Abstract
Interstitial cells of Cajal (ICC) are pacemaker cells in the small bowel, and therefore this cell type must express the mechanism responsible for slow wave activity. Isolated ICC were cultured for 1–3 days from the murine small intestine and identified with c-Kit-like immunoreactivity (c-Kit-LI).
Electrical recordings were obtained from cultured ICC with the whole-cell patch clamp technique. ICC were rhythmically active, producing regular slow wave depolarizations with waveforms and properties similar to slow waves in intact tissues.
Spontaneous activity of c-Kit-LI cells was inhibited by reduced extracellular Na+, gadolinium, and reduced extracellular Ca2+. The activity was not affected by nisoldipine. Voltage clamp studies showed rhythmic inward currents that were probably responsible for the slow wave activity. The current-voltage relationship showed that the spontaneous currents reversed at about +17 mV. These observations are consistent with the involvement of a non-selective cation current in the generation of slow waves, but do not rule out contributions from other conductances or transporters.
A Ba2+-sensitive inwardly rectifying K+ current in c-Kit-LI cells that may be involved in slow wave repolarization and maintenance of a negative potential between slow waves was also found. Similar pharmacology was observed in studies of intact murine intestinal muscles.
Cultured ICC may be a useful model for studying the properties and pharmacology of some of the ionic conductances involved in spontaneous rhythmicity in the gastrointestinal tract.
Phasic gastrointestinal muscles are characterized by continuous, rhythmic electrical activity known as slow waves (e.g. Szurszewski, 1987). These events consist of a rapid upstroke depolarization, partial repolarization, and then a sustained, ‘plateau’ potential that can last several seconds. Slow waves occur at frequencies ranging from a few cycles per minute to more than 30 cycles min−1, depending upon the species. These events have been referred to as myogenic because they occur spontaneously without input from the enteric nervous system (Szurszewski, 1987). Recent studies have shown that the term ‘myogenic’ is misleading because slow waves are generated by interstitial cells of Cajal (ICC) and propagate into electrically coupled smooth muscle cells (Langton et al. 1989; Ward et al. 1994; Huizinga et al. 1995; Torihashi et al. 1995). The mouse small intestine has become a model for studies of slow wave origin because of the facility with which ICC populations can be manipulated in mutant animals (see Sanders, 1996, for review). Despite attempts for many years to understand the mechanisms for slow wave activity in the small bowel, the ionic mechanisms for these events still remain unclear.
In previous studies we isolated pacemaker ICC from the submucosal border of the circular muscle layer in the canine colon (Langton et al. 1989). Patch clamp studies of these cells revealed a unique population of ionic conductances that might contribute to rhythmicity (Lee & Sanders, 1993), and a mechanism for slow waves in the colon was proposed (see Sanders, 1992). Although this mechanism seemed to fit the canine colon, and others have applied similar ideas to the small intestine (Malysz et al. 1995; Cayabyab et al. 1996), certain features of slow wave activity in the small bowel and stomach do not agree with the mechanism proposed for colonic slow waves. For example, low Ca2+ inhibits slow waves in the small bowel (Malysz et al. 1995; Cayabyab et al. 1996), but the sensitivity of slow waves in this organ is much less than reported for the colon (E. Flynn & K. Sanders, unpublished results). These observations suggest that other ionic mechanisms may be responsible for slow waves in the small intestine.
In the present study we have returned to the approach of studying ICC isolated from the musculature to investigate the pharmacology and ionic dependence of slow waves of the small intestine. In contrast to ICC from the canine colon, ICC from the small bowel are difficult to identify in cell suspensions. Therefore, we isolated and cultured small intestinal ICC. These cells grow into well-defined networks within 1–3 days that are morphologically similar to the myenteric plexus network of ICC in the intact small intestine. After formation of ICC networks, we identified the cells as ICC with c-Kit immunoreactivity (see Torihashi et al. 1995) and recorded electrical activity. We have compared the spontaneous rhythmicity of cultured ICC with the activity of intact muscle strips from the small intestine. There are significant similarities in the activities of the two preparations, suggesting that the events retained in culture provide a new model to study the mechanism and pharmacology of slow waves.
METHODS
BALB/C mice (9–15 days old) of either sex were anaesthetized with chloroform and killed by cervical dislocation. The small intestine, from 1 cm below the pyloric ring to the caecum, was removed, opened along the myenteric border, and the lumenal contents washed with Krebs-Ringer bicarbonate solution (KRB; see ‘Intracellular recordings from intact muscles’). Tissues were pinned to the base of a Sylgard dish and the mucosa removed by sharp dissection.
Culture of ICC
Small strips of intestinal muscle were equilibrated in Ca2+-free Hanks' solution consisting of (mm): 125 NaCl, 5.36 KCl, 15.5 NaHCO3, 0.336 Na2HPO4, 0.44 KH2PO4, 10 glucose, 2.9 sucrose and 11 Hepes adjusted to pH 7.2 with NaOH, for 30 min. The buffer was replaced with an enzyme solution containing: collagenase (Worthington Type II), 1.3 mg ml−1; bovine serum albumin (Sigma), 2 mg ml−1; trypsin inhibitor (Sigma), 2 mg ml−1; and ATP, 0.27 mg ml−1. The tissues were placed in a 37°C water bath for 25 min without agitation. After three to four washes with Ca2+-free Hanks’ solution to remove the enzyme, the tissues were triturated through a series of three blunt pipettes of decreasing tip diameter. The resulting cell suspension was plated onto murine collagen-coated (2.5 μg ml−1, Falcon/BD) sterile glass coverslips, in 35 mm culture dishes. The cells were allowed to settle for 10 min before adding culture medium. The culture medium used was SMGM (Clonetics Corp., San Diego, CA, USA) supplemented with 2 % antibiotic/antimycotic (Gibco) and murine stem cell factor (SCF, 5 ng ml−1; Sigma). The cells were incubated at 37°C in a 95 % O2-5 % CO2 incubator. The medium was changed after 24 h to SMGM containing SCF without antibiotic/antimycotic, and subsequently the medium was changed every other day until the cells were used for electrophysiological experiments.
