Abstract
Properties, kinetics and functions of large conductance calcium-activated K+ channels (BKCa) were investigated by the patch-clamp technique in small neurones (Aδ- and C-type) of a dorsal root ganglion (DRG) thin slice preparation without enzymatic treatment.
Unitary conductance of BKCa channels measured in symmetrical high K+ solutions (155 mm) was 200 pS for inward currents, and chord conductance in control solution was 72 pS. Potentials of half-maximum activation (V1/2) of the channels were linearly shifted by 43 mV per log10[Ca2+]i unit (pCa) in the range of −28 mV (pCa 4) to +100 mV (pCa 7). Open probabilities increased e-times per 15–32 mV depolarization of potential.
In mean open probability, fast changes with time were mainly observed at pCa > 6 and at potentials > +20 mV, without obvious changes in the experimental conditions.
BKCa channels were half-maximally blocked by 0.4 mm TEA, measured by apparent amplitude reductions. They were completely blocked by 100 nm charybdotoxin and 50 nm iberiotoxin by reduction of open probability.
Two subtypes of small DRG neurones could be distinguished by the presence (type I) or absence (type II) of BKCa channels. In addition, less than 10 % of small neurones showed fast (∼135 V s−1) and short (∼0.8 ms) action potentials (AP).
The main functions of BKCa channels were found to be shortening of AP duration, increasing of the speed of repolarization and contribution to the fast after-hyperpolarization. As a consequence, BKCa channels may reduce the amount of calcium entering a neurone during an AP.
BKCa channel currents suppressed a subsequent AP and prolonged the refractory period, which might lead to a reduced repetitive activity. We suggest that the BKCa current is a possible mechanism of the reported conduction failure during repetitive stimulation in DRG neurones.
In studies on K+ currents in dorsal root ganglion (DRG) neurones the repolarization phase of the action potential (AP) was usually found to be modulated by voltage-dependent K+ currents (Kostyuk et al. 1981; Safronov et al. 1996; Gold et al. 1996a). A calcium-activated K+ current was described as contributing to the slow after-hyperpolarization (AHP) but not to the AP in some types of sensory neurone (Higashi et al. 1984; Gold et al. 1996b). This conductance was found in whole-cell experiments to be sensitive to apamin and therefore the underlying conductance was suggested to be the small conductance calcium-activated K+ channel (e.g. Gold et al. 1996b). Earlier it was concluded that calcium currents and especially a fast calcium-dependent K+ current were responsible for the conduction failure during high-frequency stimulation in DRG neurones (Lüscher et al. 1994a, b). Therefore we searched for the large conductance calcium-activated K+ channels (BKCa) in DRG neurones, and investigated their contribution to an AP. Unfortunately, no information for single-channel recordings from DRG neurones was available for calcium-activated K+ channels and most of the whole-cell recordings were done in sympathetic neurones (Davies et al. 1996), which differ in function and structure. Up until now, it has been unclear whether the data for conductances from sympathetic neurones are similar to those in DRG neurones. Since differences in gating kinetics were reported in different preparations, we investigated the single-channel properties of the BKCa channel. Nevertheless, only one clone of cDNA has been found for BKCa channels.
In the present study we investigated small DRG neurones under more physiological conditions, with an enzyme-free method, the recently described thin slice preparation (Safronov et al. 1996). Small DRG neurones have been attributed mostly to Aδ- and C-type innervating nociceptors (Harper & Lawson, 1985a). We used the patch-clamp technique to investigate the single-channel properties as well as the whole-cell currents and APs. In addition, the specific blockers charybdotoxin (CTX) and iberiotoxin (IbTX) were applied to calcium-free control solution.
In addition to our finding of two subtypes of small DRG neurones with and without BKCa channels, the contribution of the channels to APs is demonstrated. Thus the observed shortening of AP duration has led to the suggestion that on the one hand the BKCa channels are responsible for reduction of the amount of calcium entering the neurone during an AP, and on the other hand they might be the conductance that is responsible for conduction failure during repetitive activity in DRG neurones.
Part of the work has been presented in abstract form (Gruß et al. 1997; Scholz et al. 1997b).
METHODS
Preparation
Experiments were performed on neurones by means of the patch-clamp technique (Hamill et al. 1981; Edwards et al. 1989) on thin slices (150 μm) prepared from DRG of 3- to 9-day-old rats. The slices were made in ice-cold control solution and kept as described previously (Takahashi, 1990; Safronov et al. 1996). Briefly, after the rats had been decapitated (according to German guidelines), two DRGs (Th10-L4) were taken from the opened spinal column and embedded in 2 % (w/v) agar, which was dissolved in control solution. Two small blocks of agar containing the DRG were glued (Cyanacrylat) to a glass slip and mounted in a slicer (FTB Vibracut 1.4). The slices were incubated for 30 min in 37°C warm control solution and stored at room temperature for up to 12 h. The control solution was permanently bubbled with a gas mixture of 5 % CO2 and 95 % O2. In contrast to cleaning procedures described for brain and spinal cord slices (Edwards et al. 1989; Takahashi, 1990) it was not possible to clean neurones of DRG (Safronov et al. 1996). Therefore, the present study was limited to neurones located near the surface of the slice.
To obtain a distribution of neuronal cell sizes, ganglia were fixed in 4 % (v/v) paraformaldehyde in 0.1 m phosphate buffer for 4–12 h and serial 5 μm sections were cut. Cell diameters were measured using an Olympus microscope (BX 60) at a magnification of × 100 connected to an AT compatible computer with frame grabber card. Size analysis was performed on computer screen using the program Scion Image (Scion Corp., Frederick, MD, USA). Only cell sections showing nuclei were measured and from this data frequency distribution histograms were produced (Harper & Lawson, 1985a).
Solutions
All values of concentrations are given in millimolar units.
External solutions
Control solution contained 115 NaCl, 5.6 KCl, 1 MgCl2, 11 glucose, 1 NaH2PO4, 25 NaHCO3 and 2.2 CaCl2, adjusted to pH 7.4 by bubbling with a 5 % CO2 and 95 % O2 gas mixture. Calcium-free control solution (0 Ca2+) was the same as control solution, but with 5 MgCl2 and 0 CaCl2. Control-Hepes solution contained 136.4 NaCl, 5.6 KCl, 1 MgCl2, 11 glucose, 2.2 CaCl2 and 10 Hepes, adjusted to pH 7.4 by NaOH; final Na+ 141. TEA (tetraethylammonium)-containing solutions were prepared by adding TEACl (Merck) from a 1 mol l−1 stock solution to control-Hepes. High- solution contained 5 NaCl, 152.5 KCl, 2.2 CaCl2, 1 MgCl2, 0.0002 TTX (tetrodotoxin, Latoxan) and 5 Hepes, adjusted to pH 7.4 with KOH; final K+ 155.
Internal solutions
High- solution contained 5 NaCl, 144.4 KCl, 1 MgCl2, 3 EGTA and 10 Hepes, adjusted to pH 7.3 with KOH; final K+ 155. High- BAPTA solution contained 104.5 KCl, 1 MgCl2, 10 BAPTA and 10 Hepes, adjusted to pH 7.3 with KOH; final K+ 155.
High- solutions with varying Ca2+ concentrations
High-, pCa 4 and pCa 5 solutions contained 0 EGTA, with addition of 0.1 or 0.01 CaCl2. High-, pCa 6 and pCa 7 solutions contained 0.08 EGTA and 0.0744 or 0.045 CaCl2, respectively, which were prepared and calculated according to methods described previously (Martell & Smith, 1975; Barrett et al. 1982).
