Abstract
In chloralose-anaesthetized, artificially ventilated dogs, the splenic pedicle was tied and the carotid sinuses were vascularly isolated and perfused at controlled pressures. In Series 1 experiments, the hepatosplanchnic circulation was perfused through the abdominal aorta with a tie on the aorta separating it from the caudal circulation, which was perfused through the femoral arteries. The two circulations were drained from cannulae in the inferior vena cava and the femoral veins, with a tie on the inferior vena cava separating the two. In Series 2, the splanchnic circulation drained from the portal vein. In both series, inflows and outflows were measured and integrated to derive volume changes. Capacitance responses were assessed during constant flow, and capacitance plus passive responses were obtained during constant pressure perfusion.
In Series 1, an increase in carotid sinus pressure (from 8 to 26 kPa) during constant flow and constant pressure perfusion increased hepatosplanchnic volume by 2.5 and 5.7 ml (kg body weight)−1, respectively. The volume of the subdiaphragmatic circulation did not increase during constant flow, but during constant pressure it increased by 2.0 ml (kg body weight)−1.
In Series 2, increasing carotid pressure during constant flow and constant pressure increased the volume of the splanchnic circulation by 0.5 and 4.2 ml (kg body weight)−1, respectively.
These results confirm that carotid baroreceptor stimulation causes larger volume changes during constant pressure perfusion than during constant flow perfusion. Also, the active capacitance change in the splanchnic circulation is small in relation to the passive response. We propose that in dogs (following splenic ligation), the major active capacitance control is from the liver. However, large passive changes in splanchnic volume occur due to changes in flow.
Vascular capacitance refers to the volume of blood contained within a vascular bed at a particular vascular distending pressure (Rothe, 1983; Hainsworth, 1986). It relates to the quantity of blood that is dependent on the degree of vasoconstriction of the capacitance vessels. This is in contrast to the volume held passively, which is dependent on the vascular distending pressure and the compliance of the blood vessels. A change in activity in vasomotor nerves may influence regional blood volumes by both mechanisms. The vessels containing the blood, mainly veins, actively constrict and thereby reduce their capacitance. In addition, constriction of resistance vessels reduces blood flow, thereby reducing the transmural pressure of the compliant blood vessels, so that their volume decreases further.
In dogs, the abdominal circulation contains a large proportion (about 25 %) of the total blood volume (Delorme et al. 1951; Johnstone, 1956; Chien, 1963) and is particularly important in capacitance control. Indeed, it has been shown to account for most, if not all, of the active capacitance regulation of the entire body (Shoukas & Sagawa, 1971; Karim & Hainsworth, 1976; Hainsworth & Karim, 1976; Brunner et al. 1981). Other regions, in particular muscle and skin, do not seem to make any contribution at all (Lesh & Rothe, 1969; Hainsworth et al. 1983). The abdominal circulation is also highly compliant, contributing about half of the total vascular compliance of the body (Drees & Rothe, 1974; Larochelle & Ogilvie, 1976; Karim & Hainsworth, 1976). Increases in efferent sympathetic activity result in decreases in abdominal blood volume both by direct constriction of capacitance vessels and by a reduction in their distending pressure caused by a decrease in blood flow. We have recently reported that sympathetic stimulation during constant flow perfusion (when volume changes are assumed to be due only to constriction of capacitance vessels) causes a reduction in volume of about 5 ml (kg body weight)−1. During constant pressure perfusion, however, the same stimulus decreases the volume by twice as much, indicating that the passive effect was of comparable importance to the active response (Noble et al. 1997). The active volume changes are due partly to changes in the capacitance of the spleen (Opdyke & Ward, 1973; Donald & Aarhus, 1974; Noble et al. 1997) and the liver (Bennett et al. 1982; Noble et al. 1998). However, the splanchnic circulation (the gastrointestinal circulation and associated viscera which drain through the portal vein) is also likely to make a contribution. There have been reports that either direct or reflex stimulation of sympathetic nerves reduces intestinal weight or volume (Donald & Aarhus, 1974; Rothe et al. 1978), but these reports did not distinguish between the active response and volume changes secondary to changes in blood flow, so the role of the splanchnic circulation in capacitance control remains unclear.
The aim of the present study, therefore, was to evaluate the contribution of the splanchnic vascular bed to the volume changes in the abdominal circulation and to assess both the active capacitance responses and the volume changes occurring secondary to the reflex changes in flow. We first determined the responses in the entire abdominal circulation (with the spleen ligated), partitioned into the hepatosplanchnic circulation and the extra-splanchnic circulation (mainly renal and musculocutaneous). Then in a further series of experiments, we determined responses in the vascularly isolated and perfused splanchnic circulation.
