Abstract
The effect of transcranial magnetic stimulation (TMS) on the soleus H reflex was investigated in the stance phase of walking in seventeen human subjects. For comparison, measurements were also made during quiet standing, matched tonic plantar flexion and matched dynamic plantar flexion.
During walking and dynamic plantar flexion subliminal (0.95 times threshold for a motor response in the soleus muscle) TMS evoked a large short-latency facilitation (onset at conditioning-test interval: −5 to −1 ms) of the H reflex followed by a later (onset at conditioning-test interval: 3–16 ms) long-lasting inhibition. In contrast, during standing and tonic plantar flexion the short-latency facilitation was either absent or small and the late inhibition was replaced by a long-lasting facilitation.
When grading the intensity of TMS it was found that the short-latency facilitation had a lower threshold during walking than during standing and tonic plantar flexion. Regardless of the stimulus intensity the late facilitation was never seen during walking and dynamic plantar flexion and the late inhibition was not seen, except for one subject, during standing and tonic plantar flexion.
A similar difference in the threshold of the short-latency facilitation between walking and standing was not observed when the magnetic stimulation was replaced by transcranial electrical stimulation.
The lower threshold of the short-latency facilitation evoked by magnetic but not electrical transcranial stimulation during walking compared with standing suggests that cortical cells with direct motoneuronal connections increase their excitability in relation to human walking. The significance of the differences in the late facilitatory and inhibitory effects during the different tasks is unclear.
In the cat and other mammals it now seems well established that the basic alternating extensor-flexor rhythm under-lying locomotion is generated by a local network in the spinal cord (Grillner, 1981). Corticospinal cells contribute facultatively to this basic rhythm with a patterned discharge during the locomotor cycle and are of major (obligatory) importance for the visual guidance of locomotion and adaptation of the walking pattern to environmental and motivational influe(Armstrong, 1988; Drew, 1991; Arms & Marple-Horvat, 1996).
Little is known about the central control of human locomotion. Accumulating evidence, however, suggests that primates, including man, possess a similar spinal locomotor centre, but that the network is more difficult to activate pharmacologically and electrically than in other species and may be more dependent on specific control from supraspinal centres (for experiments in monkey see Eidelberg et al. 1981; Fedirchuk et al. 1998; for observations in man see Calancie et al. 1994; Bussel et al. 1996; Gerasimenko et al. 1996).
Recently, the corticospinal function during human locomotion has been evaluated using transcranial magnetic stimulation (TMS) of the motor cortex (Capaday et al. 1996; Schubert et al. 1997). In both studies it was concluded that transmission in the corticospinal tract to ankle dorsiflexors appeared to be comparable during walking and voluntary tonic contraction of the same muscles as judged from the size of motor responses (MEPs) evoked by TMS. In contrast to this, soleus MEPs have been found to be depressed in the stance phase of the gait cycle (Capaday et al. 1996; C. Capaday, personal communication).
Although a significant part of these motor responses is likely to be caused by activation of direct monosynaptic projections from the motor cortex to the spinal motoneurones under investigation, other indirect pathways probably also make a significant contribution (Nielsen et al. 1993; Pierrot-Deseilligny, 1996). The rise time of the composite excitatory postsynaptic potential (EPSP) underlying the spinal H reflex has a duration of 2–3 ms (Burke et al. 1984) and it is not unreasonable to expect that the EPSP underlying the MEP has a rise time at least equally long. As pointed out by Burke et al. (1984) in the case of the H reflex there is thus sufficient time for indirect effects to contribute to the size of the compound potential. In the case of the magnetic stimulation it is known that inhibitory effects may be observed with a latency only 1–2 ms longer than the initial short-latency facilitation (Cowan et al. 1986; Iles & Pisini, 1992; Nielsen et al. 1993). The modulation of motor responses evoked by TMS thus reveals only the net effect of the activation of several different corticospinal pathways, and information on the modulation of activity in any single pathway may not be obtained. For this purpose a technique with a better temporal resolution is necessary. As argued by Nielsen et al. (1993), H reflex testing of the effect of TMS may provide one such technique. With this technique a time course of the effect of subthreshold TMS on the excitability of the spinal motoneurones may be obtained. Short-latency, presumably monosynaptic, effects may thereby be discerned from effects with a longer latency and a probable indirect origin. It has, furthermore, been demonstrated that task-related changes in the short-latency facilitation of the reflex are very likely to reflect the susceptibility of the fast conducting corticomotoneuronal pathway to magnetic stimulation (Nielsen et al. 1993, 1995). Measurements of the short-latency facilitation thus seem to permit a selective estimation of changes in excitability of this specific population of corticospinal neurones.