To prevent non-specific binding of the peptide toxins, 0.05 % (w/v) bovine serum albumin (BSA) was added to control and to test solutions containing charybdotoxin (CTX; Latoxan) or iberiotoxin (IbTX; Latoxan), which were diluted from stock solutions (50 μmol l−1). Inside-out patches were investigated in a small side chamber after the recording pipette had been moved through the air from the main chamber in order to avoid damaging the slice by long-lasting perfusion with high K+ concentrations (Safronov & Vogel, 1995). The advantage of the side chamber was its fast rate of solution exchange due to its small volume of 0.24 ml, and the fast rate of perfusion (1.6 ml min−1). Experimental solutions were introduced in the main and side chambers using a gravity-driven perfusion system and a 6-way sliding Teflon valve with a total dead volume of ∼0.15 ml. Chemicals were purchased from Sigma, except where specified otherwise.
Current and voltage recordings
Patch-clamp pipettes were made from borosilicate glass tubing (GC 150, Clark Electromedical Instruments, Pangbourne, UK) using a horizontal puller (Sutter) and were fire-polished to final resistances of 2.5–13.0 MΩ (whole cell) and 3.8–20.0 MΩ (single channel) measured after filling with internal solution. Pipettes used for single-channel recordings were mostly coated with Sylgard 184 (Dow Chemical). Recordings were obtained using an Axopatch-200A (Axon Instruments) patch-clamp amplifier and data were digitized with a 12-bit AD/DA converter Digidata 1200A (Axon Instruments) after filtering at least at two times lower frequency than sample frequency by a 10-pole low-pass Bessel filter. Voltage and current steps and data acquisition were controlled online by a PC/AT computer with pCLAMP 6 software (Axon Instruments). Continuous single-channel recordings were directly stored at 48 kHz on a DAT recorder (DTR 1204, Biologic) and were replayed through a 10-pole low-pass Bessel filter to the computer. Whole-cell recordings were performed as described by Hamill et al. (1981) and in most experiments additional series resistance compensation (55–80 %) was applied. Whole-cell and single-channel recordings were limited to small and some medium-sized neurones, 15–28 μm in mean diameter, whereas large neurones in our preparation exceeded 40 μm in diameter (Safronov et al. 1996). Currents evoked by voltage jumps were digitally corrected for leakage and transients using averaged episodes of recordings that were evoked by hyperpolarizing impulses.
V1/2 was obtained by fitting Popen-V curves to a Boltzmann distribution:
| (1) |
where G is the conductance, V is the applied voltage, z is the effective valency determining the slope factor, and F, R and T have their usual thermodynamic meanings.
Voltages are expressed as true transmembrane potentials (V), except for the cell-attached patches from neurones exposed to control solution where voltages are expressed as relative transmembrane potentials, uncorrected for the cell Vrest. In the figures, resting potentials of the neurones are given in front of the current-clamp recordings and are indicated by a dotted line.
Data of the concentration-effect curve were fitted by a Hill function:
| (2) |
where Imax is the maximum current, IC50 is the half-maximum inhibitory concentration, c is the concentration and nH is the Hill factor.
After the continuous current recordings were visually inspected, durations of the open and closed intervals were measured with half-amplitude threshold analysis, as described by McManus & Magleby (1989).
Evaluations, fits and statistical analysis were carried out with the programs pCLAMP 6 (Axon Instruments), Origin 4.1 (Microcal Software) and Excel 7 (Microsoft). Data are expressed as means ± standard error of the mean (s.e.m.) unless stated otherwise, and statistical significance was tested using Student's t test. Experiments were performed at room temperature (22–25°C).
RESULTS
More than half of all excised patches contained no BKCa channels, even when tested with 2.2 mm Ca2+ at the cytoplasmic membrane side. Currents of single BKCa channels were recorded from seventy-eight inside-out and outside-out patches excised from small neurones of DRG with a mean overall diameter of 21.2 ± 0.4 μm. These patches contained up to six BKCa channels, the mean resistance of pipette was 10.9 ± 0.4 MΩ and on average we observed 1.9 ± 0.2 channels per patch.
Properties of single BKCa channels
The selectivity of single BKCa channels for K+ ions and their conductance are shown in Fig. 1. The reversal potential of BKCa channel shifted from −86 mV in control solution, estimated from a fit of slope in the potential range from −80 to −50 mV, to 0 mV in symmetrical high-K+ solutions (155 mm; Fig. 1B), indicating a strong selectivity for K+ ions. Transitions from the open state to sub-conductance levels were observed more often in symmetrical high-K+ than in control solution. These BKCa channels also revealed a typical dependence of the open probability on voltage (Fig. 1A).
Figure 1. Single-channel recordings and conductance of BKCa channels of small DRG neurones.
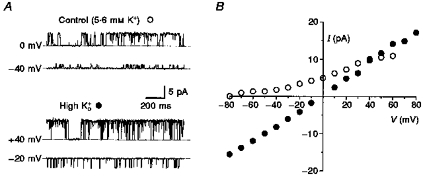
A, recordings of a single BKCa channel in an outside-out patch in control solution, and from the same channel in symmetrical high-K+ solutions at the potentials given. Dotted lines indicate the closed level; filter frequency, 1 kHz; pipette solution, high-, pCa 6; 23 °C. B, I–V relationship of single BKCa channels in inside-out and outside-out patches. Most amplitudes were measured from point-amplitude histograms at potentials V. A few amplitudes were measured directly from original recordings displayed on a computer screen using two cursors. ○, control solution; •, symmetrical high-K+ solutions. In control solution the chord conductance was 71.8 ± 3.0 pS, the mean slope conductance measured below −10 mV was 53.8 ± 5.3 pS and above +10 mV it was 106.9 ± 9.1 pS (4–19 measurements). In symmetrical high-K+ solutions, the mean conductance was 198.9 ± 1.5 pS for inward currents (n = 4–16), and for outward currents it was 222.7 ± 4.4 pS (n = 4–13). Error bars smaller than symbol size were not plotted.
Voltage dependence and sensitivity to internal Ca2+
The channel amplitudes at different potentials (V) were measured mainly from fits of a Gaussian function to point amplitude histograms and few measurements were made with two cursors on the computer screen. Their plot in the I–V relationship did not display a clear linear correlation in control solution. Although the chord conductance in control solution was 71.8 ± 3.0 pS, the slope conductance in the potential range from −80 to −10 mV was 53.8 ± 5.3 pS and found to be doubled between +10 and +60 mV to 106.9 ± 9.1 pS (11 patches). In contrast, in symmetrical high-K+ solutions there is a proper linear correlation for inward currents with a conductance of 198.9 ± 1.5 pS and for outward currents with a slightly greater conductance of 222.7 ± 4.4 pS (10 patches).
Another property of the BKCa channel, its dependence on internal Ca2+ ions, is shown in traces from an inside-out patch (Fig. 2A). Recordings (10 s duration) were analysed to obtain curves of open probability (Popen) vs. voltage at different internal Ca2+ concentrations (9 patches). From single-channel openings relative Popen values were calculated using the half-amplitude threshold analysis as described in Methods. The mean values were fitted with a Boltzmann distribution (eqn (1)) revealing voltages of half-maximum activation (V1/2) of −28, +17, +56 and +100 mV per log10[Ca2+]i unit (pCa) 4–7, respectively (Fig. 2B). The slope factors of the curves were 20, 30, 33 and 15 mV per e-fold change in Popen at pCa 4–7, respectively. This increasing open probability with increasing cytoplasmic [Ca2+] is quantitatively demonstrated in Fig. 2C, where a linear correlation of V1/2 values with cytoplasmic calcium concentration of 43 mV per log10[Ca2+]i is shown. Relative Popen values of four selected test potentials were plotted against [Ca2+]i in Fig. 2D. The cytoplasmic calcium concentrations, which were necessary to reach half-maximum open probability, were ∼0.7 μmol l−1 at +60 mV and ∼2 μmol l−1 at +40 mV.
Figure 2. Open probability is dependent on [Ca2+]i.

