Abstract
The adaptations of the ankle dorsiflexor muscles and the behaviour of single motor units in the tibialis anterior in response to 12 weeks of dynamic training were studied in five human subjects. In each training session ten series of ten fast dorsiflexions were performed 5 days a week, against a load of 30–40 % of the maximal muscle strength.
Training led to an enhancement of maximal voluntary muscle contraction (MVC) and the speed of voluntary ballistic contraction. This last enhancement was mainly related to neural adaptations since the time course of the muscle twitch induced by electrical stimulation remained unaffected.
The motor unit torque, recorded by the spike-triggered averaging method, increased without any change in its time to peak. The orderly motor unit recruitment (size principle) was preserved during slow ramp contraction after training but the units were activated earlier and had a greater maximal firing frequency during voluntary ballistic contractions. In addition, the high frequency firing rate observed at the onset of the contractions was maintained during the subsequent spikes after training.
Dynamic training induced brief (2–5 ms) motor unit interspike intervals, or ‘doublets’. These doublets appeared to be different from the closely spaced (±10 ms) discharges usually observed at the onset of the ballistic contractions. Motor units with different recruitment thresholds showed doublet discharges and the percentage of the sample of units firing doublets was increased by training from 5.2 to 32.7 %. The presence of these discharges was observed not only at the onset of the series of spikes but also later in the electromyographic (EMG) burst.
It is likely that earlier motor unit activation, extra doublets and enhanced maximal firing rate contribute to the increase in the speed of voluntary muscle contraction after dynamic training.
Chronic activity patterns such as strength training produce marked adaptations not only in the muscular but also in the nervous system (for reviews, see McDonagh & Davies, 1984; Sale, 1988; Enoka, 1996). One line of evidence that substantiates the significant role played by the neural mechanism during training is the increase in strength that is often greater than can be accounted for by only a change in the muscle cross-sectional area (Jones & Rutherford, 1987). In addition to an increase in muscle force generating capacity as the result of heavy resistance training (Duchateau & Hainaut, 1984), another common observation is an enhanced electromyographic (EMG) level of muscle activity (Hakkinen & Komi, 1983; Davies et al. 1985; Narici et al. 1989). In the absence of electrically induced EMG (M wave) modification (Duchateau & Hainaut, 1984) this latter change, which usually precedes muscle enlargement, suggests that the neural drive is intensified by training and so contributes to the increase in force.
Training with small loads at maximal movement velocity also induces muscular and neural adaptations. This method, usually called dynamic or ‘explosive’ training, involves ballistic contractions, characterized by short times to peak tension, high rates of tension development and high single motor unit discharge frequencies (Desmedt & Godaux, 1977). It has previously been shown that dynamic training induces changes in the contractile properties of the human adductor pollicis muscle (Duchateau & Hainaut, 1984), an increase in maximal force and rate of tension development, and shortening of the twitch time to peak of single motor units, without any change in the order of recruitment (Hainaut et al. 1981). Hakkinen et al. (1985) investigated the influence of dynamic training on the time course of the isometric force and EMG activity in the leg extensor muscles and observed a greater rate of tension development associated with enhanced EMG activity in the early stage of the onset of contraction. More recently, Behm & Sale (1993) suggested that it is the intended movement velocity rather than an actual ballistic movement that determines the degree of adaptation in the velocity of a mechanical response.
Despite ample evidence of a significant role for neural mechanisms in the neuromuscular adaptations associated with training, there has been less progress in identifying the specific mechanisms responsible for these adaptations. The most commonly proposed explanation is that the improvement in force or power is due to (1) a change in muscle synergy and/or an agonist-antagonist activation pattern; (2) an increased activation of the muscle as a result of changes in motor unit recruitment or firing patterns (Hakkinen & Komi, 1983; Rutherford & Jones, 1986; Carolan & Cafarelli, 1992; Moritani, 1993; Zehr & Sale, 1994). These interesting suggestions, however, are rather speculative in view of the lack of experimental data.
The purpose of the present study was to analyse the effects of 3 months of dynamic training on the neuromuscular adaptations of the ankle dorsiflexor muscles, and particularly in connection with the rate of tension development during voluntary contractions. In these muscles, we analysed the maximal voluntary contraction (MVC; i.e. the maximal voluntary torque), the speed of ballistic contractions, the EMG activity during MVC and voluntary ballistic contractions and the electrically induced twitch in order to distinguish neural adaptations from contractile changes. Single motor unit properties and behaviour pattern were also investigated in the tibialis anterior with special emphasis on a possible change in discharge frequency during ballistic contractions.
METHODS
Subjects and experimental design
Five subjects (3 female and 2 male) aged between 18 and 22 years took part in this investigation and were tested on at least three occasions before and also after training. All the subjects were familiar with the experimental procedures. Their mean ±s.d. height and weight were 168 ± 7.1 cm and 62.4 ± 11.7 kg, respectively. A control group of five subjects (1 female and 4 male; height, 178.2 ± 5.5 cm; weight, 73.0 ± 9.8 kg) did not train and were retested after 1 and 6 weeks in order to assess the reliability of the observations. The dorsiflexor muscles of the left leg (the non-dominant side) were tested. This study was approved by the University Ethics Committee and the subjects gave their informed consent prior to participation in the investigation. All the experimental procedures were performed in accordance with the Declaration of Helsinki.
Training programme
The subjects trained the dorsiflexor muscles of one leg for a period of 12 weeks at a frequency of five sessions per week. During each session they executed ten sets of ten fast dorsiflexion contractions (from 90 deg to full dorsiflexion) against a load that was 30–40 % of the maximum that could be lifted once (one repetition maximum; 1 RM). The subjects were instructed to attempt to move the footplate as fast as possible at each repetition. To avoid fatigue, a 2–3 s rest period was interposed between the individual contractions of a set, with a 2–3 min rest period between sets. The maximal force (1 RM) was tested every month and the training load was adjusted accordingly. At the end of the training session the average load lifted by the subjects had increased by 34.4 ± 3.5 % (mean ±s.e.m.).