Labelling of cultured ICC by c-Kit immunofluorescence
Immunohistochemical identification of ICC was performed on cells cultured for 2–3 days. Cells grown on glass coverslips were immersed in acetone and phosphate-buffered saline (PBS; 1 : 1) for 2 min. Following fixation, cells were washed in PBS (4 × 15 min) prior to preincubation in goat non-immune serum for 1 h (10 % in PBS). The cells were then incubated with a monoclonal antibody against c-Kit protein (ACK2, Nishikawa et al. 1991; Gibco, 5 μg ml−1 in PBS) at 4°C overnight. Immunoreactivity was detected with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (goat anti-rat, 1 : 100, 1 h, room temperature; Vector Laboratories Inc., Burlingame, CA, USA). Controls were prepared in a similar manner, omitting ACK2 from the incubation solution. Labelled cells were examined using a Leitz Diaplan Microscope with fluorescence and phase-contrast microscopy. Images were digitized with Metamorph software version 3 (Universal Imaging, West Chester, PA, USA) and images were processed and printed with Photoshop (Adobe, Mountain View, CA, USA) and a dye sublimation printer (Tektronix 440). The structures and appearance of ICC and ICC networks were distinct from other cell types present in the cultures. Therefore, once the appearance of these cells was verified by ACK2, it was possible to identify the cells with phase-contrast microscopy (see Fig. 1A and C).
Figure 1. Phase contrast and fluorescence micrographs of cultured ICC (2 days).
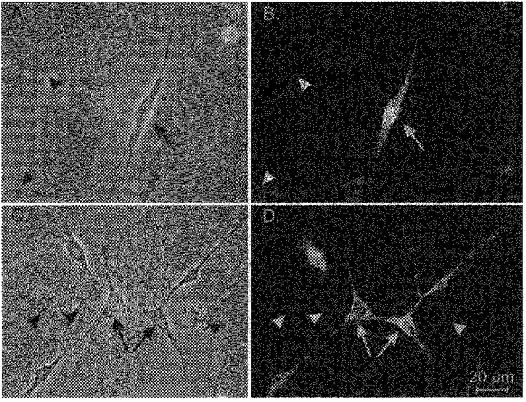
A, single, multi-processed cell (arrow) that expressed strong c-Kit-like immunoreactivity (c-Kit-LI; B, arrow). Occasionally, we observed apparent connections between cells with c-Kit-LI and non-c-Kit positive cells (see phase image of cell noted with arrowhead in A, and lack of immunoreactivity in B). C, the networks formed by cells with c-Kit-LI. Multiple cells of similar morphology (arrows) formed distinctive network structures via long thin processes. D shows that these cells possessed c-Kit-LI. The cells that formed networks had similar properties and morphology to ICC in situ. Other cells within the field of view did not possess c-Kit-LI (arrowheads).
Electrophysiological experiments
Patch clamp experiments
We used the whole-cell patch clamp technique to record membrane potential or currents from cultured ICC. Potentials or currents were amplified with standard patch clamp amplifiers (List EPC-7, or Axopatch 1A, Axon Instruments) and digitized with a 12-bit A/D converter (TL-1, DMA interface, Axon Instruments). Data were stored on videotapes or digitized on-line using pCLAMP software (version 5.5.1 or 6.03, Axon Instruments). Data were filtered at 1 kHz using an 8-pole Bessel filter. Cultured cells were bathed in a solution containing (mm): 5 KCl, 135 NaCl, 2 CaCl2, 10 glucose, 1.2 MgCl2, and 10 Hepes, adjusted to pH 7.4 with Tris. NaCl was replaced with various concentrations of N-methyl-D-glucamine (NMDG). CaCl2 was also replaced with MnCl2. The pipette solution contained (mm): 110 potassium gluconate, 20 KCl, 5 MgCl2, 2.7 K2ATP, 0.1 Na2GTP, 2.5 creatine phosphate (disodium salt), 5 Hepes and 0.1 EGTA, adjusted to pH 7.2 with Tris. Nisoldipine, cyclopiazonic acid, BaCl2, gadolinium chloride, and DIDS (all from Sigma) were added to the bath at the concentrations stated.
Results were analysed using pCLAMP 6 (Axon Instruments) and GraphPad Prizm (version 2.01, San Diego, CA, USA) software.
Intracellular recordings from intact muscles
Sheets of intestinal muscle (10 mm × 10 mm) were pinned to the floor of an electrophysiological chamber with the mucosal aspect of the muscle facing upwards. The chamber was constantly perfused with oxygenated KRB of the following composition (mm): 118.5 NaCl, 4.5 KCl, 1.2 MgCl2, 23.8 NaHCO3, 1.2 KH2PO4, 11.0 dextrose and 2.4 CaCl2, pH 7.4 at 37 ± 0.5°C. The pH of the KRB was 7.3–7.4 when bubbled with 95 % O2-5 % CO2. After pinning, the muscles were left to equilibrate for at least 1 h before experiments were begun. For Na+ replacement experiments, the KRB was changed to a Hepes-buffered solution to facilitate a broader range of Na+ exchange. The Hepes solution was similar to that previously described (see ‘Patch clamp experiments’). NaCl was exchanged with equimolar concentrations of NMDG.
Impalement of cells was carried out using glass microelectrodes with resistances of 30–90 MΩ. Transmembrane potential was recorded with a standard electrometer (Intra 767, World Precision Instruments, Sarasota, FL, USA). Data were recorded on digital tape (Vetter, Robersburg, PA, USA) and hard copies were made by replaying the tapes through a polygraph (Gould RS 3200). Nifedipine (1 μm) was included in the perfusion solution to reduce contraction and facilitate impalement of cells. Nifedipine had no resolvable effect on slow wave activity.