A, traces of a single BKCa channel at +40 mV at different calcium concentrations at the cytoplasmic membrane side recorded from an inside-out patch in the small side chamber. Dotted lines indicate closed level, filter frequency 1 kHz, symmetrical high-K+ solutions. B, voltage dependence of mean Popen values obtained at different calcium concentrations. Relative values were calculated with a half-amplitude threshold criterion from recordings of 10 s duration. Data points were fitted with a Boltzmann distribution (eqn (1)) giving the voltages for half-maximum activation (V1/2) of −28.4 ± 3.0 mV at pCa 4 (□, n = 6–56), +17.0 ± 7.1 mV at pCa 5 (○, n = 6–30), +56.0 ± 8.4 mV at pCa 6 (▵, n = 6–31) and +100.3 ± 1.4 mV at pCa 7 (▿, n = 8–18). Filter frequency, 2 kHz. C, V1/2 values as obtained in B fitted with a linear correlation of 42.8 ± 2.3 mV per log10[Ca2+]i. D, mean Popenversus[Ca2+]i at four potentials. E, example of fast mean Popen changes in a single BKCa channel at +80 mV in high- pCa 6 in an inside-out patch. Popen values are plotted for two consecutive seconds of channel activity. Without obvious changes of experimental conditions, Popen changed spontaneously between high and low values. Symmetrical high-K+ solutions, filter frequency 2 kHz.
The large variations in Popen values in Fig. 2B were due to a phenomenon that is shown by an example in Fig. 2E. The mean open probabilities showed fast changes (within 2 s) without any obvious modification of the experimental conditions. This was observed in 26/91 measurements, mostly at pCa > 6 and potentials > +20 mV.
Kinetics of the BKCa channel
To examine the activation kinetics of the BKCa channel, averages were calculated from single-channel events evoked by voltage jumps to +40 mV (Fig. 3). The time constants derived from exponential fits decreased with increasing cytoplasmic calcium concentration from 9.9 ± 1.2 ms at pCa 7 to 2.8 ± 0.3 ms at pCa 6 (n = 7). No inactivation kinetics were seen in the averaged current traces lasting 180 ms. From all traces (54) in each experiment, the open time was measured with a half-amplitude threshold method (as described in Methods) and normalized to the total time of all traces. Interestingly, this relative mean Popen as low as 0.03–0.08 in cell-attached recordings during 120 mV voltage jumps positive to the resting potential was reached within 2–3 ms, which can be seen in the averaged current traces (Fig. 3A).
Figure 3. Activation kinetics of a single BKCa channel.
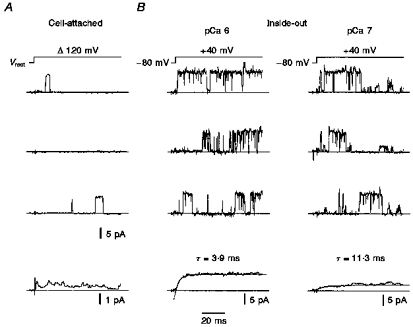
A, traces from a cell-attached patch with channel openings elicited by 120 mV depolarizing voltage jumps from resting potential Vrest. Fifty-four episodes are averaged and shown as the lowermost trace; note the different scale bars of amplitude. Pipette filled with high-, filter frequency 1 kHz. B, same patch in inside-out configuration at two concentrations of internal Ca2+. Recordings of the single BKCa channel activated by test impulses to +40 mV from a holding potential of −80 mV. Average traces fitted with a mono-exponential function (dotted line). Symmetrical high-K+ solutions, current traces corrected for transients and leakage, filter frequency 3 kHz.
Pharmacological blockade of single channels
TEA, known as a fast blocker of the BKCa channel, reversibly reduced the apparent single-channel amplitude within 10 s (Fig. 4A and B). Therefore the concentration- effect curve of TEA was built from data of the relative apparent single-channel reduction, measured from point-amplitude histograms. The fit of the data by eqn (2) revealed a half-maximum block at 0.40 ± 0.04 mm TEA (Fig. 4C). The slope factor of 0.98 ± 0.09 indicated a 1 : 1 stoichiometry of blocking molecule to channel protein.
Figure 4. Single BKCa channel blockade by TEA.

A, recording from an outside-out patch in control solution at 0 mV containing one channel. The corresponding point amplitude histogram is plotted below the recording. N, number of events. B, same patch in the presence of 0.5 mm TEA. The histogram showed an apparent reduction of amplitude by 53 %. The effects of all TEA concentrations tested were reversible. Pipette solution, high-, pCa 4; bath solution, control-Hepes; filter frequency, 2 kHz. C, data from measurements at 0 mV obtained as shown in B at different TEA concentrations. Fit of the data points by eqn (2) revealed a half-maximum inhibitory concentration (IC50) of 0.40 ± 0.04 mm, with a Hill factor of 0.98 ± 0.09 (n = 5–30).
The more selective blocker charybdotoxin (CTX), a polypeptide, completely blocked BKCa channels at 100 nmol l−1 in five patches containing 1–3 channels within 2 min (Fig. 5A). In the presence of CTX, the BKCa channel did not show a reduction of amplitude but the open probability of channels was reduced. The time necessary to achieve the former activity of the channels during washout of the CTX containing control solution was ≤ 15 min. The highly selective blocker iberiotoxin (IbTX), also a polypeptide, reduced the complete channel activity at 50 nmol l−1 in six patches (Fig. 5B). Again the open probability was reduced, but the channel amplitude was not, and the time necessary for washout of IbTX was ≤ 20 min.
Figure 5. Charybdotoxin and iberiotoxin blocked single BKCa channels.
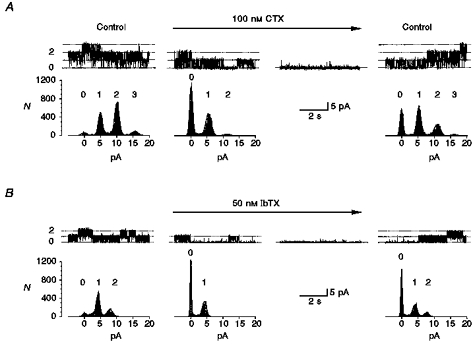
A, 100 nmol l−1 charybdotoxin (CTX; horizontal arrow) blocked three channels in an outside-out patch by reducing the open probability (second trace from left; 0 mV). Point-amplitude histograms are obtained as in Fig. 4. Amplitudes of the first current level in control, CTX and washout were 4.9, 5.1 and 5.1 pA, revealed by Gaussian fits to the histograms. Complete suppression was reached in ∼1.5 min, shown in the third trace from the left. Complete recovery was achieved after 10 min. Pipette solution, high-, pCa 6; bath solution, control-Hepes with 0.05 % BSA; filter frequency, 1 kHz. B, in another outside-out patch 50 nmol l−1 iberiotoxin (IbTX) blocked two channels completely and reversibly (0 mV). Amplitudes of the first current level in control, IbTX and washout were 4.8, 4.8 and 4.9 pA. Complete recovery was achieved after 20 min. Pipette solution, high-, pCa 4; bath solution, control-Hepes with 0.05 % BSA; filter frequency, 1 kHz. Dotted lines and numbers indicate the channel levels.
Application of the local anaesthetic lidocaine (lignocaine) did not show significant changes in channel activity in concentrations ≤ 300 μmol l−1 (n = 4). Ethanol, which is described as activating BKCa channels in neurohypophysial terminals (Dopico et al. 1996), was tested in concentrations ≤ 80 mm (∼4.6 vol 5), but no clear results were obtained (4 patches).