EMG recordings
Motor unit action potentials were recorded by a selective electrode made up of 50 μm diamel-coated nichrome wires inserted into the muscle by means of a hypodermic needle (Duchateau & Hainaut, 1990). The electrode was inserted through the skin in the mid part of the muscle belly. During each experimental session the same needle electrode was inserted in at least three separate locations and at each location it was manipulated to different depths and angles to record the action potentials from different motor units. The signals were amplified by a custom-made differential amplifier and filtered (100 Hz to 10 kHz) before being displayed on a Tektronix TAS 455 oscilloscope. The surface EMG was recorded by means of two disk electrodes (8 mm in diameter) placed 2–3 cm apart on either side of the needle electrode. The surface EMG signal was amplified (×1000) and filtered (10 Hz to 5 kHz) by a custom-made differential amplifier.
Mechanical recordings
During the pre- and post-training recordings the subject sat on a chair in a slightly reclining position with one foot strapped to a footplate which was inclined at an angle of 45 deg to the floor. The ankle and knee angles were set at about 90 and 110 deg, respectively. The foot was tightly attached to the plate by means of two straps and held in place by a heel block. One strap was placed around the ankle and the other around the foot 1–2 cm proximal to the metatarsophalangeal joint. Under these conditions the force measurement was nearly isometric and contamination by other extensor muscles (extensor digitorum longus and extensor hallucis longus) reduced to a minimum. The isometric force developed by the dorsiflexor muscles was measured by connecting the footplate to a strain gauge transducer (TC 2000, Kulite, Basingstoke, UK) and the signal was amplified (AM 502, Tektronix, Beaverton, OR, USA; bandwidth DC - 300 Hz). The transducer was attached at the level of the metatarsophalangeal joint of the big toe (lever arm, 17 cm). The sensitivity of the force transducer was 30 mV N−1 (linear range, 0–500 N). The force signal was then high-gain-AC amplified and filtered (1 Hz to 100 Hz) by means of an AM 502 plug-in Tektronix amplifier to obtain the mechanical contribution of single motor units.
Muscle twitch torque was induced by a rectangular supramaximal (10–20 % above torque responses) electrical pulse (0.1 ms in duration) delivered by a custom-made stimulator triggered by a digital timer (model 4030, Digitimer Ltd, Welwyn Garden City, UK). The peroneal nerve was stimulated by two electrodes (silver disks 8 mm in diameter), with the cathode being placed over the proximal border of the tibialis anterior while the anode was fastened to the fibular head. In order to avoid the activation of the peroneal muscles the nerve was stimulated beyond their innervation collateral and the absence of any peroneal muscle activity was assessed by means of the EMG recording.
The mechanical recording of the single motor units was assessed by the spike-triggered averaging method (Milner-Brown et al. 1973). Briefly, this method consists of triggering the sweep of an averager (model 4094c, Nicolet, Madison, WI, USA) with the action potential of the selected unit during a steady contraction, and recording the corresponding filtered isometric force. The contribution of the motor units to the net force was then extracted by means of averaging. Because a low steady firing rate of the motor unit is necessary to avoid the mechanical summation of the mechanical responses (Nordstrom et al. 1989), the subjects were provided with visual and auditory feedback. A hardware rate limiter was also used to average the mechanical responses from the same motor unit, a process which separated the individual responses by at least 100 ms (Duchateau & Hainaut, 1990).
Experimental procedure
To determine the maximal voluntary torque of the dorsiflexor muscles each experimental session began with the subjects performing three MVCs of 4–5 s duration separated by 2–3 min pauses. The motor units were subsequently identified by having the subject perform two or three isometric ramp contractions. Once the action potentials had been clearly identified (usually 2 to 4 at each site), the subject maintained a level of torque for each motor unit just sufficient to maintain the discharge frequency at a constant low rate. The mechanical contribution of each motor unit to the net torque was then determined by averaging, and its recruitment threshold was assessed for three to five voluntary isometric contractions of 10 % MVC s−1; target torque information was shown on an oscilloscope screen. The subjects then performed twenty to thirty voluntary ballistic contractions randomly at different torques. The subjects were instructed to perform each contraction as fast as possible without involvement of the big toe and were informed of the torque level but not of the speed of contraction. Three to five minutes rest was allowed between two successive trials. All the signals were stored on video tape (Vetter 620, Rebersburg, PA, USA; bandwidth, DC - 9 kHz for EMG signals and DC - 100 Hz for the force signals).
Data analysis
The data were analysed off-line from the taped records. The maximal voluntary torque was determined from the trial that yielded the largest value. The twitch torque, the time to peak and the maximal rate of tension development (dT/dt) in response to voluntary ballistic and electrically induced contractions were measured. The half-relaxation time was also measured in electrically induced contractions. During the MVCs, the EMG root mean square (r.m.s.) was measured during a 2 s epoch, while during ballistic contractions, the EMG was integrated for the entire activation. The peak-to-peak amplitude of the M wave was determined.
