing 100 %β-MHC than in myocardium expressing predominantly α-MHC (inset, Fig. 3A and B). The approximate 2.5-fold difference in the maximal rate of tension development observed in the present study (Table 3) is similar to the 3-fold greater ATPase activity of α-MHC versusβ-MHC (Pope et al. 1980; Harris et al. 1994).
Diazo-2, a photolabile Ca2+ chelator that rapidly buffers Ca2+ following flash photolysis, has been used to examine the rate of tension relaxation of skinned smooth (Khromov et al. 1995) and cardiac muscle (Zhang et al. 1995). In the present study, we assessed the rate of relaxation from a single level of steady-state force (∼0.5P0) and found that the half-time of tension relaxation was 2-fold slower in myocardium expressing 100 %β-MHC than in myocardium expressing predominantly α-MHC. In fact, the half-time of tension relaxation determined for rat myocardium expressing 100 %β-MHC in the present study (139 ± 6 ms) is similar to that determined in porcine myocardium expressing 100 %β-MHC (110 ± 10 ms; Zhang et al. 1995). Tension relaxation is a complex process governed by a number of interrelated factors, including the rate of cross-bridge detachment, reattachment of previously detached cross-bridges and Ca2+ dissociation from TnC. Of these, the intrinsic rate of cross-bridge detachment most probably dominates the rate of relaxation in rat myocardium, for several reasons. First, the rate of Ca2+ dissociation from TnC can be discounted, since the off-rate of Ca2+ from either skeletal or cardiac TnC (i.e. koff) is approximately 350 s−1 (Dong et al. 1996), which is significantly faster than the relaxation rates measured in the current study. Second, the expression of the cardiac-specific TnC isoform is unaffected by thyroid deficiency and yet the relaxation rates of skinned myocardium from euthyroid and thyroid deficient rats differed significantly. Third, since there is an almost instantaneous decrease in intracellular [Ca2+] following flash photolysis of diazo-2 (Adams et al. 1989) and the rate of attachment of myosin cross-bridges is comparatively slow, there is low probability that previously detached cross-bridges will reattach within the time required for relaxation. Therefore, it is apparent that the rate of tension relaxation in rat myocardium is primarily governed by the rate of cross-bridge detachment, an intrinsic property of the myosin heavy chain molecule. The fact that the rate of relaxation was slowed further by increased β-MHC expression (Table 3) is consistent with this idea.
Twitch characteristics and Ca2+ handling in intact ventricular myocytes
In addition to its impact on ventricular MHC expression, thyroid hormone is thought to modulate the Ca2+ handling properties of ventricular myocardium by regulating the expression of a number of key components including L-type Ca2+ channels, β-adrenergic receptors, SR Ca2+-ATPase and phospholamban (Kim et al. 1987; Beekman et al. 1988; Kiss et al. 1994). Thyroid deficiency markedly decreases the rate of SR Ca2+ uptake by increasing the expression of phospholamban while reducing the expression of the SR Ca2+-ATPase pump (Kiss et al. 1994). Therefore, since thyroid hormone influences the kinetics of Ca2+ handling by the SR and also affects the relative distribution of ventricular MHC isoforms, it was of interest to assess the relative contribution of myofibrillar and SR changes to myocardial contraction.
Modifying the relative distribution of ventricular MHC isoforms by altering thyroid status did not significantly alter the extent of myocyte shortening either in the absence or in the presence of 70 nm isoproterenol (Table 4). However, the time course of the twitch was significantly affected by MHC expression. Myocytes expressing 100 %β-MHC had markedly prolonged half-times of myocyte shortening and relengthening relative to myocytes predominantly expressing α-MHC. These data from intact myocytes are in excellent agreement with the slowing of the rates of tension development and tension relaxation observed in skinned myocardium expressing 100 %β-MHC (Table 3).
The prolonged time course of twitch contraction in living myocytes from thyroid deficient rats is most probably due to either a slowing in Ca2+ uptake by the SR, as a result of changes in the level of phospholamban expression (Kiss et al. 1994), or depressed kinetics of cross-bridge interaction due to increased expression of β-MHC, or both. In this regard, the amplitude of the Ca2+ signal during a twitch (peak fura-2 fluorescence ratio) and the levels of intracellular Ca2+ following a twitch (resting fura-2 fluorescence ratio) were unaffected by ventricular MHC distribution. However, the rate of Ca2+ uptake (inferred from the half-time for fura-2 fluorescence decay) was significantly slower in myocytes from thyroid deficient rats. In the absence of β-adrenergic stimulation, the prolonged decay of the fura-2 fluorescence signal most probably arises from the combined effects of increased phospholamban expression, which in its non-phosphorylated state would tend to inhibit the SR Ca2+-ATPase pump (Kiss et al. 1994), and increased β-MHC expression, which slows the rate of cross-bridge detachment (Fig. 4B) and may therefore decrease the rate of Ca2+ dissociation from TnC (Wang & Fuchs, 1994).