Two subtypes of small DRG neurone
During single-channel recording it was noticeable that some small neurones did not show any BKCa channel, even though we tried up to ten patches at the same neurone. In order to reveal whether a calcium-activated K+ current is activated by the calcium influx through voltage-dependent Ca2+ channels, we recorded whole-cell currents from 112 neurones in different modes in the presence or absence of external calcium. In voltage-clamp experiments, the total outward currents were larger in 2.2 mm Ca2+-containing control solution than in calcium-free control solution in 34/50 investigated neurones. A typical example including the difference current (Δ) is displayed in Fig. 6A. In this type of neurone, type I, the additional outward current (Iout) activated by invading calcium was detected at potentials positive to −40 mV (Fig. 6A; right) and was ≤ 2 nA at +30 mV. In contrast, another sixteen neurones showed a larger total outward current in calcium-free control solution than in 2.2 mm Ca2+-containing control solution (Fig. 6B). In these type II neurones the difference current was inwardly directed (Fig. 6B) and showed typical properties of known voltage-activated Ca2+ currents (Fox et al. 1987). For type I neurones the density of BKCa channels was calculated to be 0.1–0.2 channels μm−2 as a lower limit calculated from the difference current divided by the cell surface.
Figure 6. Two types of small DRG neurone.

A, single traces of whole-cell recordings in control solution (2.2 mm Ca2+; •) and in calcium-free control solution (○), where calcium ions were replaced by Mg2+ ions to give a final concentration of 5 mm, are shown superimposed. The difference between currents in 2.2 mm Ca2+ and calcium-free solution is plotted in the middle (Δ). Neurones showing an outward difference current are classified as type I. The I–V relationship of steady-state currents measured at the end of a test impulse (180 ms) is displayed on the right (washout: ▪; difference current: ▵). Pipette solution, high-, pCa 6; filter frequency, 2 kHz. B, another small DRG neurone showed an inward current (Δ) after analogous subtraction. Such neurones displaying an inward difference current are classified as type II. Pipette solution, high-, pCa 6; filter frequency, 2 kHz.
The two types of neurone not only differed in voltage-clamp recordings, but also in current-clamp recordings. Ninety-seven of 112 neurones were investigated in current-clamp mode without internal BAPTA and could be attributed to one of the three types described as follows.
Fifty-five of these ninety-seven neurones were tested in the presence of 2.2 mm Ca2+ or in the absence of calcium. In the calcium-free solution, 5 mm Mg2+ was substituted for the calcium ions to avoid surface charge effects (Hille, 1992), which also blocked Ca2+ channels (Hess et al. 1986).
In type I DRG neurones the mean AP duration was prolonged from 2.42 ± 0.10 ms in 2.2 mm Ca2+-containing control solution to 2.94 ± 0.14 ms (measured at 0 mV; P < 0.00005; n = 35) in calcium-free control solution (Fig. 7A, left). In these neurones the prolongation of AP duration at 0 mV in calcium-free control solution was accompanied by a 16 ± 11 % (s.d.; P < 0.001; n = 35) reduction of the maximum rate of repolarization from 42.3 ± 10.3 V s−1 (s.d.) compared with 36.6 ± 11.0 V s−1 (s.d.) in 2.2 mm Ca2+ (Fig. 7A, left). The maximum rate of depolarization of 53.7 ± 16.7 V s−1 (s.d.; n = 35) was not significantly changed, and the amplitude of AP was 3.8 ± 2.3 mV (s.d.) larger in calcium-free control solution with 105.4 ± 11.8 mV (s.d.; P < 0.01; n = 35). In contrast, the action potentials of type II DRG neurones showed a more prominent calcium shoulder that disappeared in calcium-free control solution. The mean AP duration at 0 mV in type II DRG neurones was reduced by 0.47 ± 0.19 ms (s.d.; P < 0.015; n = 13) from 3.0 ± 0.73 (s.d.) to 2.53 ± 0.64 ms (s.d.) in calcium-free control solution (Fig. 7A, right). In these neurones the maximum rates of re- and depolarization as well as the AP amplitudes were not influenced significantly by changes of external calcium concentration (Table 1).
Figure 7. Action potentials of two types of small DRG neurone.
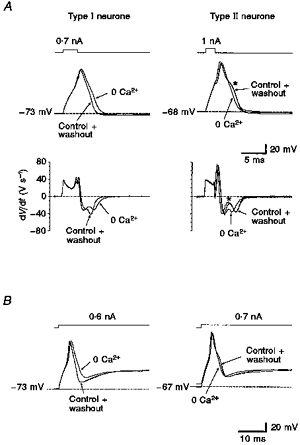
A, action potentials of a type I neurone evoked by short current injections (left, 3 ms) were prolonged reversibly by 0.6 ms (measured at half-amplitude) in calcium-free control solution (0 Ca2+) compared with action potentials in control solution (2.2 mm Ca2+). Action potentials of a type II neurone (right; stimulus duration, 2 ms) were shortened reversibly by 0.45 ms and their typical hump during repolarization (asterisk) disappeared. In the first derivative of the recordings (dV/dt) the maximum velocity of repolarization in the type I neurone was reduced from 45 to 35 V s−1 in calcium-free solution, whereas in the type II neurone it remained unchanged. B, same neurones as in A stimulated by long current injections (80 ms). Amplitude of fast after-hyperpolarization (AHP) of the type I neurone was reversibly reduced by 10 mV in calcium-free solution, whereas in the type II neurone it remained unchanged. All traces (control, 0 Ca2+, washout) are superimposed. Pipette solution, high-, 0 Ca2+; filter frequency, 3 kHz; sample frequency, 20 kHz.
Table 1.
Comparison of small DRG neurones
| Neurone type | APD control (ms) | AP in 0 Ca2+ (ms) | Max.repol control (V s−1) | Max.repol in 0 Ca2+ | Max.depol control (V s−1) | APD in CTX | APD in ibTX | lout in 0 Ca2+ | BKCa density (nμ−2) | Er (mV) | Mean size (μm) | Proportion of neurones |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | 2.42 ± 0.10 | 2.94 ± 0.14* | −42.3 ± 10.3 | (−)* | 53.7 ± 16.7 | (+)† | (+)† | (−) | 0.1–0.2 | −69.3 ± 3.5 | 22.2 ± 4.2 | 63% |
| (n = 35) | (n = 35) | (n = 35) | (n = 35) | (n = 61) | (n = 56) | |||||||
| II | 3.0 ± 0.7 | 2.53 ± 0.64 † | −41.9 ± 14.3 | (0) | 62.9 ± 29.2 | (0) | (0) | (+) | Not | −67.9 ± 3.8 | 20.7 ± 4.3 | 30% |
| (n = 13) | (n = 13) | (n = 13) | (n = 13) | found | (n = 31) | (n = 29) | ||||||
| Fast | 0.81 ± 0.30 | 0.51 ± 0.17 | −107 ± 11 | (0) | 137 ± 23 | (0) | (0) | (+) | Not | −64.6 ± 2.6 | 23.1 ± 2.6 | 7% |
| (n = 7) | (n = 7) | (n = 7) | (n = 7) | found | (n = 7) | (n = 7) |
Values are given as means ±s.d. AP duration (APD) was measured at 0 mV. (−), reduction (0), no changes, (+), prolongation of AP duration, of maximum speed of repolarization (Max. repol.) or steady-state outward current (Iout, measured as in Fig. 6) compared with measurements in control solution.
P < 0.001
P < 0.05 compared with measurements in control solution.
During longer-lasting current injections, the modulation of the fast after-hyperpolarization (AHP) by BKCa channels became evident as another function. In type I neurones the fast AHP amplitude was up to 10 mV more negative in 2.2 mm Ca2+-containing control solution than in calcium-free control solution, whereas fast AHP amplitudes in type II neurones did not show a difference (Fig. 7B).