All electrophysiological data are expressed as means ±s.e.m. Differences in the data were evaluated by Student's t test. P values less than 0.05 were taken as a statistically significant difference. The n values reported in the text refer to the number of cells used in patch clamp experiments or the number of tissues used in intracellular electrophysiological experiments.
RESULTS
Identification of interstitial cells in culture
Cultures of cells (1–3 days) dispersed from the muscularis externa of 10-day-old mice contained single cells and networks of cells that had gross morphological properties similar to ICC in situ, including fusiform cell bodies, large, prominent nuclei with little perinuclear cytoplasm, and multiple, thin processes extending from the nuclear region that were often interconnected with processes of neighbouring cells. Single cells and networks with this morphology were immunopositive for c-Kit protein (Fig. 1).
Spontaneous rhythmicity and current-voltage relation of cultured ICC
Recordings were made from ICC with the patch clamp technique as soon as the network-like structures shown in Fig. 1 were observed in cultures (1–3 days). At this time, spontaneous rhythmicity was routinely recorded from cultured ICC under current clamp conditions. Recordings were made from cells within networks and from single cells that had morphologies similar to the cells that were immunopositive for c-Kit. The single cells had resting potentials of −53 ± 1.8 mV (n = 14), capacitances of 29 ± 4 pF (n = 27; includes cells that were studied with voltage and current clamp), and input resistances of 250 ± 30 MΩ (n = 8). The isolated cells displayed small, spontaneous membrane potential oscillations in current clamp mode (2–6 mV in amplitude, 5.2 ± 0.9 cycles min−1). Under voltage clamp, the isolated cells displayed small current oscillations averaging 27 ± 6 pA and 6.1 ± 0.6 cycles min−1 at a holding potential of −60 mV (n = 3).
ICC within networks had a more robust electrical rhythmicity, and tissue-like spontaneous slow waves were recorded from these cells. The maximum diastolic potential (MDP) of the ICC in networks was −56.9 ± 1.3 mV. The spontaneous slow waves were 23 ± 1.6 mV in amplitude, and these events occurred at a frequency of 6.6 ± 0.3 cycles min−1 (n = 27) at room temperature (20–22°C). The slow waves were temperature dependent, and when temperature was increased to 29°C, MDP hyperpolarized to −61.2 ± 1.0 mV, and slow wave amplitude and frequency increased to 29 ± 0.8 mV and 13.5 ± 0.6 cycles min−1, respectively (n = 11; Fig. 2A). At higher temperatures, seals between the patch pipettes and the cells were difficult to maintain and sustained recording became impossible. Therefore, we were unable to characterize the temperature dependence of these events at or near physiological temperatures.
Figure 2. Spontaneous membrane potential and current oscillations from ICC in murine small intestine.
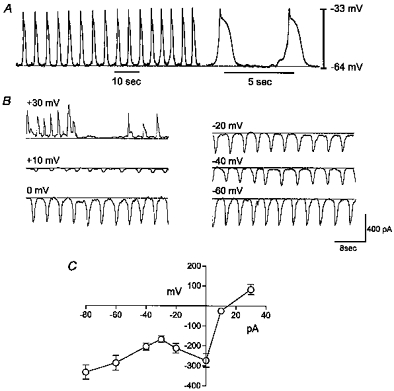
A, typical slow wave oscillations in membrane potential recorded from ICC under current clamp. B, spontaneous inward currents recorded under voltage clamp. Currents were obtained at holding potentials from −60 to +30 mV. C, summary current-voltage relationship for 4 cells.
Voltage clamp experiments were performed on small networks consisting of a few cells. These networks had average input resistances of 23 ± 7 MΩ (n = 5). Under voltage clamp the cells displayed rhythmic inward currents averaging −286 ± 37 pA at a holding potential of −60 mV (approximately the resting potential of cells in intact muscle strips; see Ward et al. 1994). The frequency of the spontaneous currents at the holding potential was 11 ± 0.6 cycles min−1. The spontaneous inward currents occurred at approximately the same frequency as the spontaneous voltage oscillations observed at 29°C in these cells (i.e. 13.5 ± 0.6 cycles min−1). Thus, it is likely that the rhythmic currents are the underlying source of the depolarizations of spontaneous slow waves.
Cells were stepped to potentials ranging from −80 to +30 mV to test the effects of membrane potential on the rhythmic currents. The amplitude of the spontaneous inward currents was −334 ± 35 pA at −80 mV and −168 ± 16 pA at −30 mV. The latter was the calculated chloride equilibrium potential (ECl), suggesting the inward current at this potential was not due to the movement of chloride ions. At potentials between −30 and 0 mV, the amplitude of the current increased such that with steps to 0 mV, the spontaneous currents averaged −273 ± 34 pA at 0 mV. At positive potentials, the current decreased in amplitude and reversed at approximately +17 mV. In contrast to the amplitude, the frequency of the spontaneous currents appeared to be independent of voltage (e.g. the frequency was 10.7 ± 0.8 cycles min−1 at 0 mV (n = 4), which was not different from the frequency at −60 mV). Figure 2B shows a representative example of the spontaneous currents and the effects of holding potential on the amplitude of the current. The current-voltage relationship (Fig. 2C) shows an ‘N-shaped’ function.