METHODS
Dogs weighing 14–20 kg were given a sedative dose of sodium pentobarbitone (0.1 mg kg−1) and anaesthetized with α-chloralose (100 mg kg−1; Vickers Laboratories, Leeds, UK) dissolved in isotonic saline and infused through a catheter, inserted under local anaesthesia through a saphenous vein so that its tip lay in the inferior vena cava. Anaesthesia was maintained by a continuous infusion of α-chloralose at 0.5–1.0 mg kg−1 min−1 and the level of anaesthesia was assessed regularly by pedal withdrawal, blink reflex and reflex movement in response to a table tap. At the end of the experiment animals were killed by exanguination.
The neck was opened, the trachea cannulated and the lungs ventilated with positive pressure by a Starling ‘Ideal’ pump using oxygen-enriched air (inspired O2 fraction, FI,O2, 0.4). The carotid sinuses were vascularly isolated by ligating all branches of the common carotid arteries except the lingual arteries, which were subsequently cannulated to drain the region.
The chest was widely opened in the left side, and the aorta was mobilized by tying and dividing the lowest four pairs of intercostal arteries. The inferior vena cava was also mobilized. A small incision was made in the diaphragm, and the spleen was pulled through, ligated at its pedicle and returned to the abdomen. The diaphragm was then sutured closed.
The abdomen was opened through a longitudinal left-side incision. A thread was placed around the aorta, between the origins of the cranial mesenteric and right renal arteries. A snare tube was inserted through a small abdominal stab wound and the aortic snare was passed through it. The caudal mesenteric artery was identified and ligated.
Two series of experiments were carried out. In one, the circulations to the hepatosplanchnic and the remainder of the subdiaphragmatic regions were separated, and in the other the intestinal circulation alone was isolated and perfused.
Series 1: separation of hepatosplanchnic and remainder of subdiaphragmatic (‘extra-splanchnic’) circulations
In addition to the common procedures, a second snare was placed around the inferior vena cava immediately below the liver.
The perfusion circuit (Fig. 1) was filled with a mixture of mammalian Ringer solution, dextran (MW, 90 000) in 5 % dextrose solution, and washed blood cells obtained from a previous experiment. The animal was given heparin (500 i.u. kg−1) and the circuit was connected. A cannula was inserted into the cardiac end of the thoracic aorta to convey blood to a pressurized reservoir. Blood from this reservoir was pumped (505U pump, Watson Marlow Ltd, Falmouth, UK) into a second reservoir maintained at a controlled pressure, from which blood perfused the regions of the carotid bifurcations. Blood draining from the main arterial reservoir also perfused the hepatosplanchnic region (Watson Marlow 604U pump) through a cannula inserted into the aorta immediately above the diaphragm. Perfusion could be either at constant pressure, by pumping the blood through a pressurized reservoir at a rate automatically controlled to maintain a constant level, or at constant flow by bypassing the reservoir (clips placed at A and removed at B in Fig. 1). Blood from the hepatosplanchnic circulation passed through a cannula inserted into the inferior vena cava immediately above the diaphragm and into an open venous reservoir from which it was returned by pump (604U) to the external jugular veins.
Figure 1. Diagram of perfusion circuit used for Series 1 experiments.
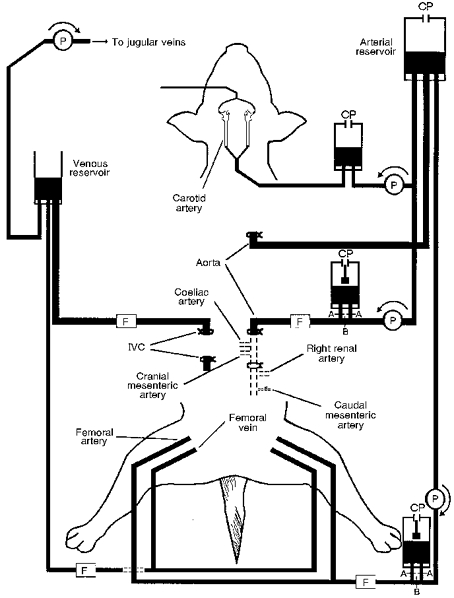
A cannula in the cardiac end of the thoracic aorta conveys blood to the arterial reservoir for distribution to various regions. Blood is pumped into a reservoir maintained at controlled pressures and leading to cannulae in both carotid arteries. Blood is pumped into the abdominal aorta immediately above the diaphragm. With clips in position A (B removed) perfusion is at constant flow, and removing the clips at A and clipping at B causes blood to pass through the constant pressure reservoir. A similar system perfuses the central ends of the femoral arteries. Blood drains from the inferior vena cava just above the liver and from the femoral veins into an open reservoir to be returned to the animal through the external jugular veins. All three constant pressure perfusion reservoirs and the venous reservoir incorporate electronic switches which control the pumps to keep the blood levels constant. Note the ties on the aorta below the cranial mesenteric artery, on the caudal mesenteric artery, and on the inferior vena cava immediately below the liver. Abbreviations: P, pump; CP, constant pressure; F, flow transducer; IVC, inferior vena cava.