Of ninety-seven neurones, sixty-one could be distinguished as type I neurones with a mean diameter of 22.2 ± 4.2 μm (s.d.; n = 56 measurements; 3–9 days old) with a mean resting potential Vrest of −69.3 ± 3.5 mV (s.d.; n = 61 measurements). Twenty-nine of ninety-seven neurones were classified as type II neurones without significant differences in mean diameter of 20.7 ± 4.3 μm (s.d.; n = 29; 3–9 days old) compared with type I. Also, no significant difference in mean resting potentials has been observed in type II with Vrest of −67.9 ± 3.8 mV (s.d.; n = 26 measurements). When tested in voltage-clamp experiments, type I (n = 6) and type II (n = 3) DRG neurones expressed more than 90 % of total Na+ current as TTX-resistant current, and no significant differences between the two types could be detected. Under light microscopy without staining, the majority of both types of small DRG neurones were dark in colour and less than 5 % were light in colour. The findings regarding type I and type II neurones are summarized in Table 1.
The frequency distribution of soma diameter was obtained from fixed DRG to compare the cell sizes of DRG neurones at this age with results of previous investigations, mainly obtained from adult rats (Harper & Lawson, 1985a). The resulting size distribution could be fitted with a double Gaussian function with peaks at 21.5 and 32.2 μm (Fig. 9C). Staining of these neurones with an antibody against neurofilament revealed that the majority of the larger neurones were labelled, whereas the majority of small neurones were not labelled.
Figure 9. Slow and fast APs of small DRG neurones.
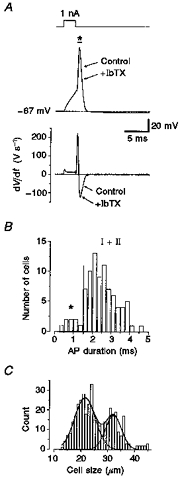
A, small DRG neurone displaying a fast AP with a duration shorter than 1 ms (measured at 0 mV) and high maximum velocity of depolarization (210 V s−1) and repolarization (125 V s−1). IbTX (50 nmol l−1) did not affect the shape of the AP in this neurone, as shown superimposed. Pipette solution, high-, 0 Ca2+; bath solution, control-Hepes, 0.05 % BSA. B, in the histogram of AP durations, fast type neurones (marked by an asterisk) displayed a mean AP duration of 0.81 ± 0.11 ms (n = 7). Mean AP duration of type I and II neurones (all data right of the dashed line) was longer, at 2.56 ± 0.08 ms (n = 79). C, frequency distribution of soma diameter for fixed neurones (n = 298) in 5 μm sections from DRG of three different rats (6 days old). The peaks of a double Gaussian fit were at 21.5 and 32.2 μm.
Pharmacological evidence for the function of BKCa channels
Since the I–V relationship of total outward current in voltage-clamp recordings indicated that the calcium-dependent component was voltage dependent, we also investigated by means of the selective blockers CTX and IbTX whether the effects described in the preceding section could be attributed to BKCa channels. Indeed, in type I neurones the mean AP duration was prolonged by 0.4 ms (n = 13) after application of 100 nmol l−1 CTX and by 0.35 ms after application of 50 nmol l−1 IbTX (n = 7; Fig. 8A). The maximum rate of repolarization in these type I neurones was reduced by 21 % (n = 9) by CTX and in most of the neurones the maximum rate of depolarization was slightly increased. Also, the amplitude of the fast AHP was diminished by CTX and IbTX when tested with long-lasting current injections.
Figure 8. CTX and IbTX affect action potentials and whole-cell currents.

A, in a type I neurone 100 nmol l−1 CTX prolonged the AP duration by 0.3 ms (measured at half-amplitude; left). In another type I neurone 50 nmol l−1 IbTX prolonged the AP duration by 0.5 ms (right). Pipette solution, high-, 0 Ca2+; bath solution, control-Hepes, 0.05 % BSA. Stimulus duration, 3.5 ms (left) and 2.5 ms (right). B, in another type I neurone 100 nmol l−1 CTX (left) reduced the total outward current by 1.44 nA (17 %; measured at 180 ms). Whole-cell current recordings were made at +30 mV; holding potential, −80 mV. In another type I neurone 50 nmol l−1 IbTX reduced the total outward current by 1.1 nA (34 %; measured at 180 ms; right). The difference currents are shown as the lowermost traces (Δ). Currents are corrected for leakage and transients. Filter frequency, 3 kHz; pipette solution, high-, 0 Ca2+; bath solution, control-Hepes, 0.05 % BSA.
In whole-cell recordings the amplitude of outward current was reduced by ≤ 2 nA both in the presence of 100 nmol l−1 CTX and 50 nmol l−1 IbTX (Fig. 8B). The current of the blocked component (Δ) was fully activated within 6 ms and showed no measurable inactivation during long-lasting test impulses. Due to a problem of exchange of high affinity toxins during whole-cell recordings in the slice preparation, complete recovery could not be achieved even after 30 min of wash-out of CTX and IbTX.
Fast type of action potential
During this investigation, seven of ninety-seven small DRG neurones could be separated on the basis of a short AP duration of 0.81 ± 0.30 ms (s.d.; n = 7; measured at 0 mV; Fig. 9B). A typical recording of this type is shown in Fig. 9A, displaying higher rates of maximum depolarization of 137 ± 23 V s−1 (s.d.; n = 7) and a maximum rate of repolarization of −107 ± 11 V s−1 (s.d.; n = 7). In addition, in four experiments the shape of the AP of this type was not influenced by 50 nmol l−1 IbTX (Fig. 9A), indicating the absence of BKCa channels. The mean size of fast type neurones was 23.1 ± 2.6 μm (s.d.) and their mean resting potential was −64.6 ± 2.6 mV (s.d.; n = 7) (summarized in Table 1).
Source of Ca2+ for activation of the BKCa channel
To test the hypothesis that Ca2+ ions entering the neurone during an AP might activate BKCa channels, we used the Ca2+ chelator BAPTA, which is 100 times faster in the binding of Ca2+ than the slower EGTA (Pape et al. 1995; Zhang et al. 1995). In eight out of nine neurones we observed a shortening of AP duration in calcium-free control solution (Fig. 10A). This indicates that a current through BKCa channels was absent during the AP in the presence of internal BAPTA, because otherwise this current would have shortened the AP in calcium-containing control solution and the AP would be prolonged in calcium-free control solution like in type I neurones. Experiments with 50 nmol l−1 IbTX confirmed these results, showing an unchanged shape of AP in all six neurones examined (Fig. 10B). The amplitudes of the fast AHP were unchanged in four neurones investigated, when tested either in calcium-free control solution or in 50 nmol l−1 IbTX (data not shown).
Figure 10. BKCa channels, source of calcium, refractory period and repetitive activity.

A, in the presence of internal 10 mm BAPTA, a fast Ca2+ chelator, AP duration (measured at half-amplitude) was shortened reversibly by 0.35 ms in calcium-free control solution (stimulus duration, 2.75 ms). Pipette solution, high-, BAPTA. B, in another neurone AP duration was not prolonged by 50 nmol l−1 IbTX. Pipette solution, high-, BAPTA; bath solution, control-Hepes, 0.05 % BSA; filter frequency, 3 kHz. C, in a type I neurone a second AP could not be evoked until 42.5 ms after the first impulse (upper trace) in control conditions (control-Hepes, 0.05 % BSA with 2.2 mm Ca2+). In the presence of 50 nmol l−1 IbTX, a second AP could be activated 30 ms after the first impulse (lower trace). Stimulus duration, 2.25 ms; 0.95 nA; pipette solution, high-, 0 Ca2+; filter frequency, 5 kHz; 24 °C. D, in a type II neurone in control conditions (control with 2.2 mm Ca2+), the second AP could be elicited 32 ms after the first impulse (upper trace), whereas in calcium-free control solution over 42 ms no further AP could be elicited (lower trace). Pipette solution, high-, 0 Ca2+; filter frequency, 10 kHz; 24 °C. E, during a long current injection into a type I neurone, a second AP was seen only in calcium-free control solution, which was reversible. Pipette solution, high-, 0 Ca2+; bath solution, control; filter frequency, 3 kHz. F, in another type I neurone, higher repetitive firing (6 APs) could be elicited in the presence of 50 nmol l−1 IbTX in contrast to control conditions (two APs in control-Hepes with 2.2 mm Ca2+). Pipette solution, high-, 0 Ca2+; 24 °C; filter frequency, 3 kHz.