The effect of Na+ replacement on spontaneous rhythmicity
The current-voltage relation for the spontaneous currents suggests that a non-selective cation conductance could be involved in the generation of spontaneous slow wave activity. Therefore, we tested the effects of replacing external Na+ with equimolar NMDG on spontaneous slow waves. In these experiments MDP and slow wave amplitude and frequency were −59 ± 3 mV, 28 ± 2 mV and 14 ± 1 cycles min−1, respectively, under control conditions (n = 6, Fig. 3A). After replacement of Na+, the spontaneous oscillations went through a period of waxing and waning, and depolarization (to a mean of −41 ± 3 mV), and slow waves were reduced to small, irregular fluctuations in membrane potential (Fig. 3B). In three preparations, all spontaneous oscillations stopped. Recovery of spontaneous oscillations was slow upon restoration of extracellular Na+, requiring up to 25 min for return of control-like events (e.g. Fig. 3C).
Figure 3. Effects of Na+ replacement on spontaneous slow wave activity of ICC.
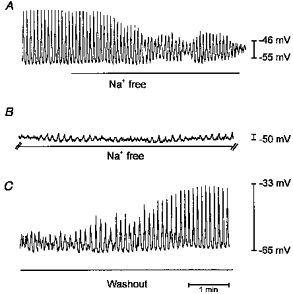
A, normal membrane potential oscillations before and after switching to a Na+-free solution (indicated by bar). After a brief period of waxing and waning, slow waves were greatly reduced in amplitude to events of only a couple of millivolts (B). A break of 5 min exists between the end of the record in A and the beginning of B. C begins 15 min after the start of washout of Na+-free conditions, and shows restoration of normal frequency and amplitude of slow waves.
Pharmacology of spontaneous rhythmicity
Previous studies have shown that dihydropyridines do not block spontaneous slow waves in small intestinal muscles (Ward et al. 1994; Malysz et al. 1995; Cayabyab et al. 1996). We examined the effect of dihydropyridines on cultured ICC (Fig. 4A). In these experiments, MDP and slow wave amplitude and frequency were −52 ± 3 mV, 15 ± 4 mV and 8.6 ± 1.6 cycles min−1, respectively, under control conditions (n = 4). After treatment with nisoldipine (1 μm), there was no difference in slow wave parameters from control values during 30 min of observation. Replacement of Ca2+ with Mn2+ depolarized MDP to −39 ± 3 mV (from a control in this group of cells of −54 ± 2 mV, n = 4) and blocked spontaneous slow waves (Fig. 4B). Treatment of cells with cyclopiazonic acid (CPA, 50 μm), a Ca2+-ATPase inhibitor, hyperpolarized MDP from a control level of −56 ± 3 to −59 ± 3 mV, and increased slow wave amplitude from 15 ± 5 to 22 ± 5 mV and frequency from 5.3 ± 0.7 to 8.9 ± 0.9 cycles min−1 (n = 4; Fig. 4C). The non-selective cation channel blocker gadolinium (Gd3+, 50 μm) induced depolarization (from −55 ± 2 mV in control to −48 ± 3 mV in the presence of Gd3+). Slow wave frequency decreased, and eventually spontaneous rhythmicity stopped (Fig. 4D). DIDS (50 μm) significantly decreased spontaneous slow wave amplitude (from 22 ± 3 mV in control to 10 ± 3 mV in the presence of DIDS) and frequency (by 35 ± 3 %, P < 0.001; n = 4). DIDS also induced a slight hyperpolarization of 3 mV but this did not reach statistical significance. DIDS did not block spontaneous rhythmicity in any preparation (Fig. 4E).
Figure 4. Pharmacology of slow wave oscillations in cultured ICC.
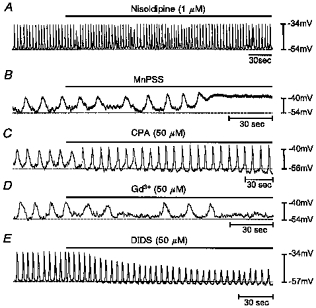
A, lack of effect of nisoldipine (1 μm) on slow waves. B, inhibition of slow waves when cells were exposed to a bathing solution in which Ca2+ was replaced by Mn2+ (in physiological saline solution, MnPSS). C, effects of cyclopiazonic acid (CPA). CPA increased frequency and produced a small hyperpolarization in MDP. D, gadolinium (Gd3+) blocked slow wave activity. E, DIDS reduced the amplitude of slow waves, caused a small hyperpolarization in MDP, but did not block spontaneous rhythmicity.
Evidence for a Ba2+-sensitive inwardly rectifying K+ conductance in ICC
Further tests investigated whether an inward rectifier K+ current participates in the regulation of the MDP in ICC. In these experiments MDP and slow wave amplitude and frequency were 59 ± 2 mV, 30 ± 2 mV and 13.5 ± 1.3 cycles min−1, respectively, under control conditions. Addition of Ba2+ (10–100 μm) to the bathing solution caused membrane depolarization (Fig. 5A). At a mid-range concentration of 50 μm Ba2+ MDP was depolarized to −51 ± 1 mV and slow wave amplitude was reduced to 23 ± 1 mV (n = 4). The maximum depolarizations during slow waves and the frequencies of these events were not affected by Ba2+. These data suggested that ICC may express inwardly rectifying K+ channels, and this hypothesis was studied with voltage clamp experiments. Cells were held at −80 mV, stepped to −100 mV and then ramped to +20 mV (Fig. 5B). This protocol was repeated fifteen times and current responses were averaged. The current response showed a marked inwardly rectifying current at negative potentials (Fig. 5C). Addition of Ba2+ (10 μm) reduced the magnitude of the current in response to the ramp protocol. The responses after Ba2+ were subtracted from the control responses, and the Ba2+-sensitive difference current is shown in Fig. 5D. These data show substantial Ba2+-sensitive outward current positive to the potassium equilibrium potential (EK), suggesting that a Ba2+-sensitive inward rectifier current contributes to the membrane potential of these cells
Figure 5. Evidence that a Ba2+-sensitive inward rectifier current participates in the regulation of membrane potential in cultured ICC.