Motor unit discrimination was accomplished with a computer-based template-matching algorithm (Signal Processing Systems, SPS 8701, Malvern, Victoria, Australia). Single motor unit action potentials identified on the basis of their waveform shapes were used to trigger the averaging sweeps of the force signal. Usually, 100–300 sweeps were averaged, depending on motor unit size. For large motor units 50–100 sweeps were often sufficient, and in this case the contractions were interrupted by one or more short pauses to minimize fatigue. We measured the peak amplitude and the time to peak of the mechanical response of each motor unit. The motor unit recruitment threshold during voluntary activation was defined as the level of torque at which the action potential of the selected unit appeared for the first time on the EMG trace. The recruitment threshold was measured during the isometric ramp contractions and expressed either as an absolute value or as a percentage of the MVC torque. The motor unit discharge frequency was analysed at the onset of the ballistic contractions, and the times of the first three interspike intervals were measured. For each subject, the motor unit data acquired during the different recording sessions were pooled before or after training. The day to day reliability, estimated with an interclass correlation coefficient (r) of the slopes of the force-recruitment threshold relationship, was 0.94.
Statistics
The significance of any differences before and after training was determined either by Student's t test (for paired or unpaired data), or by the non-parametric Kolmogorov-Smirnov two-sample test where appropriate. The effects of training on the relationship between the rate of tension development and torque during ballistic contractions as well as the motor unit torques and their recruitment thresholds during ramp contractions were tested by the Student's t test by comparing the slope and ordinate intercepts of the linear regressions. Data from the control group at 1 and 6 week intervals were compared with those of the pre-test by means of a repeated-measures analysis of variance (ANOVA). In the text, values are given as means ±s.e.m. and a significance level of P < 0.05 was used for all statistical comparisons.
RESULTS
Muscle adaptations
Twelve weeks of dynamic training increased (P < 0.05) the MVC torque and the EMG by 30.2 % and 19.6 %, respectively, though without any significant change in M wave amplitude (Table 1). The twitch torque induced by the supramaximal electrical stimulation of the motor nerve did not change significantly after training (Table 1). The increase in the MVC torque and the EMG were clearly related to a training effect because the control group did not show any significant change either at 1 or at 6 week intervals.
Table 1.
MVC and twitch parameters of the ankle dorsiflexor muscles before and after training for the 5 subjects
| Before training | After training | Percentage change | |
|---|---|---|---|
| MVC | |||
| Torque (N m) | 48.0 ± 9.9 | 59.1 ± 7.5 | +30.2 ± 4.2 * |
| EMG (r.m. s.) (μV) | 61.0 ± 7.6 | 71.9 ± 7.5 | +19.6 ± 2.8 * |
| Twitch | |||
| Torque (N m) | 3.4 ± 0.6 | 3.2 ± 0.5 | −4.1 ± 6.6 |
| Time to peak (ms) | 71.0 ± 10.1 | 67.0 ± 12.2 | −6.7 ± 8.1 |
| Half-relaxation time (ms) | 70.0 ± 9.6 | 78.0 ± 16.4 | +4.7 ± 8.4 |
| Maximal rate of tension development (N m s−1) | 50.2 ± 11.8 | 55.8 ± 20.3 | +8.6 ± 10.7 |
| M wave amplitude (mV) | 5.5 ± 0.6 | 5.2 ± 0.5 | −4.8 ± 6.0 |
Values are means ±s.e.m.
P < 0.05, Student's t test for paired data.
The adaptation of the contraction velocity was assessed by measuring the maximal rate of tension development during ballistic contractions at different torque levels. When expressed either as an absolute value (not illustrated) or as a percentage of the maximal torque (Fig. 1A), the rate at which tension developed was augmented after training at a proportionally comparable level of increase, whatever the torque level. In the control group these relationships were comparable at 1–6 week intervals since neither the slopes (before: 0.0046 ± 0.0002 % MVC ms−1; 1 week: 0.0051 ± 0.0002 % MVC ms−1; 6 weeks: 0.0051 ± 0.0002 % MVC ms−1) nor the ordinate intercepts (before: 0.073 ± 0.008 % MVC ms−1; 1 week: 0.055 ± 0.007 % MVC ms−1; 6 weeks: 0.064 ± 0.008 % MVC ms−1) changed significantly. Although there is a small (non-significant) difference in the slopes in the control group, it cannot be compared with the large and significant (P < 0.001) increase observed in the trained group. The comparison of two ballistic contractions of similar torque levels (expressed as a percentage of the maximal torque) showed that the increase in the maximal rate of tension development was associated with a decrease in the time-to-peak tension after training (Fig. 1B). The mean maximal rate of tension development of the five fastest ballistic contractions in each subject was increased by 82.3 % (0.17 ± 0.11 vs. 0.31 ± 0.13 N m ms−1; P < 0.01; Student's t test for paired data) and by 52.9 % (0.333 ± 0.018 vs. 0.509 ± 0.024 % MVC ms−1; P < 0.001) when expressed as absolute or relative values, respectively, and the time-to-peak tension was shortened by 15.9 % (135.8 ± 6.9 vs. 114.2 ± 3.8 ms; P < 0.05). After training, these mechanical adaptations were associated with an increase in EMG activity in the early stage of the contraction and the whole EMG appeared to be more segmented (Fig. 1B). The integrated EMG was increased by 42.7 % (13.9 ± 2.1 vs. 19.8 ± 3.2 μV s; P < 0.001) and the time taken to reach its half-integrated maximal value was reduced by 15.6 % (130.4 ± 13.0 vs. 110 ± 11.4 ms; P < 0.01). In contrast, neither the time to peak nor the half-relaxation time of the twitch nor its maximal rate of tension development changed significantly in the trained group (Table 1). When mechanical and EMG parameters recorded during voluntary or electrically induced contractions were tested in the control group no significant difference was observed.
Figure 1. Rate of tension development as a function of torque and comparison of torque and rectified EMG during ballistic contractions.