Thus BAPTA prevented the activation of BKCa channels in small DRG neurones in contrast to EGTA, which is too slow to bind the Ca2+ entering the neurone. It was expected that about 63 % of the neurones would respond as type I neurones, but in the presence of BAPTA only 11 % of the neurones responded like a type I neurone, and 0 % responded to IbTx. Thus in the presence of internal BAPTA, the type I neurones responded like a type II neurone.
Influence of BKCa channels on repetitive APs
As well as the functions of BKCa channels described above, we also observed another possible function in a small subpopulation of these type I neurones. Interestingly, during a longer-lasting current injection, a second AP could be elicited (Fig. 10E) in calcium-free control solution, which could not be elicited in 2.2 mm Ca2+ control. This effect of higher firing frequencies was also seen in the presence of the specific blocker IbTX (Fig. 10F). This indicates that the current through BKCa channels prevents further membrane depolarizations. The prolonged AP duration and the reduced AHP amplitude of the first AP recorded in calcium-free control solution characterized these neurones as type I. Thus the antagonizing effect of the BKCa current could be seen in the prolongation of the refractory period of type I neurones (Fig. 10C and D). In neurones containing BKCa channels we could not elicit a second AP in double-pulse experiments within 50 ms, whereas after addition of the BKCa channel blocker IbTX to the control solution a second AP could be elicited earlier. A subsequent AP was reversibly suppressed in calcium-free control solution (data not shown), when internal BAPTA prevented the activation of the BKCa current. We conclude that the subsequent AP was generated by sodium and calcium currents and therefore it failed in calcium-free control solution. Due to the presence of internal BAPTA the BKCa current could not suppress the second AP in 2.2 mm Ca2+-containing control solution, which is in contrast to the experiment of Fig. 10C.
On the other hand, there were some neurones that displayed APs of shorter duration than those in Fig. 10A–D. The subsequent AP could be elicited in either control solution (data not shown). We conclude that these APs were generated by sodium currents only, and that there was no BKCa current present that could suppress the subsequent AP.
DISCUSSION
The recently described slice preparation (Safronov et al. 1996) allowed access to a more intact preparation of DRG neurones, especially those connected to Aδ- and C-fibres conducting afferent input like pain, temperature and tactile information. It was thus possible to describe single-channel properties as well as whole-cell currents with their functional contribution without enzymatic pretreatment of the neurones. The question whether the large BKCa channels, beside the apamin-sensitive calcium-activated K+ current, would be present in DRG neurone, remained still open. Different kinetic and gating properties of BKCa channels have been described (DiChiara & Reinhart, 1995; Morales et al. 1995; Silberberg et al. 1996; Tanaka et al. 1997). In addition, we characterized here a fast activating BKCa channel type, which contributed to the repolarization phase of AP (shortening the AP duration), prolonged the refractory period and modulated the fast AHP of DRG neurones.
Single-channel kinetics
The deviation of the I–V curve in asymmetric K+ solutions (control) from linear correlation as observed in symmetric K+ solutions (Fig. 1) might be attributed to the Goldman-Hodgkin-Katz current equation, which could explain the 2-fold higher conductance at potentials > +40 mV compared with the conductance at potentials < −10 mV. The smaller single-channel amplitudes in cell-attached recordings (Fig. 3A) compared with those in inside-out recordings might be due to higher cytoplasmic Mg2+ or Na+ ion concentrations in the neurones, which reduce the amplitudes, especially at potentials positive to 0 mV (Morales et al. 1996).
Most previous studies described the BKCa current as a slow one, which needed up to some hundreds of milliseconds for full activation, but in order to obtain fast activation, 40–100 μmol l−1 Ca2+ at the cytoplasmic membrane side was necessary (Egan et al. 1993; Gola & Crest, 1993; Morales et al. 1996). Since in neuronal cells BKCa channels are said to contribute to the action potential, it is evident that they should activate quickly at depolarizing potentials. Not only did we find fast kinetics of single BKCa channels even at low [Ca2+]i, but also a short latency of channel openings after depolarizing voltage steps in cell-attached recordings (Fig. 3A). These differences observed in kinetics might be attributed to different types of BKCa channel, as already described for their different clones dslo, hslo or mslo (Cox et al. 1997; DiChiara & Reinhart, 1997), or may be due to the modulating effect of β-subunits of the channel protein in addition to modulating effects on their calcium sensitivity (Tanaka et al. 1997).
Calcium sensitivity and Popen fluctuations
The cytoplasmic calcium concentration that we estimated for half-maximum activation was within the range found for BKCa channels composed of α- and β-subunits, but below the values found for BKCa channels composed of α-subunits only (Tanaka et al. 1997). Even though these calcium concentrations were close to physiological values, the potentials of half-maximum activation (Fig. 2) were more positive than reported for cloned channels (Meera et al. 1996) or for native channels in other preparations (Kang et al. 1996a, b; Tanaka et al. 1997). Only in the peripheral myelinated axon were they found to be at more positive potentials (Jonas et al. 1991). Even if the potentials of half-maximum activation are relatively positive in BKCa channels of DRG neurones, the channels could contribute effectively to modulation of the AP already near its peak (Fig. 7). In addition, these potentials showed a linear correlation with [Ca2+]i (Fig. 2C), whereas in a number of other BKCa channels a smaller decrease of potentials with increasing [Ca2+]i was found (DiChiara & Reinhart, 1995). This might be explained by the influence of the β-subunit (Meera et al. 1996) on the calcium sensitivity of the channel, whereas BKCa channels of DRG neurones might be composed only of an α-subunit and therefore showed the linear correlation with [Ca2+]i and the low calcium sensitivity.
It should be noted that the open probability curves of the BKCa channel of DRG neurones did not perfectly correlate due to the spontaneous and fast fluctuations of Popen during the measurements illustrated in Fig. 2E. In 26/91 measurements fast Popen fluctuations were observed more often at potentials > +20 mV and with pCa > 6. We did not study this issue in further detail and the underlying mechanism is still unclear. In our case we could not observe a calcium-dependent depression of the current amplitude (Eckert & Ewald, 1982) because at pCa ≥ 5 we measured open probabilities close to 1. Another possibility might be that different types of BKCa channel exist, as found for developing central neurones where the channels constantly showed different kinetic properties (Kang et al. 1996a, b). In our case this explanation is unlikely because in the same patch from DRG neurones a single channel showed two different gating modes, one with a higher Popen, and a second one with a lower Popen. Fluctuation of Popen of a single BKCa channel was also reported by (Silberberg et al. 1996) and was called ‘wanderlust’. In those cloned BKCa channels changes of Popen were reported within minutes, whereas we observed the changes within seconds, which reflects the main difference between both phenomena. Interestingly, Silberberg et al. (1996) had to reject a model of switching between low and high calcium sensitivity because it could not explain the wanderlust kinetics, but in our case this model reflects the observation of fast fluctuations of mean Popen very well.
In general, the differences in kinetic properties described for BKCa channels in different cells might be causally related with alternative splicing of the known complementary DNA encoding the α-subunit, which results in natural variants (Butler et al. 1993; Silberberg et al. 1996; Tanaka et al. 1997).