Based on this background, it was the aim of the present study to investigate changes in the effect of TMS on the soleus H reflex during walking. Given that the corticomotoneuronal pathway bypasses spinal networks, including possible rhythm generators, it was of particular interest to investigate how transmission in this pathway changes during the different phases of walking and in comparison to other motor tasks. Part of the material in this study has been presented in abstract form (Petersen et al. 1997).
METHODS
General experimental arrangements
Seventeen healthy human subjects participated in the experiments. All gave informed consent to the experimental set-up, which was approved by the local ethics committee.
Electromyographic (EMG) activity was recorded from the tibialis anterior (TA) and soleus muscles using Ag-AgCl surface electrodes (1 cm2 recording area; 2 cm between poles) placed over the respective muscles. The effect of transcranial magnetic stimulation (TMS) on the soleus H reflex was investigated during quiet standing, the stance phase of walking, tonic voluntary plantar flexion and dynamic voluntary plantar flexion.
Quiet standing. The subjects were standing relaxed with their hands supported on bars in front of them. It was confirmed that there was no EMG activity in the TA or soleus muscles.
Stance phase of walking. The subjects walked on a treadmill at a speed of 4 km h−1. With a pressure-sensitive trigger placed under the heel of the shoe, touch-down of the heel was used to trigger the computer, which controlled the different stimuli (see below). A variable delay could be introduced after this triggering in order to give the stimuli at the desired time of the gait cycle.
In each subject the soleus EMG activity without stimulation was amplified (10 000–50 000 ×), band-pass filtered (25–1000 Hz), rectified and integrated (time constant: 96 ms). Thirty walking cycles were averaged and stored on a personal computer for later display on an oscilloscope (see below).
Tonic voluntary plantar flexion. The subjects were seated in a reclining armchair with the right leg fixed to a foot plate, which was connected to a torque meter. The hip was flexed to 120 deg, the knee flexed to 160 deg and the ankle was plantar flexed to 110 deg. The torque and the rectified and integrated soleus EMG activity (amplified and rectified as described above) was monitored on an oscilloscope placed in front of the subject. On the same oscilloscope the level of background soleus EMG activity measured at different times of the gait cycle could be displayed (see above). The subject's task was to match this level of EMG activity. The EMG activity produced during tonic contraction was measured and compared with that during walking. Usually a rather strong tonic contraction was required in order to match the EMG activity obtained during walking. The EMG activity was quantified off-line in order to check that the subject had matched the EMG activity during walking correctly. In separate control experiments the effect of TMS on the soleus H reflex was investigated during tonic voluntary plantar flexion in standing subjects. This made no difference for the results reported in the study.
Dynamic voluntary plantar flexion. The subjects were seated as described above. The profile of the soleus EMG activity measured in the stance phase of the gait cycle (see above) was displayed on the oscilloscope and the subject's task was to match both the level and the rate of change of the EMG activity. Stimuli (described below) were applied at a time which corresponded to that during walking with regard to the EMG profile.
Test reflex
The soleus H reflex was evoked by electrical stimulation of the posterior tibial nerve (PTN) through a monopolar electrode placed in the popliteal fossa and measured by surface electrodes placed over the muscle (see above). The indifferent electrode was placed just below the patella. For these measurements the EMG activity was amplified (500–5000 ×) and band-pass filtered (25–1000 Hz) before being recorded on a personal computer. The size of the H reflex was measured as the peak-to-peak amplitude and expressed as a percentage of the maximal M-response (Mmax), which was measured in the beginning of each experiment and checked several times throughout the experiment. It was routinely checked that measuring the area of the responses made no difference to the results. Since the size of conditioning effects on the H reflex varies with the size of the control H reflex, we ensured that the control H reflex had the same size in all investigated situations (15–25 % of Mmax; see Crone et al. 1990). It was therefore not possible (or desirable for the purpose of the experiments) to use an M-response as a monitor of the constancy of the test stimulus (Capaday, 1997). Within each task, control and conditioned H reflexes were, however, randomly alternated with each other (see below), making it unlikely that movement of the stimulating electrode could explain the observations. Furthermore, in some subjects a small M-response could be recorded with the stimulation intensities used and in these subjects we ensured that the M-response had the same size in control and conditioned trials.