A similar system was used to perfuse the remainder of the subdiaphragmatic circulation (extra-splanchnic circulation) at either constant pressure or constant flow. The blood perfused the central ends of both femoral arteries and was drained into the venous reservoir through cannulae in the central ends of the femoral veins.
After connecting the perfusion circuit, the snares already placed around the aorta and vena cava were tightened to separate the splanchnic and extra-splanchnic circulations. The regions were initially perfused at constant flows and the pumps were set to restore the pressures near to the value of arterial pressure before connecting the circuit. Adequate isolation was confirmed by stopping each pump in turn and observing that the pressure fell to below 4 kPa and venous outflow fell to less than 10 % of the previous value.
Series 2: vascular isolation of intestinal circulation
In these experiments the abdomen was opened in the mid-line. A snare was placed around the aorta and the caudal mesenteric artery was tied as in Series 1. Threads were also placed around the hepatic artery and the gastroduodenal artery (anastomosis between hepatic and cranial mesenteric arteries). The portal vein was dissected free and a catheter, for recording portal venous pressure, was inserted into a splenic venous branch.
After heparinization of the animal, the perfusion circuit was filled as described above and connected to the animal. The aorta was cannulated immediately above the diaphragm, the femoral arteries were cannulated centrally, and both regions were perfused as described above. The peripheral end of the portal vein was cannulated, and blood drained into the venous reservoir to be returned to the external jugular veins.
After completion of the cannulations the hepatic and gastroduodenal arteries were tied and the snare tightened around the aorta. The pumps perfusing the intestinal and extra-splanchnic regions were set and the effectiveness of the intestinal vascular isolation was confirmed as described above. The pumps were then set so that the arterial pressures were equal and similar to those before cannulation.
Measurements
Blood pressure was determined using Gould-Statham (P23ID) transducers connected to catheters attached to the carotid perfusion cannula, thoracic aorta and the splanchnic and extra-splanchnic perfusion cannulae. In Series 1 experiments, venous pressures were recorded from the inferior vena cava and the femoral vein, and in Series 2 from a splenic vein. Perfusion inflows and venous outflows were recorded using cannulating ultrasonic flow meters (Transonic Systems Inc., NY, USA) for splanchnic flows and electromagnetic flow meters (Narco Bio-systems, Houston, TX, USA) for extra-splanchnic flows. Pressures and flows were recorded on a direct writing electrostatic recorder (Model ES1000, Gould, Ballainvilliers, France) and on VHS tape (V-store, Racal Recorders, Southampton, UK). Data were analysed using a real-time data acquisition unit (Fastdaq, Lectromed, Letchworth, UK).
Arterial blood gases and pH were measured frequently throughout the experiment using a blood gas analyser (model 1610, Instrumentation Laboratory, Lexington, MA, USA). Pa,O2 was maintained above 15 kPa, Pa,CO2 between 5 and 6 kPa, and pH between 7.35 and 7.45, by adjustments of FI,O2, respiratory pump stroke, and infusion of molar sodium bicarbonate as required. The temperature of the animal was recorded by a thermistor probe (Yellow Springs Instruments, Yellow Springs, OH, USA) in the oesophagus. This was maintained at 37–39°C by heat exchangers in the circuit and by heaters under the table. The haematocrit of the blood after connecting the circuit was 21.5 ± 1.8 %.
Experimental procedure
Similar protocols were used for the two series of experiments. After connection of the circuit and adjustment of the constant flow perfusion rates, the animal was allowed to stabilize for approximately 30 min, during which time blood gases and temperature were determined and corrected as necessary.
Carotid sinus pressure was then increased to 24 kPa and held at that level for 2 min before decreasing it in a single step to 8 kPa. This test was carried out initially during constant flow perfusion of both regions. Perfusions were changed to constant pressure, and after about 5 min, to allow pressures to stabilize, another test of an increase and a decrease in carotid pressure was carried out. After this, another test was performed during constant flow perfusion. This entire sequence was carried out twice in each animal and all responses were averaged.
In some animals, carotid pressure was held constant and the perfusion flows were changed by amounts similar to those observed during the reflex responses to changes in carotid pressure at constant pressure perfusion.
Calculation of volume changes
During constant flow perfusion, volume changes were calculated by integration of the change in outflow from the time when baroreceptor pressure was changed to when flow, having transiently changed, had returned to baseline. During constant pressure perfusion, volume changes were calculated from the differences between inflow and outflow. Initially a control period was taken before stimulation and any difference of less than 10 % between the inflows and the outflows was assumed to represent an unchanged volume. The change in this subtracted signal was then integrated starting from the time of the change in baroreceptor pressure and continued until a new steady level had been achieved. Responses were calculated to both increases and decreases in carotid pressure. These were averaged and, for clarity, reported as the changes to carotid pressure increases. All results are given as means ±s.e.m., and significance of responses was assessed by Student's t test for paired data.
RESULTS
Series 1: hepatosplanchnic and extra-splanchnic circulations
Experiments were carried out on eight dogs.