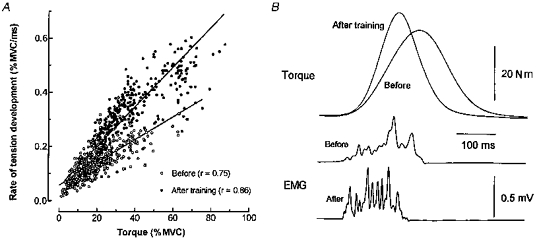
A, relationship for all subjects between the rate of tension development and torque (expressed as a percentage of MVC) during ballistic contractions. The linear regression lines are, respectively, y = 0.0042x + 0.057 (r = 0.75; P < 0.001) and y = 0.0065x+ 0.063 (r = 0.86; P < 0.001) before and after training. The slopes of the relationships before and after training are significantly different (P < 0.001). B, comparison of the torque and rectified EMG recorded in one subject during a ballistic contraction with a similar MVC percentage (41 vs. 44 %), before and after training.
Motor unit mechanical adaptations
In our five subjects, a large sample of motor units was collected before (n = 256) and after training (n = 269). The contribution of each subject to the total motor unit population lay between 16.0 and 24.2 %. The recruitment thresholds of these motor units, which were recorded during linearly increasing voluntary contractions, displayed a skewed distribution and ranged from less than 1 % up to 87 % of MVC (Fig. 2A and B). Even if after training, the range of the recruitment threshold distribution, expressed in absolute values, showed a tendency to be greater (0.2–59.9 N m) than pre-training values (0.4–49.5 N m), there was, however, a small but significant (P < 0.05; Kolmogorov-Smirnov two-sample test) shift of the distribution towards lower values (10.9 ± 0.7 N m vs. 13.4 ± 0.6 N m in control). When expressed as a percentage of MVC, the recruitment threshold distribution was significantly shifted to the left (17.8 ± 1.2 %vs. 29.6 ± 1.4 % in control; P < 0.001) after training (Fig. 2B). The comparison of the mechanical properties of the motor units before and after training showed a significant shift (P < 0.001; Kolmogorov-Smirnov two-sample test) in force distribution towards larger values, and the mean twitch torque of the motor unit was respectively 32.2 ± 1.9 mN m (range, 0.7–170.9 mN m) and 57.3 ± 2.5 mN m (range, 1.9–197.8 mN m; Fig. 2C). A Gaussian distribution was observed for the time-to-peak torque (Fig. 2D) and no significant change (Student's t test for unpaired data) was found since the mean values of 41.3 ± 0.9 ms (range, 19–89 ms) and 41.9 ± 0.8 ms (range, 19–88 ms) were similar before and after training. During spike-triggered averaging, the motor unit discharge frequency did not differ significantly before (7.1 ± 0.1 Hz) and after (7.2 ± 0.1 Hz) training.
Figure 2. Distribution of single motor unit recruitment thresholds and contractile properties.
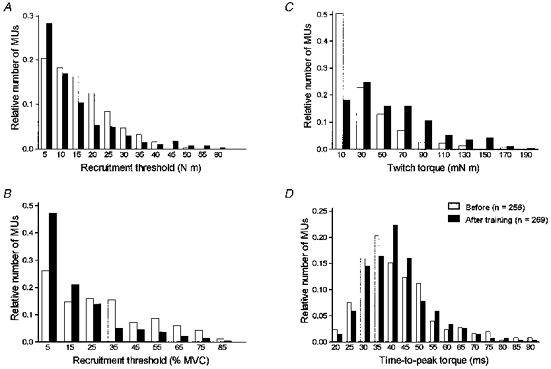
Histograms showing the distribution of single motor unit (MU) recruitment thresholds for all subjects expressed either as an absolute value (A) or as a percentage of MVC (B), twitch torques (C) or the time-to-peak torque (D) before and after training. Whereas no significant difference was observed before and after training for the time-to-peak torque distributions (Student's t test), the distributions of the recruitment thresholds and the twitch torques are significantly different (Kolmogorov-Smirnov two-sample test).
Figure 3 illustrates the relationship between the recruitment threshold, expressed as a percentage of MVC, and the twitch torque of the motor unit before and after training. This figure shows that after training, all motor units increased their force and suggests that the size principle (Henneman et al. 1965) was respected, since after dynamic training the motor units appeared to remain recruited according to their contractile force (size principle). Although the results are illustrated in one subject, they were similar in all of our subjects. In all of them, the linear correlation coefficients (r) of these relations ranged from 0.51 to 0.89 and from 0.55 to 0.83 before and after training, respectively. When tested for all subjects, the regression lines (slopes and intercepts) were significantly (P < 0.05) different after training, expressed as either absolute or relative values.
Figure 3. Comparison of motor unit twitch torque in one subject before and after training.
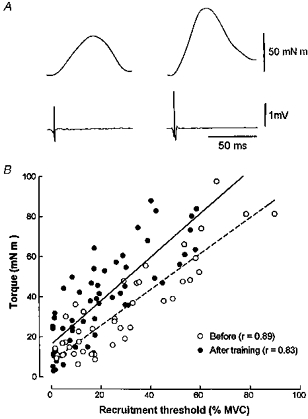
A, comparison of two motor units recruited in one subject at a similar threshold (58 vs. 56 % of MVC) before (left-hand traces) and after training (right-hand traces). The motor unit action potential and the corresponding mechanical contribution extracted by spike-triggered averaging (200 sweeps) are illustrated. B, the motor unit twitch torque in the same subject before (42 units) and after training (60 units) plotted as a function of the recruitment threshold (expressed as a percentage of MVC) during a ramp contraction. The linear regression lines are, respectively, y = 0.89x + 7.8 (r = 0.89; P < 0.001) and y = 1.07x+ 16.3 (r = 0.83; P < 0.001) before and after training. The ordinate intercepts of the relationships before and after training are significantly different (P < 0.05).