Conditioning stimuli
Transcranial magnetic stimulation (TMS) was applied by a Magstim 200 (Magstim Company Ltd, Dyfed, UK) using a prototype of the figure-of-eight coil (loop diameter, 9 cm). The position of the coil was systematically adjusted at the beginning of each experiment to find the optimum location for activation of the soleus muscle. In general the optimum position was 0–2 cm lateral to the vertex. The position of the coil with respect to the head was secured by a harness (special courtesy of Volker Dietz, Zurich and Balgrist Tec, Zurich, Switzerland; for details, see Schubert et al. 1997) worn by the subject. In addition, a reference grid marked on the scalp was used to ensure that the coil remained in the same position in the same way as described by Lavoie et al. (1995). It was further checked several times throughout the study that the threshold of the MEP in the soleus muscle during quiet standing with support was constant. The intensity of the magnetic stimulation was expressed as percentage of the maximal stimulator output (2 tesla).
In some experiments transcranial electrical stimulation was applied by a digitimer D180A stimulator (Digitimer Ltd, Welwyn Garden City, UK). The anode was placed 2 cm left of the vertex and the cathode 5 cm anterior to the vertex. With this electrode arrangement MEPs in lower limb muscles evoked by electrical cortical stimulation consistently have a shorter latency than MEPs evoked by TMS, which suggests that the two types of stimulation activate corticospinal cells at different sites (Nielsen et al. 1995). The stimulation electrodes were firmly positioned on the scalp using adhesive electrode gel and further secured by elastic straps. It was checked several times throughout the study that the threshold of an MEP in the soleus muscle during quiet standing was constant. The intensity of the stimulation was expressed as a percentage of the maximal stimulator output (1500 V).
Stimulus protocol and data analysis
Conditioned and unconditioned stimuli were delivered in a random order. At least twenty reflex responses were averaged for each alternative with inter-stimulus intervals of 4 s. The mean and standard deviation of the responses were calculated on-line. After testing whether the data were normally distributed, statistically significant differences between control and conditioned reflexes were determined using a Student's paired t test. Pooled data from different subjects were compared using an analysis of variance (ANOVA). A two-way ANOVA test was used to determine significant differences in the time course of the effect of TMS on the soleus H reflex in different situations.
RESULTS
Time course of the effect of TMS on the soleus H reflex
Figure 1 demonstrates a time course of the effect of TMS on the soleus H reflex in a single subject during quiet standing with support (Fig. 1A), during tonic plantar flexion (Fig. 1B), in the stance phase of walking 300 ms after heel trigger (Fig. 1D) and during dynamic plantar flexion at a comparable time after the onset of EMG activity as during walking (Fig. 1C). We ensured that the level of background EMG activity was comparable in the latter three tasks. The intensity of TMS was adjusted to 0.95 × MEP threshold in all situations and the control H reflex was adjusted to 20 % of Mmax. In this subject, TMS evoked a short-latency facilitation of the H reflex at a conditioning-test interval of −3 ms in all situations, followed during standing and walking by a short-lasting inhibition at a conditioning-test interval of −1 ms. As argued in previous studies (Cowan et al. 1986; Iles & Pisini, 1992; Nielsen et al. 1993) the short-latency facilitation is probably caused by activation of corticomotoneuronal projections to the soleus motoneurones, whereas the inhibition is probably - to a large extent - mediated disynaptically through I a inhibitory interneurones. The most pronounced effect observed during standing and tonic plantar flexion was, however, a long-lasting facilitation, which in this subject appeared at a conditioning-test interval of 3 ms and had a duration of at least 17 ms. In contrast to this, TMS evoked a clear inhibition with an onset at conditioning-test intervals around 10 ms and lasting for at least 10 ms during walking and dynamic plantar flexion. Using a two-way ANOVA test it was determined that the time courses in the four situations differed significantly (P < 0.05) from each other except for the time courses during dynamic plantar flexion and walking. This confirms findings by Nielsen & Petersen (1995) in the case of tonic and dynamic plantar flexion.