In a recent study, Ca2+ handling properties of cardiac myocytes were altered by constructing a transgenic murine model in which phospholamban was selectively overexpressed in cardiac muscle (Kadambi et al. 1996): the amplitude of the systolic Ca2+ transient was depressed and the decay of the fura-2 fluorescence signal was significantly prolonged in the transgenic compared with the wild-type myocytes under control conditions. However, during β-adrenergic stimulation, both the amplitude of the systolic Ca2+ transient and the rate of decline of fura-2 fluorescence were equivalent between transgenic and wild-type myocytes, suggesting that the inhibitory effects of phospholamban overexpression could be overcome by phosphorylation of phospholamban. Likewise, the maximal rates of left ventricular relaxation (-dP/dt) of isoproterenol-stimulated hypothyroid and euthyroid hearts were comparable (Kiss et al. 1994).
In contrast to these earlier observations, in the present study β-adrenergic stimulation did not restore a normal time course for fura-2 fluorescence decay in myocytes expressing 100 %β-MHC, despite using the protocol for β-adrenergic stimulation that previously was effective in overcoming the inhibitory effects of phospholamban overexpression (Kadambi et al. 1996). The half-time for the decay of fura-2 fluorescence was reduced but was still markedly longer in myocytes from thyroid deficient rats, which most probably is a consequence of the reduced SR Ca2+-ATPase content. Another factor that may contribute to the prolonged Ca2+ transient in myocytes from thyroid deficient rats is an increase in Ca2+-binding affinity of cardiac TnC, which could slow the rate of Ca2+ dissociation and subsequent Ca2+ re-uptake by the SR. It is well established that an increased number of strongly bound cross-bridges enhances the Ca2+-binding affinity of cardiac TnC (Wang & Fuchs, 1994). Since the rate of relaxation of myocardium expressing 100 %β-MHC is significantly slower than that expressing ∼80 %α-MHC (Fig. 4), it is reasonable to suppose that the β-MHC cross-bridges remain attached to the thin filament for an extended period of time, perhaps due to a longer duty cycle (VanBuren et al. 1995). Thus, in contrast to α-MHC, β-MHC once strongly bound to the thin filament undergoes a slower transition from the force-generating to the non-force-generating state, thereby slowing the apparent rate of dissociation of Ca2+ from TnC. This phenomenon would manifest as a prolonged half-time in the decay of the fura-2 fluorescence signal in living myocardium.
Acknowledgments
This study was supported by grants HL-54581 from the National Institutes of Health (R. L. M.) and 97-GB-90 from the American Heart Association, Wisconsin Affiliate (D. P. F.). The authors would also like to thank Dr James Graham for SDS-PAGE analysis of the myocardial preparations.