Adaptations of the motor unit discharge rate
The frequency of the instantaneous motor unit discharge at the onset of ballistic contractions was analysed in 475 motor units before, and 633 motor units after, dynamic training. This analysis was limited to the first three interspike intervals because (1) few motor units fired more than 4 times before training, (2) at that time the recording was not contaminated by possible electrode movements, and (3) the maximal rate of tension development was reached (see Discussion). Figure 4A illustrates the behaviour of a single motor unit in untrained muscle during the first four spikes in a ballistic contraction and shows an increase in the interspike intervals. The mean discharge rates for the first, second and third intervals were 125, 43 and 28 Hz, respectively. As illustrated in Fig. 4B, the decline in the instantaneous discharge frequency of the motor unit was smaller in the trained than in the untrained muscles. Among the motor units recorded before training, only 5.2 % showed the presence of two close spikes less than 5 ms apart (a ‘doublet’) at the onset of the EMG burst. In contrast, in the trained muscles, 32.7 % of the motor unit recorded population started to fire with interspike intervals between 2 and 5 ms (Fig. 5A). Interestingly enough, some motor units did not display this doublet at the onset but did so later during contraction (Fig. 5B).
Figure 4. Behaviour of single motor units during ballistic contractions.
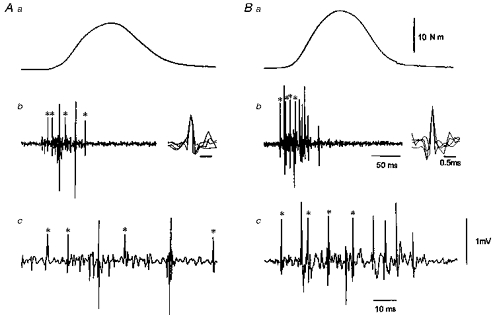
Behaviour of single motor units during ballistic contractions of similar torque levels (41 vs. 44 % of MVC) before (A) and after (B) dynamic training. The traces correspond to the mechanical force (a) and the intramuscular EMG plotted at slow (b) and fast (c) speeds. In A, a typical example of the firing pattern of a single motor unit in untrained muscle shows a short time lapse between the first two spikes followed by longer interspike periods. The first three interspike intervals are 8, 23 and 36 ms, respectively. B illustrates the usual motor unit behaviour in trained muscle, showing that the high onset of the instantaneous firing rate is maintained during the subsequent spikes. The first three interspike intervals are 11.8, 10 and 11 ms, respectively. The asterisks indicate the discharge of the same motor unit and their traces are superimposed with an extended display (b).
Figure 5. Examples of doublet discharges of single motor units during ballistic contraction after dynamic training.
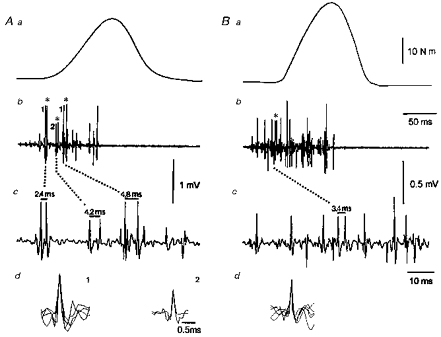
The traces correspond to the mechanical force (a), and the intramuscular EMG plotted at slow (b) and fast (c) speeds. A illustrates two different motor units which started to discharge with a doublet. Motor unit 1 fired two doublets (2.4 and 4.8 ms, respectively) while motor unit 2 discharged with a double spike of 4.2 ms interval. B illustrates a doublet which appeared later during the contraction. The motor unit illustrated showed three single firings at interspike intervals of, respectively, 14, 12.5 and 6 ms, followed by a 3.4 ms doublet. The asterisks indicate double discharges and their traces are superimposed with an extended display (d). Note that in Bd, the double discharge has been superimposed on the first 3 single spikes.
Figure 6 illustrates the distribution of the first three interspike intervals of the motor unit populations. It appears from these histograms that training induced a drastic shift (P < 0.001; Kolmogorov-Smirnov two-sample test) of the distribution to the left. This shift was larger for the third than for the second and first interspike intervals. The mean discharge frequency in the motor units during the first three intervals was, respectively, 98.0 ± 2.5 Hz (n = 474), 75.1 ± 3.0 Hz (n = 350) and 58.0 ± 1.9 Hz (n = 243) before training, and 182.1 ± 5.2 Hz (n = 609), 127.9 ± 5.8 Hz (n = 412) and 130.3 ± 7.7 Hz (n = 252) after training. If doublets of fewer than 5 ms are not considered, the mean discharge frequency of the motor units during the first three interspike intervals was, respectively, 84.6 ± 1.9, 64.8 ± 2.0 and 59.2 ± 2.6 Hz before training, and 90.2 ± 2.1, 89.4 ± 2.5 and 89.2 ± 3.3 Hz after training. Interestingly, the change in the discharge frequency was not related either to the motor unit time to peak or to the recruitment threshold.
Figure 6. Distribution of the first three interspike intervals during ballistic contractions.
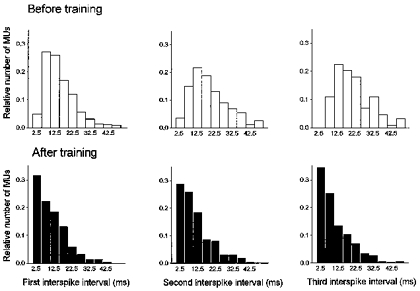
Histograms showing the distribution of the first three interspike intervals during ballistic contractions for the whole motor unit (MU) population studied both before and after training. The comparison of the distributions before and after training are significantly different (P < 0.001; Kolmogorov-Smirnov two-sample test) for the three interspike intervals.