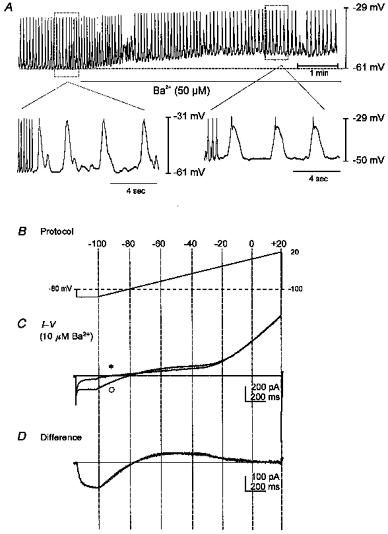
A, effect of adding Ba2+ (50 μm) to a spontaneously active network of ICC under current clamp conditions. Ba2+ caused depolarization of the MDP. B, ramp clamp protocol utilized to show inward rectification in isolated ICC. C, current responses of an isolated cell to voltage ramps before (○) and in the presence (*) of Ba2+ (10 μm). D, difference current obtained by subtracting the response during the exposure to Ba2+ from the control response. The difference current demonstrates the presence of a Ba2+-sensitive inwardly rectifying current.
Comparison of spontaneous rhythmicity in cultured ICC with slow waves in intestinal muscles
We performed parallel experiments on intact sheets of intestinal muscles and recorded slow wave activity with intracelluar microelectrodes to compare the characteristics of slow waves in situ with the events recorded from cultured ICC. Cells were impaled and spontaneous slow wave activity was recorded. Under control conditions MDP was −66 ± 2.4 mV, and the frequency, amplitude and duration of slow waves were 35.9 ± 1 cycles min−1, 29 ± 2.6 mV and 1.2 ± 0.06 s, respectively. Nifedipine had no effect on slow wave activity as previously noted (Ward et al. 1994; Malysz et al. 1995). We tested the effects of reduced extracellular Na+, since this was shown to attenuate and eventually halt slow wave activity in cultured ICC. To allow reduction of extracellular Na+ to low levels, a Hepes-buffered solution was used. Replacement of the KRB with a Hepes solution containing normal extracellular ionic concentrations did not significantly affect electrical activity (i.e. MDP was −62 ± 2.3 mV, and slow wave frequency was 34 ± 1.8 cycles min−1, amplitude was 28 ± 2.2 mV, and duration was 1.3 ± 0.06 s after addition of Hepes-buffered solution; n = 11). Extracellular Na+ was replaced by NMDG, and the effects of Na+ replacement were tested over the range of 50 mm to nominally 0 mm Na+. Reducing Na+ caused a concentration-dependent reduction in slow wave amplitude and frequency (Fig. 6), consistent with the effects of reduced Na+ on slow waves recorded from cultured ICC. The effects of reducing external Na+ on slow waves are summarized in Table 1.
Figure 6. Effects of reduced Na+ on slow waves from intact small intestinal muscles.
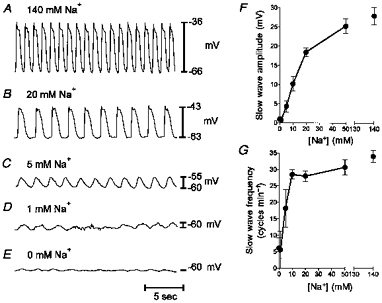
A-E, similar to the cultured ICC, there was a reduction in slow wave amplitude when extracellular Na+ was reduced from 140 to 0 mm. Distinct oscillations in membrane potential were still present in 1 mm Na+. F, summary of the relationship between extracellular [Na+] and slow wave amplitude. G, relationship between extracellular [Na+] and slow wave frequency. Data in F and G are means ± s.e.m. (n = 5).
Table 1.
Effects of extracellular [Na+] on electrical slow waves in intact intestinal muscles
| [Na+] (mm) | MDP (mV) | Amplitude (mV) | Frequency (mV) | Duration (s) |
|---|---|---|---|---|
| 140 | −62 ± 2.3 | 28 ± 2.2 | 34 ± 1.8 | 1.3 ± 0.06 |
| 50 | −62 ± 3.0 | 25 ± 1.9 | 31 ± 2.4 | 1.5 ± 0.15 |
| 20 | −63 ± 2.0 | 19 ± 1.1 | 28 ± 1.6 | 1.8 ± 0.17 |
| 10 | −63 ± 1.9 | 10 ± 2.0 | 29 ± 1.4 | 1.8 ± 0.18 |
| 5 | −61 ± 1.1 | 4 ± 1.5 | 18 ± 5.8 | 1.3 ± 0.4 |
| 1 | −62 ± 2.1 | 1 ± 0.8 | 6 ± 5.6 | 0.4 ± 0.4 |
| 0 | −59 ± 1.6 | 1 ± 0.5 | 6 ± 3.1 | 0.5 ± 0.2 |
Reduction of Na+ to very low levels (< 1 mm) did not block slow waves in some preparations. In three experiments the reduction in Na+ (to 10 mm) was combined with a simultaneous reduction in extracellular Ca+ (to 0.1 mm). Under these conditions, resting membrane potential depolarized from −64 ± 4.5 to −49 ± 4.6 mV, and there was a reduction in slow wave amplitude from 27 ± 2.6 to 4.3 ± 0.3 mV and frequency from 33 ± 4.7 to 14.6 ± 1.7 cycles min−1. Thus, large reductions in both of the cations that could provide inward current via a non-selective cation conductance reduced, but failed to block, slow waves.