Constant flow perfusion
In these experiments hepatosplanchnic and extra-splanchnic flows were held constant at 923 ± 131 and 260 ± 39 ml min−1, respectively. At the low carotid sinus pressure (8.2 ± 0.45 kPa) hepatosplanchnic and extra-splanchnic perfusion pressures were 16.9 ± 0.82 and 16.4 ± 1.13 kPa. Following an increase in carotid pressure to 26.4 ± 1.89 kPa, there were decreases in hepatosplanchnic and extra-splanchnic perfusion pressures to 12.0 ± 0.88 kPa (−29 ± 3.7 %, P < 0.05) and 10.2 ± 1.12 kPa (−35 ± 7.1 %; P < 0.05), respectively. These pressures returned to levels not significantly different from the original within 1 min of decreasing carotid pressure. Following the increase in carotid pressure there was a transient decrease in outflow from the hepatosplanchnic region, which gradually then returned to its original level (e.g. Fig. 2). The regional volume changes, determined from the changes in the two flows to both increases and decreases in carotid pressure, and expressed as the response to carotid pressure increase, were +2.51 ± 0.18 ml (kg body weight)−1 (P < 0.005) from the hepatosplanchnic region but only −0.04 ± 0.14 ml (kg body weight)−1 (P > 0.5) from the extra-splanchnic region.
Figure 2. Original traces from one animal showing responses to a step increase and decrease in carotid sinus pressure during constant flow perfusion of the hepatosplanchnic region and remainder of caudal circulation (extra-splanchnic circulation).
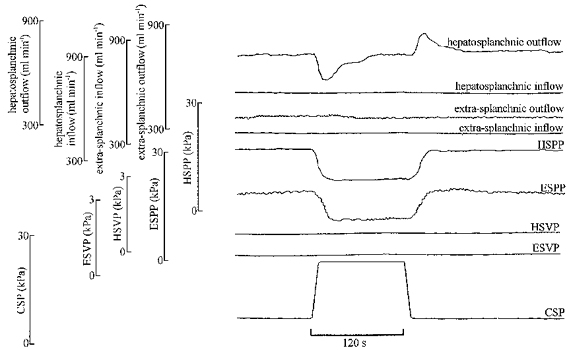
Abbreviations: HSPP, hepatosplanchnic perfusion pressure; ESPP, extra-splanchnic perfusion pressure; HSVP, hepatosplanchnic venous pressure (inferior vena cava at the level of the liver); ESVP, extra-splanchnic venous pressure (inferior vena cava caudal to the liver); CSP, carotid sinus pressure. Note that the increase in carotid pressure transiently decreased hepatosplanchnic outflow, denoting a reduction in regional blood volume. However, there was almost no effect on extra-splanchnic outflow. There were decreases in both perfusion pressures.
Throughout these tests inferior vena caval pressure and femoral venous pressure remained unchanged at 1.01 ± 0.34 and 1.11 ± 0.29 kPa.
Constant pressure perfusion
In these experiments hepatosplanchnic and extra-splanchnic perfusion pressures were held constant at 17.2 ± 0.59 and 17.8 ± 0.72 kPa, respectively. At the low carotid pressure (8.2 ± 0.24 kPa) hepatosplanchnic and extra-splanchnic flows were 882 ± 162 and 249 ± 26 ml min−1. Following an increase in carotid pressure to 27.8 ± 2.13 kPa, the flows increased to 1274 ± 258 and 408 ± 56 ml min−1, indicating changes in vascular resistance of 49 ± 16 % (P < 0.05) and 66 ± 26 % (P < 0.05). After decreasing carotid pressure these flows returned close to their original levels. As may be seen from careful inspection of Fig. 3, for both the increases and decreases in flow, the changes in the inflows preceded the outflow changes, denoting changes in the regional blood volumes. The regional volume changes, averaged from both the increases and decreases in carotid pressure and expressed as the response to carotid pressure increase, were +5.67 ± 0.63 and +2.04 ± 0.31 ml (kg body weight)−1 (P < 0.05 for both).
Figure 3. Original traces from the same animal as in Fig. 2 but during constant pressure perfusion.
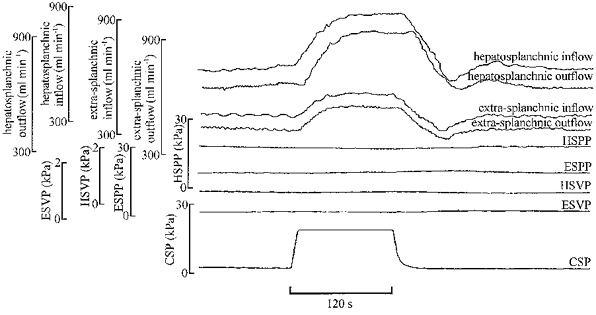
Abbreviations as in Fig. 2. The increase in carotid pressure resulted in increases in inflow to and outflow from both regions. Careful inspection of the traces reveals that the changes in inflow preceded those of outflow, and this implies that the volume in both regions increased.