DISCUSSION
The main findings of the present study are that in the tibialis anterior training by dynamic contractions induces (1) no change in the recruitment sequence of the motor units during ramp contractions, (2) an earlier muscle EMG activity, (3) an increase in the maximal firing frequency of the motor units, and (4) brief interspike intervals (doublets) in the EMG burst of ballistic contractions. In the absence of change in the control group, these results indicate that neural adaptations are likely to mediate some of the increases observed in the speed of contraction of the dorsiflexor muscles after dynamic training.
In our experimental conditions the observation that after training, the greater torque induced during an MVC was associated with an increase in EMG activity is consistent with previous studies (Hakkinen & Komi, 1983; Davies et al. 1985) and, in the absence of M wave modification, supports the viewpoint that neural adaptations mediate some of the increases in strength. These changes thus contribute to the muscle torque increment by adaptation mechanisms other than the increase in contractile force (Jones & Rutherford, 1987; Behm & Sale, 1993). A comparison of the mechanical twitch elicited by single supramaximal electrical stimulation and the corresponding M wave indicates that in the tibialis anterior the training programme did not affect the excitation-contraction coupling processes, which were found to be intensified in the adductor pollicis during repetitive activation (Desmedt & Hainaut, 1968) and dynamic training (Duchateau & Hainaut, 1984), since in these latter experiments the increase in the speed of tension development was recorded without any change in the M wave. In the present experiment there was no significant change in mechanical twitch and M wave responses. Such different behaviour patterns in the tibialis anterior and the adductor pollicis could be explained by functional differences in the two muscles. In the adductor pollicis most motor units are recruited at forces below 50 % of MVC, and the remaining force results from increased rate coding (Kukulka & Clamann, 1981). In the tibialis anterior, motor units are recruited up to 80–90 % of MVC. It could thus be expected that the rate coding ‘adaptability’ during training would be larger in the tibialis anterior and the voluntary torque and speed increases would be more closely related to neural changes than in the adductor pollicis. In the latter muscle, these increases were more closely related to changes in electro-mechanical processes than in the tibialis anterior since in this muscle there was no change in the time to peak of the motor units. Nonetheless, these points support the idea of training-induced changes in the neural drive to the muscles during training with ballistic contractions. We only experimented on the tibialis anterior, which contributes to about 60 % of the dorsiflexion torque (Andreassen & Arendt-Nielsen, 1987), but there is no evidence that other dorsiflexors (extensor digitorum longus and extensor hallucis longus) would adapt differently to such types of training since they have similar twitch time courses (Marsh et al. 1981; M. Van Cutsem, J. Duchateau and K. Hainaut, unpublished observations).
It is surprising that after training, motor units and MVC torques were increased without significant change in the muscle twitch, but such different behaviour has also been observed in adductor pollicis after training (Hainaut et al. 1981) and immobilization (Duchateau & Hainaut, 1990). Training reduced muscle twitch torque with increased motor units and tetanic torques, but after immobilization the muscle twitch was not changed although motor units and MVC torques were decreased. In the present experiments, the ‘paradox’ could be explained by a sampling bias towards motor units of higher recruitment threshold after training. In fact, the distribution was shifted in the opposite way and a similar range of recruitment threshold was observed before and after training (Fig. 2A and B). Another explanation would be that dynamic training induces increased motor unit synchronization, but there was no evidence for this when tested by surface EMG recordings (Milner-Brown et al. 1975) during spike-triggered averaging. In fact, when recording a single motor unit during spike-triggered averaging, the activity of other motor units decreases the muscle compliance and the repeated activation progressively potentiates the motor unit force signals (staircase potentiation; see Desmedt & Hainaut, 1968). In a muscle twitch elicited by maximal electrical stimulation, all motor units are activated concomitantly and only once at resting muscle compliance. Possible training-induced changes in resting muscle compliance and/or in staircase potentiation would complicate the relationship between torques recorded in single motor units and in a single twitch elicited by electrical stimulation. During MVC, the situation is different because all motor units are activated repetitively and the contractile elements have a longer time to stretch the elastic components. Thus MVC should reflect more closely motor units than muscle twitch torque changes and this was precisely our observation in the present experiments and in previous works (Hainaut et al. 1981; Duchateau & Hainaut, 1990).
The findings of an earlier onset of muscle EMG activity, of increased maximal firing frequency of motor units and that this high initial discharge frequency was kept up during a longer period are consistent with the observed increase in velocity and the decrease in the time to peak of the voluntary ballistic contractions (Fig. 1). The more segmented aspect of the EMG activity during these contractions could be explained by a tendency of motor units to pulse at a similar frequency after training (Fuglevand et al. 1993).
The observation of greater motor unit firing frequencies than those generally reported in the literature (Desmedt & Godaux, 1977; Bawa & Calencie, 1983) can be explained by the greater torques and speeds tested in the present experimental conditions. In addition, Marsden et al. (1971) reported similar high (150–200 Hz) motor unit discharge frequencies at the onset of sustained contractions, and the doublet responses of short interval (3.2–4.0 ms) during magnetic cortical stimulation have also been described by other authors (Gandevia & Rothwell, 1987; Day et al. 1989; Bawa & Lemon, 1993). These instantaneous high frequencies, which were also observed at the onset of motoneurone firing induced by fast ramp-current injection (Baldissera et al. 1987), correspond to the ‘secondary’ range of motoneurone discharges described by Kernell (1965). Before training, double motor unit discharges (cf. Denslow, 1948) less than 5 ms apart were observed infrequently during ballistic contractions in the tibialis anterior, and this is consistent with data from the literature indicating that these discharges are typical for motor units in fast muscles and are rarely present in the discharges of slow muscles (Kudina & Churikova, 1990). Our finding that, after ballistic training, double discharges were present in motor units with different recruitment thresholds indicates that this muscle adapted itself and exhibited the properties of faster muscles. Because the interspike interval ranged between 2 and 5 ms and doublets were also observed later in post-training ballistic contractions, these double firings appear to be ‘true’ doublets and not the short interspike intervals observed at the onset of ballistic movements (cf. Bawa & Calancie, 1983). The presence of these post-training doublets is consistent with the previous suggestion that motor units which do not fire doublets have lower maximal firing rates whereas those which do show higher rates (Bawa & Calancie, 1983).