We also tested the effects of Gd3+ and DIDS on slow waves recorded from intact muscles. Gd3+ (1–100 μm) did not significantly affect MDP or slow wave activity (i.e. MDP was −71 ± 3.3 mV in control vs.−69 ± 3.8 mV in the presence of Gd3+; slow waves were 34 ± 1.3 mV in amplitude, 1.5 ± 0.2 s in duration, and occurred at a frequency of 31.8 ± 1.5 cycles min−1 in control conditions (37°C) vs. 26 ± 6.6 mV, 1.1 ± 0.3 s, and 24 ± 6.1 cycles min−1 in the presence of Gd3+(100 μm); P > 0.05; n = 5). Exposure to Gd3+ was continued for up to 3 h in some experiments, and there was no time-dependent increase in effects on slow waves observed. DIDS (300 μm) was similarly ineffective in decreasing slow waves (n = 2; data not shown).
We tested the presence and function of a Ba2+-sensitive conductance by exposing intact muscle strips to Ba2+ (1–100 μm). Ba2+ (100 μm) caused depolarization of MDP from −70 ± 1.4 to −60 ± 1.9 mV (P < 0.01), and reduced slow wave amplitude from 31.6 ± 2.2 to 23.8 ± 2.2 mV (P < 0.05), increased duration from 1.4 ± 0.17 to 1.7 ± 0.19 s, and reduced frequency from 40 ± 4.4 to 36 ± 4.1 cycles min−1 (n = 5; P < 0.05; data not shown).
Previous studies of electrical slow waves in feline small intestine showed that ouabain depolarized membrane potential and inhibited slow waves (Connor et al. 1974). In the murine small intestine, ouabain (10−5 M) had no effect on MDP or slow waves (i.e. MDP was −69.3 ± 2.3 before, and −71.3 ± 1.2 mV after ouabain, slow wave amplitude was 29 ± 3 and 30 ± 2.6 mV, duration was 1.75 ± 0.25 and 1.75 ± 0.25 s, and frequency was 31 ± 2.6 and 30.3 ± 2.2 cycles min−1; n = 3; P > 0.05; data not shown).
DISCUSSION
Previous studies have investigated the ionic basis for slow waves in colonic muscles, and determined that a low voltage, Ca2+ current in interstitial cells of Cajal (ICC) may be important for pacemaker activity (Huizinga et al. 1991; Ward et al. 1992a, b; Lee & Sanders, 1993). In the mammalian small intestine and stomach slow waves appear to result from different mechanisms, and experiments on tissues have led to suggestions that slow waves result from voltage-dependent mechanisms (El-Sharkawy & Daniel, 1975) and processes relatively independent of membrane potential, e.g. an oscillating Na+ pump (Connor et al. 1974), Na+-Ca2+ exchange (Ohba et al. 1977), and other ion exchange mechanisms (see Tomita, 1981). More recent studies on intact muscles of mouse and dog have suggested the involvement of Ca2+ channels in slow wave activity in the small intestine (Malysz et al. 1995; Cayabyab et al. 1996), but the type of conductance proposed in these studies was described only as ‘non-L-type’ Ca2+ channels. Slow waves of the small bowel are generated by ICC in the myenteric plexus region (IC-MY; see Ward et al. 1994; Huizinga et al. 1995); however, direct studies of pacemaker ICC from the small bowel have not been reported.
In the present study we recorded from enzymatically dispersed and cultured ICC from the murine small intestine. It was necessary to use cultured ICC because freshly dispersed ICC lose c-Kit-like immunoreactivity during enzymatic treatment. This makes it difficult to identify ICC in suspensions of freshly dispersed cells. After enzymatic treatment, c-Kit-like immunoreactivity was recovered within 24 h after putting the cells into culture. The cultured cells developed into networks that were strikingly similar to IC-MY networks in vivo (see Burns et al. 1997), and the cells were identified as ICC by c-Kit immunofluorescence (see Torihashi et al. 1995). Electrical recordings showed that IC-MY were spontaneously active, and the ‘slow waves’ recorded were similar in magnitude and waveform to slow waves recorded from intact muscles. Slow waves recorded from cultured cells were temperature dependent as observed in studies of intact guinea-pig stomach muscles (Ohba et al. 1975). The temperature dependence may explain why the frequency of slow waves in cultured cells was less than the frequency of slow waves in intact muscles, because cultured cells were studied below 30°C. Under voltage clamp conditions, spontaneous inward currents were generated that approximated the frequency of the slow waves recorded from the cells in current clamp mode. The spontaneous current oscillations were probably the cause of spontaneous slow waves. Some of the ionic mechanisms retained by cultured cells may be equivalent to the mechanisms that initiate slow waves in intact muscles. Use of isolated ICC facilitates studies of basic slow wave mechanisms without contaminating conductances from the smooth muscle cells that are coupled to ICC in situ.
Stepping membrane potential of cultured ICC to various potentials resulted in an ‘N-shaped’ whole-cell current- voltage curve for the spontaneous currents, which reversed at about +17 mV. The frequency of the spontaneous currents was not significantly affected by voltage over the physiological range of potentials (i.e. −60 to 0 mV), suggesting that the process responsible for ‘gating’ the inward currents was voltage independent. Based on the current-voltage relation for the transient inward current, at least three kinds of conductance could contribute to slow wave depolarizations: non-selective cation conductance, Cl− conductance or Ca2+ conductance. It is also possible that an unknown electrogenic ion exchange mechanism could generate inward current (see Tomita, 1981). The major ions contributing to inward currents through a non-selective cation conductance at the resting potential of cultured ICC would most probably be Na+ and Ca2+. We tested the effects of replacement of external Na+ with an impermeant ion. Slow waves of cultured ICC and intact small intestinal muscle strips were not affected by reducing external Na+ from 135 to 20 mm; however, reduction of external Na+ to lower levels reduced spontaneous slow waves. Although the amplitude and frequency of slow waves were reduced in low Na+, in a few cases small oscillations were still observed in solutions nominally free of Na+. These events also persisted under conditions of a combined reduction in extracellular Na+ (10 mm) and Ca2+ (0.1 mm). A non-selective cationic conductance may contribute to the generation of spontaneous slow wave oscillations; however, it does not appear that such a conductance can fully explain these events. These results also do not rule out possible contributions from an ion exchange transport mechanism.