Active and passive volume changes
Responses during constant flow perfusion were assumed to be active, and during constant pressure perfusion, both active and passive. The passive components were estimated from the differences between the responses at constant pressure and those at constant flow. These are given in Fig. 4, which shows that for the hepatosplanchnic circulation the active and estimated passive contributions were not significantly different. For the extra-splanchnic circulation, however, there was no significant active response at constant flow and the response during constant pressure perfusion was therefore assumed to be a solely passive effect.
Figure 4. Summary diagram showing volume changes in hepatosplanchnic and extra-splanchnic regions in response to changes in carotid pressure.
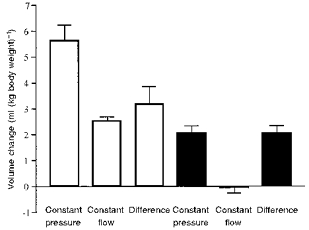
Values are means ± s.e.m. from 8 dogs and represent averages of increases in volumes to increases in carotid pressure, and decreases in volume to decreases in carotid pressure. Note that, in the hepatosplanchnic region (□), the active contribution (during constant flow) represents about half the total response (during constant pressure). In the extra-splanchnic circulation (▪), there was no active contribution and the entire response was estimated to be passive.
Series 2: splanchnic circulation
Experiments were carried out on six dogs.
Constant flow perfusion
In these experiments, splanchnic blood flow was held constant at 493 ± 47 ml min−1. At the low carotid pressure (8.2 ± 0.33 kPa) the splanchnic perfusion pressure was 18.4 ± 1.5 kPa. Following an increase in carotid pressure to 27.3 ± 1.56 kPa, splanchnic perfusion pressure decreased and there was a small transient reduction in venous outflow. On restoring carotid pressure to its previous low levels, splanchnic perfusion pressure increased and there was a small transient increase in outflow. Overall, increasing carotid pressure resulted in a decrease in splanchnic perfusion pressure from 16.7 ± 0.85 to 11.8 ± 0.77 kPa (−27 ± 5.9 %; P < 0.05) and increased the splanchnic blood volume by 0.53 ± 0.10 ml (kg body weight)−1 (P < 0.05).
Constant pressure perfusion
In these experiments splanchnic perfusion pressure was controlled at 16.3 ± 0.5 kPa. Carotid pressure was changed as in the constant flow experiments. Increasing carotid pressure increased both splanchnic inflow and outflow with the lag in the outflow denoting an increase in splanchnic blood volume. These values returned to initial levels following the decrease in carotid pressure. Overall, increasing carotid pressure resulted in an increase in splanchnic flow from 408 ± 57 to 569 ± 106 ml min−1. This represented a significant (P < 0.05) decrease in resistance of −34 ± 6.05 %, which was similar to that obtained during constant flow perfusion. The regional blood volume increased by +4.17 ± 0.33 ml (kg body weight)−1 (P < 0.05). This change in volume was significantly greater than that obtained during constant flow perfusion (P < 0.05). The difference between the changes in volume during constant pressure and constant flow perfusion, i.e. the estimated passive decrease in volume, was 3.64 ± 0.33 ml (kg body weight)−1.
Responses to changes in blood flow
Splanchnic flow was changed by altering the pump speed from 387 ± 38 to 581 ± 59 ml min−1. The flows were selected in each dog to correspond to the change induced by changing carotid pressure. This resulted in an increase in intestinal blood volume of 3.3 ± 0.3 ml (kg body weight)−1, a value that was not significantly different from the volume change following an increase in carotid sinus pressure (P > 0.05).
Figure 5 shows the changes in splanchnic blood volume to changes in carotid pressure during constant flow, to changes in carotid pressure during constant pressure, the difference between these values, and the response to changes in perfusion rate. This emphasizes the small contribution of the active response. It also confirms the relatively large passive effect, and shows that the passive effect calculated from the difference in inflow and outflow when carotid pressure changed was similar to that obtained in response to pump-induced changes in flow.
Figure 5. Summary diagram showing responses calculated from the splanchnic circulation to changes in carotid pressure and to changes in blood flow.
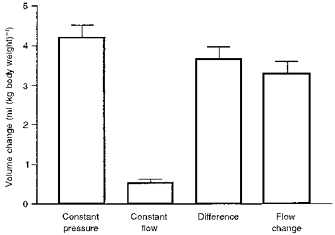
Values are means ± s.e.m. from 6 dogs. This shows that, of the total volume change in the region, the active component (during constant flow) made only a small contribution. The difference in the responses at constant flow and constant pressure was not significantly different from the change in volume occurring when flow was changed by a similar amount by altering the pump speed.