Our results were recorded after training by voluntary contractions and do not support the view that doublet discharges originate in the distal part of the axon (Roth, 1994), but rather in the motoneurone. The observation that, after training, the presence of the doublets is not related to the recruitment threshold of the motor units is not in line with the idea that increased synaptic input plays an important role, because smaller motor units with larger input resistance should show a greater tendency towards doublet discharges. Motoneurone excitability changes, possibly related to delayed depolarization, thus seem to be the best explanation (see Bawa & Calancie, 1983; Kudina & Churikova, 1990; Kudina & Alexeeva, 1992). This possibility is also consistent with the increases in motoneurone excitability observed after training (Sale et al. 1983).
It is suggested that the functional significance of these doublets is a contribution to an increase in the maximal rate of tension development and/or the enforcement of submaximal rates, depending on the moment when they appear in a burst. In fact, the maximal rate of tension development has been reported as being present during the second (or third twitch) of a tetanus (see Stein & Parmiggiani, 1979). During experiments involving electrical stimulation, the speed at which tension developed was found to increase up to a frequency of 200 Hz in the first dorsal interosseus (Miller et al. 1981) and to 250 Hz in the adductor pollicis (J. Duchateau & K. Hainaut, unpublished observations). However, it has previously been reported that at very short intervals (roughly 1 ms) the muscle action potential (M wave) elicited by two pulses was identical to that elicited by a single pulse in the adductor pollicis (Desmedt & Hainaut, 1968). Thus, a tendency of the motoneurone to fire doublets at such short intervals during voluntary contractions would be ignored by the membrane because of its refractory state. Because the doublets observed in the present experiments included an interpulse interval greater than the refractory period (roughly 1.5 ms) ranging from 2 to 5 ms, the functional significance of doublets suggested above appears to be consistent with other experimental data quoted in this section.
It is concluded that the dynamic training of the dorsiflexor muscles induces muscular and neural adaptations in the tibialis anterior. There were no changes in the time-to-peak torque and the recruitment sequence of the motor units, but obviously a different type of behaviour occurs, since during ballistic contractions they were activated earlier in the contraction stage and their maximal firing rate was greater after training. Thus, neural adaptations in the tibialis anterior muscle are likely to have contributed to the increase in strength and speed of contraction after dynamic training.
Acknowledgments
The authors gratefully acknowledge Dr E. Godaux and Dr R. Enoka for critical reading of the paper and Miss A. Deisser for assistance in the preparation of the manuscript. This work was supported by NATO CRG no. 930261, the Fonds National de la Recherche Scientifique of Belgium and the Conseil de la Recherche of the Université Libre de Bruxelles.
References
- Andreassen B, Arendt-Nielsen L. Muscle fibre conduction velocity in motor units of the human anterior tibial muscle: a new size principle parameter. The Journal of Physiology. 1987;391:561–571. doi: 10.1113/jphysiol.1987.sp016756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Campadelli P, Piccinelli L. The dynamic response of cat gastrocnemius motor units investigated by ramp-current injection into their motoneurones. The Journal of Physiology. 1987;387:317–330. doi: 10.1113/jphysiol.1987.sp016575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P, Calancie B. Repetitive doublets in human flexor carpi radialis muscle. The Journal of Physiology. 1983;339:123–132. doi: 10.1113/jphysiol.1983.sp014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P, Lemon RN. Recruitment of motor units in response to transcranial magnetic stimulation in man. The Journal of Physiology. 1993;471:445–464. doi: 10.1113/jphysiol.1993.sp019909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm DG, Sale DG. Intended rather than actual movement velocity determines velocity-specific training response. Journal of Applied Physiology. 1993;74:359–368. doi: 10.1152/jappl.1993.74.1.359. 10.1063/1.354117. [DOI] [PubMed] [Google Scholar]
- Carolan B, Cafarelli E. Adaptations in coactivation after isometric resistance training. Journal of Applied Physiology. 1992;73:911–917. doi: 10.1152/jappl.1992.73.3.911. [DOI] [PubMed] [Google Scholar]
- Davies CTM, Dooley P, McDonagh MJN, White MJ. Adaptation of mechanical properties of muscle to high force training in man. The Journal of Physiology. 1985;365:277–284. doi: 10.1113/jphysiol.1985.sp015771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. The Journal of Physiology. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow JS. Double discharges in human motor units. Journal of Neurophysiology. 1948;11:209–215. doi: 10.1152/jn.1948.11.3.209. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. The Journal of Physiology. 1977;264:673–693. doi: 10.1113/jphysiol.1977.sp011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE, Hainaut K. Kinetics of myofilament activation in potentiated contraction: staircase phenomenon in human skeletal muscle. Nature. 1968;217:529–532. doi: 10.1038/217529a0. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Isometric or dynamic training: differential effects on mechanical properties of a human muscle. Journal of Applied Physiology. 1984;56:296–301. doi: 10.1152/jappl.1984.56.2.296. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. The Journal of Physiology. 1990;422:55–65. doi: 10.1113/jphysiol.1990.sp017972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM. Neural adaptations with chronic physical activity. Journal of Biomechanics. 1996;30:447–455. doi: 10.1016/s0021-9290(96)00170-4. 10.1016/S0021-9290(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Winter DA, Patla AB. Models of recruitment and rate coding organization in motor-unit pools. Journal of Neurophysiology. 1993;70:2470–2488. doi: 10.1152/jn.1993.70.6.2470. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Rothwell JC. Knowledge of motor commands and the recruitment of human motoneurons. Brain. 1987;110:1117–1130. doi: 10.1093/brain/110.5.1117. [DOI] [PubMed] [Google Scholar]