The effects of low Na+ were accompanied by depolarization of basal resting potential, and a period of waxing and waning of the amplitude of the slow waves. In both preparations bath perfusion was used to change solutions. Therefore, we are unable to show the exact time course of the effects of Na+ replacement. However, the reduction in slow waves resulting from Na+ replacement was very slow, requiring up to 15 min to develop fully and 25 min to reverse. The slow reduction in slow waves caused by Na+ replacement could have been due to the washout of an excluded volume (e.g. caveolae) or intracellular Na+. These experiments suggest that a conductance or transport mechanism utilizing Na+ as the charge carrier or dependent upon Na+ contributes to spontaneous slow waves; however, other conductances may also participate.
Previous studies have also explored the effects of reduced extracellular Na+ on slow waves of the small intestine and stomach. However, variable results and conclusions have been reported. Liu et al. (1969) found that slow waves of the cat jejunum were blocked by Na+ replacement with Li+ or Tris, and El-Sharkawy & Daniel (1975) observed a similar result in studies of rabbit jejunal muscles. In both of these studies the effects of Na+ replacement were slow to develop and recover, suggesting that the observations in our study represent a fairly general phenomenon. In both studies, ouabain, an inhibitor of electrogenic Na+ pumping, depolarized membrane potential and blocked slow waves. One group concluded that slow waves were due to oscillations in the Na+ pump (Liu et al. 1969), and the other concluded that a Na+ conductance was responsible (El-Sharkawy & Daniel, 1975). In studies of the guinea-pig stomach, reduced Na+ (137 to 14 mm) prolonged slow waves, and total replacement caused membrane depolarization and deterioration of the slow waves (Ohba et al. 1977). Other evidence led these authors to discount the involvement of the Na+ pump and to propose that another transport mechanism might be involved (see Tomita, 1981, for review). We found that slow waves were unaffected by ouabain in the mouse, so it is unlikely that an oscillating Na+ pump is responsible. The involvement of a non-selective cation conductance is suggested by the current-voltage relationship for the spontaneous inward currents, reduction in slow wave amplitude in response to reduced Na+, and the effects of Gd3+; however, the slow onset and recovery of activity during replacement of Na+ suggests that the mechanism is more complicated than the periodic gating of an ionic conductance.
We also evaluated the role of Ca2+ currents in the generation of slow waves of cultured ICC and intact muscles. Dihydropyridines did not block slow waves in either preparation. Replacement of external Ca2+ with Mn2+ induced depolarization of cultured ICC and blockade of slow waves. A previous study of cultured ICC also found that Ca2+-free solution blocked rhythmic inward current transients (Tokutomi et al. 1995). Previous studies have evaluated the effects of reduced Ca+ on slow waves of intact murine small intestinal muscles (e.g. Malysz et al. 1995). These studies showed that exposure of intact muscles to Ca2+-free solution (without divalent replacement) blocked slow waves (Malysz et al. 1995). The effects of reduced Ca2+ were also relatively slow to develop and could have been due to depletion of intracellular Ca2+ or loss of Ca2+ from stores. We tested CPA, an inhibitor of the Ca2+-ATPase, on cultured ICC and found this compound increased the frequency of slow waves. These data suggest that intracellular Ca2+ or release of Ca2+ from stores may regulate the mechanism that initiates slow waves. It is possible that the mechanisms responsible for either the inward or the outward currents in slow waves could be Ca2+ dependent. At the present time we cannot exclude the possible involvement of a low threshold Ca2+ conductance in the initial slow wave depolarization as observed in colonic ICC (see Lee & Sanders, 1993).
Another candidate responsible for spontaneous inward currents in ICC could be a chloride conductance. Others have reported that cultured ICC express a Cl− current that was blocked by SITS (see Tokutomi et al. 1995), and suggested that Cl− efflux could be responsible for the spontaneous inward currents. Under conditions in which cationic currents were blocked, these authors found that the spontaneous inward currents reversed close to ECl. In the present study the amplitude of whole-cell spontaneous currents was significant at ECl, and the reversal potentials for the spontaneous currents were significantly positive to ECl. The differences in observations from these two studies may be due to the differences in experimental conditions. Spontaneous inward currents in cultured ICC could be due to multiple conductance and/or transport mechanisms. It should be noted that DIDS reduced slow wave amplitude in cultured ICC in the present study, but this agent had no effect on slow waves in intact muscles. This difference in the pharmacology of slow waves recorded from cultured ICC and from intact muscles may indicate that Cl− channel expression increases in cultured cells.
Outward currents responsible for repolarization of slow waves are also an important factor in rhythmicity. To maintain a resting membrane potential near −60 mV, ICC may require an inwardly rectifying K+ current. Experiments in the present study provide evidence for a Ba2+-sensitive inward rectifier K+ conductance in cultured ICC. Under voltage clamp, a significant Ba2+-sensitive current was apparent at negative potentials. This current strongly rectified at potentials positive to EK. Under current clamp conditions, Ba2+ depolarized the membrane potentials of cultured ICC, and the effects of Ba2+ were also observed in intact muscles. These data suggest that Ba2+-sensitive inward rectifiers could provide a portion of the current necessary for the repolarization of slow waves. Other conductances, such as Ca2+-dependent K+ channels or delayed rectifiers, could also contribute to the repolarization phase of the slow wave cycle. Experiments to test this hypothesis are being performed.