DISCUSSION
It has already been well established that most, if not the whole, of the active change in vascular capacitance occurs in the abdominal circulation. This is based partly on observing that responses in that region, induced by either direct or reflex stimulation of sympathetic nerves (e.g. Hainsworth & Karim, 1976; Karim & Hainsworth, 1976; Noble et al. 1997), were not very different from changes observed by others in the whole body (Shoukas & Sagawa, 1973; Brunner et al. 1981). Also, studies of responses in other regions, particularly the limb circulation (Hainsworth et al. 1983) or in perfused muscle (Lesh & Rothe, 1969), have shown that these regions do not show significant changes in volume in response to stimulation, provided that flow does not change and thereby cause secondary passive changes.
Although it is necessary to keep blood flow constant to assess active capacitance responses, under physiological conditions when vasoconstriction occurs, blood flow would decrease. The consequence of this is that the total net change in volume is likely to be considerably larger than that attributable to the capacitance response alone. The effects of flow changes have been studied in the limb circulation (Hainsworth et al. 1983) and it was shown that, even though sympathetic stimulation caused no apparent active change in capacitance, when blood flow decreased there was a small decrease in the volume of blood in the limb. This passive effect also occurs in the abdominal circulation and is much larger there, attributable to the high vascular compliance of that region (Karim & Hainsworth, 1976). In fact, comparisons of responses during constant pressure and constant flow perfusion (Brooksby & Donald, 1972; Noble et al. 1997) have shown that the passive volume changes occurring secondarily to changes in flow are of a similar magnitude to the active capacitance effects.
Previous investigations have established that, in the dog, the spleen makes a large contribution to the active response, but very little to the passive change (Noble et al. 1997). The contribution of the spleen varies greatly between species. The canine spleen is very large and contractile, whereas in humans it is normally a very much smaller organ. Because of this species variability, in all experiments in the present report we excluded responses from the spleen by firmly tying the splenic pedicle.
The other major blood-containing organ in the abdomen is the liver, and we have recently examined its capacitance role in response to electrical stimulation of the splanchnic nerves, with portal venous and hepatic flows controlled (Noble et al. 1998). These experiments showed that stimulation caused a reduction in hepatic capacitance of a magnitude similar to that seen in the canine spleen. In fact these experiments indicated that the change in volume in the liver, induced by sympathetic nerve stimulation, could account for most of the active response in the entire splenectomized abdomen.
Although the capacitance roles of the spleen and the liver are now well established, the role of the intestinal or splanchnic circulation remains uncertain. There is no doubt that the splanchnic blood volume can change in response to such interventions as haemorrhage or carotid occlusion (Hadjiminas & Oberg, 1968; Carneiro & Donald, 1977). However, the extent to which these volume changes were due to active constriction of capacitance vessels as opposed to being secondary to the flow changes is unclear as there have been no previous quantitative studies of active capacitance response in the splanchnic circulation.
In the present study, to assess the capacitance function of the splanchnic circulation, we first separated the hepatosplanchnic circulation from the remainder of the subdiaphragmatic circulation and showed that the active response to carotid baroreceptor stimulation, during constant flow perfusion, came exclusively from the hepatosplanchnic region. Both regions, however, contributed to the passive effect when flow decreased. The decrease in volume of the hepatosplanchnic region was more than that in the extra-splanchnic region despite its blood flow being much less. These results add further support to the view that it is the hepatosplanchnic circulation that is responsible for virtually all the body's capacitance response and for much of its passive blood volume change.
The second part of this study examined responses in the vascularly isolated splanchnic circulation, perfused through the coeliac and cranial mesenteric arteries and drained into the portal vein. The caudal mesenteric artery was tied to complete the vascular isolation. This artery supplies the distal part of the intestinal tract, but its exclusion is unlikely to impair perfusion of the gastrointestinal bed because, in the dog, there is a good collateral flow from the cranial to the caudal mesenteric arteries (Miller et al. 1964) and we never saw any evidence of ischaemia in the colon. The liver perfusion was also excluded in these experiments as splanchnic blood was drained from the portal vein and the hepatic artery was tied. The only structures to be included in this perfusion system would have been the gastrointestinal tract and the pancreas. The vascular isolation of the region was shown to be adequate because stopping the perfusion pump led to a decrease in arterial pressure to less than 4 kPa and near cessation of portal outflow. Furthermore, to reduce even further any possible flow along unrecognized collateral vessels, we kept the splanchnic and extra-splanchnic perfusion pressures at similar levels.
These results showed that although there was an active capacitance response in the splanchnic circulation, it was small, averaging only 0.5 ml (kg body weight)−1. This contrasts with the active response of the entire hepatosplanchnic circulation of 2.5 ml (kg body weight)−1 and the passive response in the splanchnic circulation of over 3.0 ml (kg body weight)−1.