References
- Adams SR, Kao JPY, Tsien RY. Biologically useful chelators that take up Ca2+ upon illumination. Journal of the American Chemical Society. 1989;111:7957–7968. [Google Scholar]
- Akella AB, Ding X-L, Cheng R, Gulati J. Diminished Ca2+ sensitivity of skinned cardiac muscle contractility coincident with troponin T-band shifts in the diabetic rat. Circulation Research. 1997;76:600–606. doi: 10.1161/01.res.76.4.600. [DOI] [PubMed] [Google Scholar]
- Araujo A, Walker JW. Kinetics of tension development in skinned cardiac myocytes measured by photorelease. American Journal of Physiology. 1994;267:H1643–1653. doi: 10.1152/ajpheart.1994.267.5.H1643. [DOI] [PubMed] [Google Scholar]
- Ashley CC, Mulligan IP, Lea TJ. Ca2+ and activation mechanisms in skeletal muscle. Quarterly Reviews of Biophysics. 1991;24:1–73. doi: 10.1017/s0033583500003267. [DOI] [PubMed] [Google Scholar]
- Averyhart-Fullard V, Fraker LD, Murphy AM, Solaro RJ. Differential regulation of slow-skeletal and cardiac troponin I mRNA during development and by thyroid hormone in rat heart. Journal of Molecular and Cellular Cardiology. 1994;26:609–616. doi: 10.1006/jmcc.1994.1073. 10.1006/jmcc.1994.1073. [DOI] [PubMed] [Google Scholar]
- Beekman RE, Hardeveld C, Simonides WS. Effect of thyroid state on cytosolic free calcium in resting and electrically stimulated cardiac myocytes. Biochimica et Biophysica Acta. 1988;969:18–27. doi: 10.1016/0167-4889(88)90083-3. [DOI] [PubMed] [Google Scholar]
- Dong WJ, Rosenfeld SS, Wang CK, Gordon AM, Cheung HC. Kinetic studies of calcium binding to the regulatory site of troponin C from cardiac muscle. Journal of Biological Chemistry. 1996;271:688–694. doi: 10.1074/jbc.271.2.688. 10.1074/jbc.271.2.688. [DOI] [PubMed] [Google Scholar]
- Edman KAP. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. The Journal of Physiology. 1979;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free and free from specified total ionic concentrations in aqueous solutions containing multiple metals or ligands. Methods in Enzymology. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fitzsimons DP, Bodell PW, Herrick RE, Baldwin KM. Effect of thyroid state on cardiac myosin P-light chain phosphorylation during exercise. Journal of Applied Physiology. 1990;69:313–320. doi: 10.1152/jappl.1990.69.1.313. [DOI] [PubMed] [Google Scholar]
- Geeves MA, Lehrer SS. Dynamics of the muscle thin filament regulatory switch: The size of the cooperative unit. Biophysical Journal. 1994;67:273–282. doi: 10.1016/S0006-3495(94)80478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned musle fibers of the frog. Journal of General Physiology. 1982;80:279–297. doi: 10.1085/jgp.80.2.279. 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F, Masatsugu M, Bodell PW, Qin A, McCue SA, Baldwin KM. Role of thyroid hormone and insulin in control of cardiac isomyosin expression. Journal of Molecular and Cellular Cardiology. 1997;29:559–569. doi: 10.1006/jmcc.1996.0299. 10.1006/jmcc.1996.0299. [DOI] [PubMed] [Google Scholar]
- Harris DE, Work SS, Wright RK, Alpert NA, Warshaw DM. Smooth, cardiac and skeletal muscle myosin force and motion generation assessed by cross-bridge mechanical interactions in vitro. Journal of Muscle Research and Cell Motility. 1994;15:11–19. doi: 10.1007/BF00123828. [DOI] [PubMed] [Google Scholar]
- Hofmann PA, Menon V, Gannaway KF. Effects of diabetes on isometric tension as a function of [Ca2+] and pH in rat skinned cardiac myocytes. American Journal of Physiology. 1995;269:H1656–1663. doi: 10.1152/ajpheart.1995.269.5.H1656. [DOI] [PubMed] [Google Scholar]
- Huxley AF. Muscle structure and theories of contraction. Progress in Biophysics and Biophysical Chemistry. 1957;7:255–318. [PubMed] [Google Scholar]
- Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, Walsh RA, Kranias EG. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. Journal of Clinical Investigation. 1996;97:533–539. doi: 10.1172/JCI118446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JH, Ellis-Davies CR. Photolabile chelators for the rapid photorelease of divalent cations. Proceedings of the National Academy of Sciences of the USA. 1988;85:6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khromov A, Somlyo AV, Trentham DR, Zimmermann B, Somlyo AP. The role of MgADP in force maintenance by dephosphorylated cross-bridges in smooth muscle: a flash photolysis study. Biophysical Journal. 1995;69:2611–2622. doi: 10.1016/S0006-3495(95)80132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Smith TW, Marsh JD. Effect of thyroid hormone on slow calcium channel function in cultured chick ventricular cells. Journal of Clinical Investigation. 1987;80:88–94. doi: 10.1172/JCI113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss E, Jakab G, Kranias EG, Edes I. Thyroid hormone-induced alterations in phospholamban protein expression. Circulation Research. 1994;75:245–251. doi: 10.1161/01.res.75.2.245. [DOI] [PubMed] [Google Scholar]