- Hainaut K, Duchateau J, Desmedt JE. Differential effects on slow and fast motor units of different programs of brief daily muscle training in man. In: Desmedt JE, editor. Progress in Clinical Neurophysiology. Vol. 9. Basel: Karger; 1981. pp. 241–249. [Google Scholar]
- Hakkinen K, Komi PV. Electromyographic changes during strength training and detraining. Medicine and Science in Sports and Exercise. 1983;15:455–460. [PubMed] [Google Scholar]
- Hakkinen K, Komi PV, Alen M. Effect of explosive type strength training on isometric force and relaxation-time, electromyographic and muscle fibre characteristics of leg extensor muscles. Acta Physiologica Scandinavica. 1985;125:587–600. doi: 10.1111/j.1748-1716.1985.tb07759.x. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. Journal of Neurophysiology. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Jones DA, Rutherford OM. Human muscle strength training: the effects of three different regimes and the nature of the resultant changes. The Journal of Physiology. 1987;391:1–11. doi: 10.1113/jphysiol.1987.sp016721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D. High repetitive firing of cat lumbosacral motoneurones stimulated by long-lasting injected currents. Acta Physiologica Scandinavica. 1965;65:84–86. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Kudina LP, Alexeeva NL. Repetitive doublets of human motoneurones: analysis of interspike intervals and recruitment pattern. Electroencephalography and Clinical Neurophysiology. 1992;85:243–247. doi: 10.1016/0168-5597(92)90112-o. 10.1016/0168-5597(92)90112-O. [DOI] [PubMed] [Google Scholar]
- Kudina LP, Churikova LI. Testing excitability of human motoneurones capable of firing double discharges. Electroencephalography and Clinical Neurophysiology. 1990;75:334–341. doi: 10.1016/0013-4694(90)90111-v. 10.1016/0013-4694(90)90111-V. [DOI] [PubMed] [Google Scholar]
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Research. 1981;219:45–55. doi: 10.1016/0006-8993(81)90266-3. 10.1016/0006-8993(81)90266-3. [DOI] [PubMed] [Google Scholar]
- McDonagh MJN, Davies CTM. Adaptive response of mammalian skeletal muscle to exercise with high loads. European Journal of Applied Physiology. 1984;52:139–155. doi: 10.1007/BF00433384. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Meadows JC, Merton PA. Isolated single motor units in human muscle and their rate of discharge during maximal voluntary effort. The Journal of Physiology. 1971;217:12–13P. [PubMed] [Google Scholar]
- Marsh E, Sale D, McComas AJ, Quinlan J. Influence of joint position on ankle dorsiflexion in humans. Journal of Applied Physiology. 1981;51:160–167. doi: 10.1152/jappl.1981.51.1.160. [DOI] [PubMed] [Google Scholar]
- Miller RG, Mirka A, Maxfield M. Rate of tension development in isometric contractions of a human hand muscle. Experimental Neurology. 1981;73:267–285. doi: 10.1016/0014-4886(81)90061-3. 10.1016/0014-4886(81)90061-3. [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Lee RG. Synchronization of human motor units: possible role of exercise and supraspinal reflex. Electroencephalography and Clinical Neurophysiology. 1975;38:245–254. doi: 10.1016/0013-4694(75)90245-x. 10.1016/0013-4694(75)90245-X. [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. The contractile properties of human motor units during voluntary isometric contractions. The Journal of Physiology. 1973;228:285–306. doi: 10.1113/jphysiol.1973.sp010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritani T. Neuromuscular adaptations during the acquisition of muscle strength, power and motor tasks. Journal of Biomechanics. 1993;26(suppl. 1):95–107. doi: 10.1016/0021-9290(93)90082-p. 10.1016/0021-9290(93)90082-P. [DOI] [PubMed] [Google Scholar]
- Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P. Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. European Journal of Applied Physiology. 1989;59:310–319. doi: 10.1007/BF02388334. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Miles T, Veale J. Effect of motor unit firing pattern on twitches obtained by spike-triggered averaging. Muscle and Nerve. 1989;12:556–567. doi: 10.1002/mus.880120706. [DOI] [PubMed] [Google Scholar]
- Roth G. Repetitive discharge due to self-ephaptic excitation of a motor unit. Electroencephalography and Clinical Neurophysiology. 1994;93:1–6. doi: 10.1016/0168-5597(94)90084-1. 10.1016/0168-5597(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Rutherford OM, Jones DA. The role of learning and coordination in strength training. European Journal of Applied Physiology. 1986;55:100–105. doi: 10.1007/BF00422902. [DOI] [PubMed] [Google Scholar]
- Sale DG. Neural adaptation to resistance training. Medicine and Science in Sports and Exercise. 1988;20:S135–145. doi: 10.1249/00005768-198810001-00009. [DOI] [PubMed] [Google Scholar]
- Sale DG, Macdougall JD, Upton ARM, McComas AJ. Effect of strength training upon motoneuron excitability in man. Medicine and Science in Sports and Exercise. 1983;15:57–62. [PubMed] [Google Scholar]
- Stein RB, Parmiggiani F. Optimal motor patterns for activating mammalian muscle. Brain Research. 1979;175:372–376. doi: 10.1016/0006-8993(79)91019-9. 10.1016/0006-8993(79)91019-9. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Sale DG. Ballistic movement: muscle activation and neuromuscular adaptation. Canadian Journal of Applied Physiology. 1994;19:363–378. doi: 10.1139/h94-030. [DOI] [PubMed] [Google Scholar]