The estimated contributions of the various subdiaphragmatic regions to the active and passive volume changes in response to carotid baroreceptor stimulation are summarized in Fig. 6. The average total change in volume in the entire region (excluding spleen), calculated as the sum of hepatosplanchnic and extra-splanchnic volume changes during constant pressure perfusion, was just over 7 ml (kg body weight)−1 of which 5 ml (kg body weight)−1 was from the hepatosplanchnic and 2 ml (kg body weight)−1 from the extra-splanchnic region. The response of the extra-splanchnic region was entirely passive, as had previously been shown for the limb circulation (Hainsworth et al. 1983). The active and the passive responses of the hepatosplanchnic region were approximately equal. The approximate contribution of the liver was estimated by subtraction of the response of the splanchnic circulation (Series 2) from that of the hepatosplanchnic circulation (Series 1). This revealed that, although the splanchnic circulation did make a small contribution, the major part of the active response was from the liver, approximately 2 ml (kg body weight)−1. This value is only a little less than that which we previously measured from the isolated liver in response to direct sympathetic stimulation at 16 Hz (2.4 ml (kg body weight)−1; Noble et al. 1998). Interestingly, in confirmation of our recent findings of the effects of direct sympathetic stimulation, there was no apparent passive response of the liver.
Figure 6. Summary diagram showing estimated contributions to changes in abdominal blood volumes of dogs with the spleen ligated.
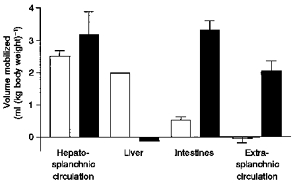
Results are means ± s.e.m. (except liver) derived from both experimental series. □, active capacitance responses (during constant flow); ▪, estimates of passive components (differences between values at constant pressure and constant flow). Note that the values for the liver were estimated by subtraction of the volume changes in Series 2 (splanchnic region) from those in Series 1 (hepatosplanchnic region). Note also that overall active and passive responses are similar. However, most of the active response is from the liver with a small contribution from the splanchnic region. The passive response is mainly from splanchnic and extra-splanchnic regions with little from the liver.
The principal original finding from this study relates to the splanchnic circulation. As far as we are aware, there have been no previous studies of active responses in that region. Earlier investigations determined changes in weight (Selkurt & Johnson, 1958; Donald & Aarhus, 1974) or volume (Folkow et al. 1963) in intestinal loops with the perfusion uncontrolled. Others have determined effects on venous perfusion pressures (Iizuka et al. 1970) which, although indicative of active responses, are impossible to quantify. Our results have shown that the splanchnic circulation does show an active capacitance response to a reflex stimulus; however, the main mechanism for blood volume mobilization is a passive change, whereby blood is released or retained simply as a consequence of a change in flow.
The conclusion to be drawn from these experiments is that, in terms of an active capacitance effect (that is, a change in vascular volume occurring as a direct result of a change in smooth muscle activity) excluding the spleen, the liver is by far the most important organ, with the splanchnic region making a small contribution. However, in the more physiological situations, in which sympathetic activity leads to a reduction in blood flow, the largest contribution to overall blood volume shifts is likely to be due to a passive change in volume in the splanchnic circulation.
Acknowledgments
This study was funded, in part, by the British Heart Foundation.
References
- Bennett TD, Macanespie CL, Rothe CF. Active hepatic capacitance responses to neural and humoral stimuli in dogs. American Journal of Physiology. 1982;242:H1000–1009. doi: 10.1152/ajpheart.1982.242.6.H1000. [DOI] [PubMed] [Google Scholar]
- Brooksby GA, Donald DE. Release of blood from the splanchnic circulation in dogs. Circulation Research. 1972;31:105–118. doi: 10.1161/01.res.31.1.105. [DOI] [PubMed] [Google Scholar]
- Brunner MJ, Shoukas AA, Macanespie CL. The effect of the carotid sinus baroreceptor reflex in the total systemic vascular bed of the dog. Circulation Research. 1981;48:274–285. doi: 10.1161/01.res.48.2.274. [DOI] [PubMed] [Google Scholar]
- Carneiro JJ, Donald DE. Blood reservoir function of dog spleen, liver, and intestine. American Journal of Physiology. 1977;232:H67–72. doi: 10.1152/ajpheart.1977.232.1.H67. [DOI] [PubMed] [Google Scholar]
- Chien S. Cell volume, plasma volume, and cell percentage in splanchnic circulation of splenectomized dogs. Circulation Research. 1963;12:22–28. doi: 10.1161/01.res.12.1.22. [DOI] [PubMed] [Google Scholar]