In conclusion, previous experiments have indicated that slow waves of the small bowel are generated by ICC. Cultured ICC from the small intestine retain phasic electrical activity that is similar in many ways to the electrical slow waves recorded from intact muscle strips. Use of cultured ICC may provide a means of characterizing the ionic apparatus responsible for electrical rhythmicity. Unfortunately, the cultured cells may not preserve the rhythmic mechanism with complete fidelity. We noted differences in the responsiveness of cultured ICC and intact muscles to Gd3+ and Cl− channel-blocking drugs, suggesting that the nature of the conductance or transporters responsible for the inward current could change somewhat in culture. In contrast to previous studies on intact murine small intestine in which a non-L-type Ca2+ current was suggested as the pacemaker current (Malysz et al. 1995), our studies suggest that a non-selective cation conductance or a transport mechanism utilizing Na+ and Ca2+ as charge carriers may be an important source of inward current in the generation of slow waves. The sensitivity of slow waves to decreased Ca2+ may indicate involvement of a Ca2+ conductance, conduction of Na+ and Ca2+ by a non-selective cation conductance, contributions from an electrogenic ion exchange mechanism requiring Ca2+, or facilitation of a conductance or ion exchange mechanism by Ca2+. Additional inward current could also be provided by a Cl− conductance. Contributions from this conductance are not obvious in intact muscles, but expression of this conductance may increase in cultured cells. Many additional experiments will be required to characterize systematically the conductances and ion exchange mechanisms present in ICC and to compare the pharmacology of identified transport mechanisms with the actions of drugs on slow waves of intact muscles. Questions about the factors that regulate the gating of the conductance or activity of the transporter responsible for the spontaneous inward current remain to be investigated.
Note added in proof
After this paper was submitted, a paper by L. Thomsen, T. L. Robinson, J. C. Lee, L. A. Farraway, M. J. Hughes, D. W. Andrews & J. D. Huizinga (Nature Medicine4, 848–851 (1998)) appeared describing spontaneous inward currents in cultured ICC from the murine small intestine. These authors suggested that the pacemaker current had the properties of a non-selective cation current. Such a conductance may be present in ICC of the small bowel; however, the present study argues that non-selective cation currents alone cannot explain the spontaneous slow waves in ICC and intact small intestinal muscles.
Acknowledgments
This project was supported by National Institute of Diabetes and Digestive and Kidney Diseases with DK41315 and DK40569. We are grateful to Mary-Frances Brennan for technical assistance in electrophysiological experiments and to Julia Bayguinov and Nancy Horowitz for preparation of the cultured ICC.
References
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell and Tissue Research. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- Cayabyab FS, deBruin H, Jimenez M, Daniel EE. Ca2+ role in myogenic and neurogenic activities of canine ileum circular muscle. American Journal of Physiology. 1996;271:G1053–1066. doi: 10.1152/ajpgi.1996.271.6.G1053. [DOI] [PubMed] [Google Scholar]
- Connor JA, Prosser CL, Weems WA. A study of pacemaker activity in intestinal smooth muscle. The Journal of Physiology. 1974;240:671–701. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy TY, Daniel EE. Ionic mechanisms of intestinal electrical control activity. American Journal of Physiology. 1975;229:1287–1298. doi: 10.1152/ajplegacy.1975.229.5.1287. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Farraway L, Den hertog A. Generation of slow-wave-type action potentials in canine colon smooth muscle involves a non-L-type Ca2+ conductance. The Journal of Physiology. 1991;442:15–29. doi: 10.1113/jphysiol.1991.sp018779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Langton P, Ward SM, Carl A, Norell M, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proceedings of the National Academy of Sciences of the USA. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Sanders KM. Comparison of ionic currents from interstitial cells and smooth muscle cells of canine colon. The Journal of Physiology. 1993;460:135–152. doi: 10.1113/jphysiol.1993.sp019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Prosser CL, Job DD. Ionic dependence of slow waves and spikes in intestinal muscle. American Journal of Physiology. 1969;217:1542–1547. doi: 10.1152/ajplegacy.1969.217.5.1542. [DOI] [PubMed] [Google Scholar]
- Malysz J, Richardson D, Farraway L, Christen MO, Huizinga JD. Generation of slow wave type action potentials in the mouse small intestine involves a non-L-type calcium channel. Canadian Journal of Physiology and Pharmacology. 1995;73:1502–1511. doi: 10.1139/y95-208. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi S-I, Kunisada T, Era T, Sakakura T, Nishikawa S-I. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit dependency during melanocyte development. EMBO Journal. 1991;10:2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M, Sakamoto Y, Tomita T. The slow wave in the circular muscle of the guinea-pig stomach. The Journal of Physiology. 1975;253:505–516. doi: 10.1113/jphysiol.1975.sp011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M, Sakamoto Y, Tomita T. Effects of sodium, potassium and calcium ions on the slow wave in the circular muscle of the guinea-pig stomach. The Journal of Physiology. 1977;267:167–180. doi: 10.1113/jphysiol.1977.sp011806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Rave Press; 1987. p. 1435. [Google Scholar]
- Tokutomi N, Maeda H, Tokutomi Y, Sato D, Sugita M, Nishikawa S, Nishikawa S, Nakao J, Imamura T, Nishi K. Rhythmic Cl− current and physiological roles of the intestinal c-kit-positive cells. Pflügers Archiv. 1995;431:169–177. doi: 10.1007/BF00410188. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrical activity (spikes and slow waves) in gastrointestinal smooth muscle. In: Bulbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle: An Assessment of Current Knowledge. Austin, TX, USA: University of Texas Press; 1981. pp. 127–156. [Google Scholar]
- Torihashi S, Ward SM, Nishikawa S-I, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell and Tissue Research. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in the murine intestine. The Journal of Physiology. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Dependence of electrical slow waves of canine colonic smooth muscle on calcium current. The Journal of Physiology. 1992a;455:307–319. doi: 10.1113/jphysiol.1992.sp019303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Upstroke component of electrical slow waves in canine colonic smooth muscle due to nifedipine-resistant Ca2+ current. The Journal of Physiology. 1992b;455:321–337. doi: 10.1113/jphysiol.1992.sp019304. [DOI] [PMC free article] [PubMed] [Google Scholar]