- Lenart TD, Allen TS, Barsotti RJ, Ellis-Davies GCR, Kaplan JH, Franzini-Armstrong C, Goldman YE. Mechanics and structure of cross-bridges during contractions initiated by photolysis of caged Ca2+ In: Sugi H, Pollack GH, editors. Mechanism of Myofilament Sliding in Muscle Contraction. New York: Plenum Press; 1993. [DOI] [PubMed] [Google Scholar]
- Lompre AM, Nadal-Ginard B, Mahdavi V. Expression of the cardiac alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. Journal of Biological Chemistry. 1984;259:6437–6446. [PubMed] [Google Scholar]
- Metzger JM, Moss RL. Calcium-sensitive cross-bridge transitions in mammalian fast and slow skeletal muscle fibers. Science. 1990;247:1088–1090. doi: 10.1126/science.2309121. [DOI] [PubMed] [Google Scholar]
- Morkin E. Regulation of myosin heavy chain genes in the heart. Circulation. 1993;87:1451–1460. doi: 10.1161/01.cir.87.5.1451. [DOI] [PubMed] [Google Scholar]
- Moss RL. Ca2+ regulation of mechanical properties of striated muscle. Circulation Research. 1992;70:865–884. doi: 10.1161/01.res.70.5.865. [DOI] [PubMed] [Google Scholar]
- Nasser R, Malouf NN, Kelly MB, Oakeley AE, Anderson PAW. Force-pCa relation and troponin T isoforms of rabbit myocardium. Circulation Research. 1991;69:1470–1475. doi: 10.1161/01.res.69.6.1470. [DOI] [PubMed] [Google Scholar]
- Pagani ED, Julian FJ. Rabbit papillary muscle myosin isozymes and the velocity of muscle shortening. Circulation Research. 1984;54:586–594. doi: 10.1161/01.res.54.5.586. [DOI] [PubMed] [Google Scholar]
- Patel JR, Diffee GM, Moss RL. Myosin regulatory light chain modulates the Ca2+ dependence of the kinetics of tension development in skeletal muscle fibers. Biophysical Journal. 1996;70:2333–2340. doi: 10.1016/S0006-3495(96)79799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JR, McDonald KS, Wolff MR, Moss RL. Ca2+ binding to troponin C in skinned skeletal muscle fibers assessed with caged Ca2+ and a Ca2+ fluorophore. Journal of Biological Chemistry. 1997;272:6018–6027. doi: 10.1074/jbc.272.9.6018. 10.1074/jbc.272.9.6018. [DOI] [PubMed] [Google Scholar]
- Pope B, Hoh JFY, Weeds A. The ATPase activities of rat cardiac myosin isoenzymes. FEBS Letters. 1980;118:205–208. doi: 10.1016/0014-5793(80)80219-5. 10.1016/0014-5793(80)80219-5. [DOI] [PubMed] [Google Scholar]
- Reiser PJ, Greaser ML, Moss RL. Troponin T isoforms are associated with functional heterogeneity among single fibres from neonatal and adult avian skeletal muscles. The Journal of Physiology. 1992;449:573–588. doi: 10.1113/jphysiol.1992.sp019102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiological Reviews. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Strang KT, Sweitzer NK, Greaser ML, Moss RL. Beta-adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats. Circulation Research. 1994;74:542–549. doi: 10.1161/01.res.74.3.542. [DOI] [PubMed] [Google Scholar]
- Swartz DR, Moss RL. Influence of a strong-binding myosin analogue on calcium-sensitive mechanical properties of skinned skeletal muscle fibres. Journal of Biological Chemistry. 1992;267:20497–20506. [PubMed] [Google Scholar]
- Swartz DR, Moss RL, Greaser ML. Calcium alone does not fully activate the thin filament for S1 binding to rigor myofibrils. Biophysical Journal. 1996;71:1891–1904. doi: 10.1016/S0006-3495(96)79388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer NK, Moss RL. Determinants of loaded shortening velocity in single cardiac myocytes permeabilized with alpha-hemolysin. Circulation Research. 1993;73:1150–1162. doi: 10.1161/01.res.73.6.1150. [DOI] [PubMed] [Google Scholar]
- VanBuren P, Harris DE, Alpert NA, Warshaw DM. Cardiac V1 and V3 myosins differ in their hydrolytic and mechanical activities in vitro. Circulation Research. 1995;77:439–444. doi: 10.1161/01.res.77.2.439. [DOI] [PubMed] [Google Scholar]
- Wang Y-P, Fuchs F. Length, force, and Ca2+-troponin C affinity in cardiac and slow skeletal muscle. American Journal of Physiology. 1994;266:C1077–1082. doi: 10.1152/ajpcell.1994.266.4.C1077. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 2. Englewood Cliffs, NJ, USA: Prentice Hall; 1984. [Google Scholar]
- Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circulation Research. 1995;76:1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]