- Delorme EJ, Macpherson AIS, Mukherjee SR, Rowlands S. Measurement of the visceral blood volume in dogs. Quarterly Journal of Experimental Physiology. 1951;36:219–231. doi: 10.1113/expphysiol.1951.sp000975. [DOI] [PubMed] [Google Scholar]
- Donald DE, Aarhus LL. Active and passive release of blood from canine spleen and small intestine. American Journal of Physiology. 1974;227:1166–1172. doi: 10.1152/ajplegacy.1974.227.5.1166. [DOI] [PubMed] [Google Scholar]
- Drees JA, Rothe CF. Reflex vasoconstriction and capacity vessel pressure-volume relationships in dogs. Circulation Research. 1974;34:360–373. doi: 10.1161/01.res.34.3.360. [DOI] [PubMed] [Google Scholar]
- Folkow B, Lundgren O, Wallentin I. Studies on the relationship between flow resistance, capillary filtration coefficient and regional blood volume in the intestine of the cat. Acta Physiologica Scandinavica. 1963;57:270–283. doi: 10.1111/j.1748-1716.1963.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Hadjiminas J, Oberg B. Effects of carotid baroreceptor reflexes on venous tone in skeletal muscle and intestine of the cat. Acta Physiologica Scandinavica. 1968;72:518–532. doi: 10.1111/j.1748-1716.1968.tb03876.x. [DOI] [PubMed] [Google Scholar]
- Hainsworth R. Vascular capacitance: it's control and importance. Reviews in Physiology, Biochemistry, and Pharmacology. 1986;105:101–173. doi: 10.1007/BFb0034498. [DOI] [PubMed] [Google Scholar]
- Hainsworth R, Karim F. Responses of abdominal vascular capacitance in the anaesthetized dog to changes in carotid sinus pressure. The Journal of Physiology. 1976;262:659–677. doi: 10.1113/jphysiol.1976.sp011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth R, Karim F, McGregor KH, Wood LM. Hind-limb vascular-capacitance responses in anaesthetized dogs. The Journal of Physiology. 1983;337:417–428. doi: 10.1113/jphysiol.1983.sp014632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka T, Mark IL, Wendling MG, Schmid PG, Eckstein JW. Differences in responses of saphenous and mesenteric veins to reflex stimuli. American Journal of Physiology. 1970;219:1066–1070. doi: 10.1152/ajplegacy.1970.219.4.1066. [DOI] [PubMed] [Google Scholar]
- Johnstone FRC. Measurement of splanchnic blood volume in dogs. American Journal of Physiology. 1956;185:450–452. doi: 10.1152/ajplegacy.1956.185.3.450. [DOI] [PubMed] [Google Scholar]
- Karim F, Hainsworth R. Responses of abdominal vascular capacitance to stimulation of splanchnic nerves. American Journal of Physiology. 1976;231:434–440. doi: 10.1152/ajplegacy.1976.231.2.434. [DOI] [PubMed] [Google Scholar]
- Larochelle P, Ogilvie RI. Effective vascular compliance and venous diameter in dogs. Canadian The Journal of Physiology and Pharmacology. 1976;54:244–247. doi: 10.1139/y76-024. [DOI] [PubMed] [Google Scholar]
- Lesh TA, Rothe CF. Sympathetic and haemodynamic effects on capacitance vessels in dog skeletal muscle. American Journal of Physiology. 1969;217:819–827. doi: 10.1152/ajplegacy.1969.217.3.819. [DOI] [PubMed] [Google Scholar]
- Miller ME, Christensen GC, Evans HE. Anatomy of the Dog. Philadelphia: W. B. Saunders Company; 1964. [Google Scholar]
- Noble BJ, Drinkhill MJ, Myers DS, Hainsworth R. Mechanisms responsible for changes in abdominal vascular volume during sympathetic nerve stimulation in anaesthetized dogs. Experimental Physiology. 1997;82:925–934. doi: 10.1113/expphysiol.1997.sp004073. [DOI] [PubMed] [Google Scholar]
- Noble BJ, Drinkhill MJ, Myers DS, Hainsworth R. Blood mobilization from the liver of the anaesthetized dog. Experimental Physiology. 1998;83:513–522. doi: 10.1113/expphysiol.1998.sp004134. [DOI] [PubMed] [Google Scholar]
- Opdyke DF, Ward CJ. Spleen as an experimental model for the study of vascular capacitance. American Journal of Physiology. 1973;225:1416–1420. doi: 10.1152/ajplegacy.1973.225.6.1416. [DOI] [PubMed] [Google Scholar]
- Rothe CF. Reflex control of veins and vascular capacitance. Physiological Reviews. 1983;63:1281–1335. doi: 10.1152/physrev.1983.63.4.1281. [DOI] [PubMed] [Google Scholar]
- Rothe CF, Johns BL, Bennett TD. Vascular capacitance of dog intestine using mean transit time indicator. American Journal of Physiology. 1978;234:H7–13. doi: 10.1152/ajpheart.1978.234.1.H7. [DOI] [PubMed] [Google Scholar]
- Selkurt EE, Johnson PC. Effect of acute elevation of portal venous pressure on mesenteric blood volumes, interstitial fluid volumes and hemodynamics. Circulation Research. 1958;6:592–599. doi: 10.1161/01.res.6.5.592. [DOI] [PubMed] [Google Scholar]
- Shoukas AA, Sagawa K. Control of systemic vascular capacity by the carotid sinus baroreceptor reflex. Circulation Research. 1973;33:22–33. doi: 10.1161/01.res.33.1.22. [DOI] [PubMed] [Google Scholar]