Two subtypes of small DRG neurone
Small DRG neurones of diameter < 28 μm were classified as Aδ- and C-type, conducting nociception with slow velocities (Harper & Lawson, 1985a; Villiere & McLachlan, 1996). It was shown that the mean cell diameter of DRG neurones increased in the first 3 weeks after birth (Lawson et al. 1974). Therefore we measured cell sizes from fixed DRG of 6-day-old rats. The results of frequency distribution of soma diameter revealed one peak for small cells, which is close to the mean cell size of electrophysiological investigated neurones. The major difference in mean diameter was found for larger cells, which were already about 10 μm larger in 3- to 5-week-old rats compared with our preparation (Villiere & McLachlan, 1996). In addition, only these larger neurones showed a positive staining of neurofilament. This might indicate that changes in mean diameter in this population of neurones is the major reason for the described increase of mean cell diameter in DRG during ageing (Lawson et al. 1974). Furthermore, support for the investigated population of neurones in this work being of small type connected with slow conducting axons comes from the measured mean AP duration, which was longer than 2 ms, similar to findings in older rats (Harper & Lawson, 1985b; Villiere & McLachlan, 1996).
These small neurones are equipped with TTX-sensitive and TTX-resistant Na+ channels (Black et al. 1996; Scholz et al. 1998), different types of Ca2+ channel (Tsien et al. 1987; Mintz et al. 1992; Scholz et al. 1997a) and delayed rectifier K+ channels (Safronov et al. 1996). In this study we subdivided the small DRG neurones into two types, a major group displaying BKCa channels (type I) and another group where no BKCa channels were observed (type II; Figs 6 and 7). In whole-cell currents of type I neurones, the inward Ca2+ current was clearly compensated by the BKCa currents. To exclude the possible influence of changed surface charge on activation of channels (e.g. Na+ channels) in calcium-free control solution we added another 4 mm Mg2+ (final 5 mm) to the external solution (Hille, 1992). Therefore the functional relevance of BKCa channels during an AP is not unexpected, but their contribution even to the peak of the AP amplitude results from their fast kinetics found in single-channel recordings. Small DRG neurones with short AP duration were rarely observed (Fig. 9) and are suggested to be A-type neurones with a fast conducting characteristic (Harper & Lawson, 1985a, b).
The amplitudes of whole-cell currents blocked by the specific toxins (Fig. 8B) were not as large as one expects from the experiments of exchange with external calcium ions (Fig. 7). This might arise from slow and incomplete diffusion of the high molecular toxins into the tissue of the slice surrounding the neurone under investigation. Therefore a washout was possible in single-channel recordings, whereas it often remained incomplete in whole-cell recordings (Figs 5 and 8).
Functions of BKCa channels
The activation of BKCa channels leads to an increase in speed of repolarization and to a shortening of AP duration in type I small DRG neurones. It is suggested that the hastening of repolarization by BKCa channels could result in a decreased amount of calcium entering the neurone during an AP. In addition, we found a suppression of following APs by the calcium-dependent K+ conductance during longer or repetitive stimulation (Fig. 10). This observation might explain conduction failures during repetitive stimulation reported by Lüscher et al. (1994b). They observed that these failures are accompanied by an intracellular accumulation of calcium ions and they suggested a summation of K+ conductances as a major mechanism. Since BKCa channels do not inactivate and are activated by internal calcium ions, they might explain the limited increase of [Ca2+]i during longer periods of repetitive stimulation (Lüscher et al. 1996). This may be in support of neurones with a small buffer capacity for internal calcium (Gabso et al. 1997) where the clearance of intracellular calcium is low and cannot remove the invaded calcium within tens of milliseconds (Lüscher et al. 1994b; Gabso et al. 1997).
A TEA-sensitive and calcium-dependent K+ conductance caused a membrane depolarization in DRG neurones when blocked by TEA (Morita & Katayama, 1996). Unfortunately we did not observe clear changes of the resting potential above 5 mV in the presence of the specific blockers CTX and IbTX. In cell-attached experiments we found an extremely low activity of BKCa channels, and therefore they might not play a major role in generating the resting potential under physiological conditions. Although Morita & Katayama (1996) measured similar resting potentials, their findings might be explained by the 10–50 times lower input resistance in their experiments with microelectrodes compared with our gigaohm-seals. The leakage of ions including calcium might have been larger in their experiments and might have activated the BKCa channels at the resting potential. Alternatively, in different DRG neurones there might exist BKCa channels of another channel subtype, with higher open probability under resting conditions, which could exert the functions observed by Morita & Katayama (1996).
Besides their role under physiological conditions, BKCa channels might become even more important under certain pathophysiological conditions. In hypoxia, for instance, the resting potential will depolarize (Grafe et al. 1994) and an increased calcium influx (Duchen et al. 1990) will be critical for the survival of the neurones. BKCa channels may counteract this process.
Acknowledgments
We are grateful to B. Agari, O. Becker and J. Beltzer for excellent technical assistance. We acknowledge the support of S. Wiegand, M. Henrich and Dr A. Fischer from the Department of Anatomy for measurements of cell size. We thank Dr R.-D. Treede and Dr M. Baker for valuable comments on the manuscript, and Dr B. Safronov for critical discussions throughout this study.
References
- Barrett JN, Magleby KL, Pallotta BS. Properties of single calcium-activated potassium channels in cultured rat muscle. The Journal of Physiology. 1982;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Dibhajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Molecular Brain Research. 1996;43:117–131. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding ‘maxi’ calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- Cox DH, Cui J, Aldrich RW. Separation of gating properties from permeation and block in mslo large conductance Ca activated K+ channels. Journal of General Physiology. 1997;109:633–646. doi: 10.1085/jgp.109.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ, Ireland DR, McLachlan EM. Sources of Ca2+ for different Ca2+-activated K+ conductances in neurones of the rat superior cervical ganglion. The Journal of Physiology. 1996;495:353–366. doi: 10.1113/jphysiol.1996.sp021599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChiara TJ, Reinhart PH. Distinct effects of Ca2+ and voltage on the activation and deactivation of cloned Ca2+-activated K+ channels. The Journal of Physiology. 1995;489:403–418. doi: 10.1113/jphysiol.1995.sp021061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChiara TJ, Reinhart PH. Redox modulation of hslo Ca2+ activated K+ channels. Journal of Neuroscience. 1997;17:4942–4955. doi: 10.1523/JNEUROSCI.17-13-04942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico AM, Lemos JR, Treistman SN. Ethanol increases the activity of large conductance, Ca2+-activated K+ channels in isolated neurohypophysial terminals. Molecular Pharmacology. 1996;49:40–48. [PubMed] [Google Scholar]
- Duchen MR, Valdeolmillos M, O'Neill SC, Eisner DA. Effects of metabolic blockade on the regulation of intracellular calcium in dissociated mouse sensory neurones. The Journal of Physiology. 1990;424:411–426. doi: 10.1113/jphysiol.1990.sp018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R, Ewald D. Residual calcium ions depress activation of calcium-dependent current. Science. 1982;216:730–733. doi: 10.1126/science.6281880. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Archiv. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Egan TM, Dagan D, Levitan IB. Properties and modulation of a calcium-activated potassium channel in rat olfactory bulb neurons. Journal of Neurophysiology. 1993;69:1433–1442. doi: 10.1152/jn.1993.69.5.1433. [DOI] [PubMed] [Google Scholar]
- Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. The Journal of Physiology. 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabso M, Neher E, Spira ME. Low mobility of the Ca2+ buffers in axons of cultured Aplysia neurons. Neuron. 1997;18:473–481. doi: 10.1016/s0896-6273(00)81247-7. [DOI] [PubMed] [Google Scholar]
- Gola M, Crest M. Colocalization of active KCa channels and Ca2+ channels within Ca2+ domains in helix neurons. Neuron. 1993;10:689–699. doi: 10.1016/0896-6273(93)90170-v. [DOI] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Characterization of six voltage gated K+ currents in adult rat sensory neurons. Journal of Neurophysiology. 1996a;75:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Role of a Ca2+ dependent slow afterhyperpolarization in prostaglandin E (2) induced sensitization of cultured rat sensory neurons. Neuroscience Letters. 1996b;205:161–164. doi: 10.1016/0304-3940(96)12401-0. [DOI] [PubMed] [Google Scholar]
- Grafe P, Bostock H, Schneider U. The effects of hyperglycaemic hypoxia on rectification in rat dorsal root axons. The Journal of Physiology. 1994;480:297–307. doi: 10.1113/jphysiol.1994.sp020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruß M, Scholz A, Vogel W. Modulation of action potential by BK-KCa channels in small neurones of rat DRG slices. Pflügers Archiv. 1997;433:R17. [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. The Journal of Physiology. 1985a;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. The Journal of Physiology. 1985b;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P, Lansman JB, Tsien RW. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. Journal of General Physiology. 1986;88:293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H, Morita K, North RA. Calcium-dependent after-potentials in visceral afferent neurones of the rabbit. The Journal of Physiology. 1984;355:479–492. doi: 10.1113/jphysiol.1984.sp015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates Inc.; 1992. [Google Scholar]
- Jonas P, Koh DS, Kampe K, Hermsteiner M, Vogel W. ATP-sensitive and Ca-activated K channels in vertebrate axons: novel links between metabolism and excitability. Pflügers Archiv. 1991;418:68–73. doi: 10.1007/BF00370453. [DOI] [PubMed] [Google Scholar]
- Kang J, Huguenard JR, Prince DA. Development of BK channels in neocortical pyramidal neurons. Journal of Neurophysiology. 1996a;76:188–198. doi: 10.1152/jn.1996.76.1.188. [DOI] [PubMed] [Google Scholar]
- Kang J, Huguenard JR, Prince DA. Two types of BK channels in immature rat neocortical pyramidal neurons. Journal of Neurophysiology. 1996b;76:4194–4197. doi: 10.1152/jn.1996.76.6.4194. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Veselovsky NS, Fedulova SA, Tsyndrenko AY. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons. III. Potassium currents. Neuroscience. 1981;6:2439–2444. doi: 10.1016/0306-4522(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Caddy KW, Biscoe TJ. Development of rat dorsal root ganglion neurones. Studies of cell birthdays and changes in mean cell diameter. Cell Tissue Research. 1974;153:399–413. doi: 10.1007/BF00229167. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Lipp P, Lüscher HR, Niggli E. Control of action potential propagation by intracellular Ca2+ in cultured rat dorsal root ganglion cells. The Journal of Physiology. 1996;490:319–324. doi: 10.1113/jphysiol.1996.sp021146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Streit J, Lipp P, Lüscher HR. Action potential propagation through embryonic dorsal root ganglion cells in culture. II. Decrease of conduction reliability during repetitive stimulation. Journal of Neurophysiology. 1994a;72:634–643. doi: 10.1152/jn.1994.72.2.634. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Streit J, Quadroni R, Lüscher HR. Action potential propagation through embryonic dorsal root ganglion cells in culture. I. Influence of the cell morphology on propagation properties. Journal of Neurophysiology. 1994b;72:622–633. doi: 10.1152/jn.1994.72.2.622. [DOI] [PubMed] [Google Scholar]
- McManus OB, Magleby KL. Kinetic time constants independent of previous single-channel activity suggest Markov gating for a large conductance Ca-activated K channel. Journal of General Physiology. 1989;94:1037–1070. doi: 10.1085/jgp.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell AE, Smith RM. Amines. Vol. 2. New York: Plenum Press; 1975. Critical stability constants; p. 416. [Google Scholar]
- Meera P, Wallner M, Jiang Z, Toro L. A calcium switch for the functional coupling between alpha (hslo) and beta subunits (Kv,Ca beta) of maxi K channels. FEBS Letters. 1996;385:127–128. doi: 10.1016/0014-5793(96)83884-1. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Morales MJ, Castellino RC, Crews AL, Rasmusson RL, Strauss HC. A novel beta subunit increases rate of inactivation of specific voltage-gated potassium channel alpha subunits. Journal of Biological Chemistry. 1995;270:6272–6277. doi: 10.1074/jbc.270.11.6272. [DOI] [PubMed] [Google Scholar]
- Morales E, Cole WC, Remillard CV, Leblanc N. Block of large conductance Ca2+ activated K+ channels in rabbit vascular myocytes by internal Mg2+ and Na+ The Journal of Physiology. 1996;495:701–716. doi: 10.1113/jphysiol.1996.sp021627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Katayama Y. Tetraethylammonium sensitive calcium sensitive potassium current in a subclass of the bullfrog dorsal root ganglion cells. Neuroscience Letters. 1996;215:193–196. doi: 10.1016/0304-3940(96)12979-7. [DOI] [PubMed] [Google Scholar]
- Pape PC, Jong DS, Chandler WK. Calcium release and its voltage dependence in frog cut muscle fibers equilibrated with 20 mM EGTA. Journal of General Physiology. 1995;106:259–336. doi: 10.1085/jgp.106.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Bischoff U, Vogel W. Single voltage-gated K+ channels and their functions in small dorsal root ganglion neurones of rat. The Journal of Physiology. 1996;493:393–408. doi: 10.1113/jphysiol.1996.sp021391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Vogel W. Single voltage-activated Na+ and K+ channels in the somata of rat motoneurones. The Journal of Physiology. 1995;487:91–106. doi: 10.1113/jphysiol.1995.sp020863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Appel N, Vogel W. R- and Q-type Ca-channels in rat dorsal root ganglion neurones. Pflügers Archiv. 1997a;433:R143. [Google Scholar]
- Scholz A, Gruß M, Vogel W. BK-KCa channels modulate the action potential of sensory neurones in thin slices of rat. In: Elsner N, Wässle H, editors. Proceedings of the 25th Göttingen Neurobiology Conference, From Membrane to Mind. I. Stuttgart, New York: Georg Thieme Verlag; 1997b. p. 85. [Google Scholar]
- Scholz A, Kuboyama N, Hempelmann G, Vogel W. Complex blockade of TTX-resistant Na+ currents by lidocaine and bupivacaine reduce firing frequency in DRG neurons. Journal of Neurophysiology. 1998;79:1746–1754. doi: 10.1152/jn.1998.79.4.1746. [DOI] [PubMed] [Google Scholar]
- Silberberg SD, Lagrutta A, Adelman JP, Magleby KL. Wanderlust kinetics and variable Ca2+-sensitivity of Drosophila, a large conductance Ca2+-activated K+ channel, expressed in oocytes. Biophysical Journal. 1996;70:2640–2651. doi: 10.1016/S0006-3495(96)79833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. The Journal of Physiology. 1990;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi Kca channels in human coronary smooth muscle: predominant α+β subunit complexes. The Journal of Physiology. 1997;502:545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RW, Fox AP, Hess P, McCleskey EW, Nilius B, Nowycky MC, Rosenberg RL. Multiple types of calcium channel in excitable cells. Society of General Physiologists Series. 1987;41:167–187. [PubMed] [Google Scholar]
- Villiere V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. Journal of Neurophysiology. 1996;76:1924–1941. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- Zhang L, Pennefather P, Velumian A, Tymianski M, Charlton M, Carlen PL. Potentiation of a slow Ca2+-dependent K+ current by intracellular Ca2+ chelators in hippocampal CA1 neurons of rat brain slices. Journal of Neurophysiology. 1995;74:2225–2241. doi: 10.1152/jn.1995.74.6.2225. [DOI] [PubMed] [Google Scholar]