Figure 1. Time course of the effect of transcranial magnetic stimulation (TMS) on the soleus H reflex during quiet standing (A), tonic plantar flexion (B), dynamic plantar flexion (C) and in the mid-stance of walking (D).
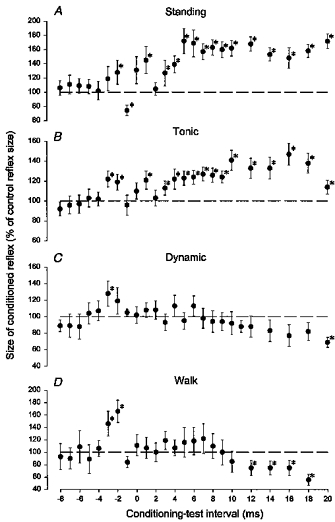
The data are from a single subject. The background EMG activities in the three ladder tasks were of similar size and the stimuli were applied at a similar time after the onset of EMG activity in C and D. In each situation the intensity of the magnetic stimulation was decreased to 0.95 × MEP threshold (54 % of maximal stimulator output in A, 61 % in B, 57 % in C and 48 % in D). In all situations the test reflex was adjusted to 20 % of the maximal motor response, Mmax. In A, the subject was standing without background EMG activity in the soleus and tibialis anterior muscles. In B, the subject maintained a constant EMG level comparable to that measured during walking. In C, the subject performed a dynamic plantar flexion with a soleus EMG activity profile similar to that recorded in the stance phase of walking. In D, the subject walked on a treadmill at a speed of 4.0 km h−1. The stimuli were applied 300 ms after heel contact. Symbols with an asterisk indicate that the conditioned reflex was significantly different (P < 0.05) from the test reflex. The vertical bars represent the s.e.m.
Essentially similar results were obtained in all seventeen investigated subjects. To give an idea of the lack of variability in the effects, data obtained during tonic plantar flexion (•) and walking (○) from four subjects are illustrated in Fig. 2. In all subjects, an inhibition was seen during walking with an onset at conditioning-test intervals of 5–10 ms and with a duration of around 10–15 ms. In contrast, during tonic plantar flexion a strong facilitation was seen at similar conditioning-test intervals. The exact onset of this facilitation was difficult to determine when the facilitation was preceded by the short-latency facilitation (cf. Fig. 2B). However, in such subjects the short-latency facilitation was often absent during standing and the onset of the long-latency facilitation could therefore be determined more exactly in that situation. The onset of the facilitation was thus found to vary between conditioning-test intervals of 2 and 6 ms and the duration of the facilitation was found to be in the order of 10–20 ms, as has also been reported by Nielsen & Petersen (1995). A two-way ANOVA test showed that the time courses in the two situations differed significantly from each other (P < 0.05) in all subjects except for the subject illustrated in Fig. 2D.
Figure 2. Time course of TMS on the soleus H reflex during tonic plantar flexion (•) and in the mid-stance of walking (○).
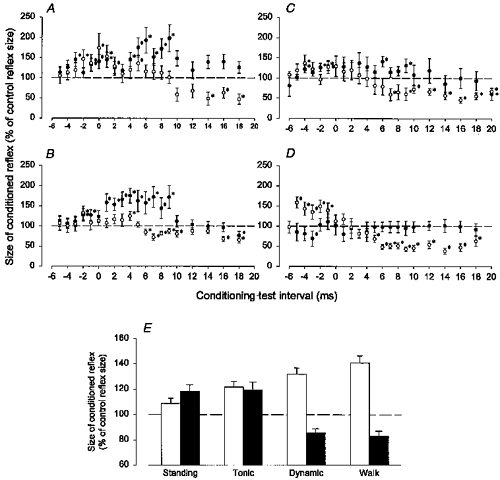
The data are from four different subjects. The experimental conditions were the same as described for B and D in Fig. 1. The intensities of the test and conditioning stimuli were adjusted in the same way as in Fig. 1. In E is shown the population mean of the conditioned reflexes (as a percentage of control reflexes) from eleven subjects. Open columns give the size of the short-latency facilitation (measured at one conditioning test interval from −4 to −2 ms) and black columns the size of the long-latency effects (measured at conditioning test intervals from 10 to 12 ms). The vertical bars represent the s.e.m.
In eleven subjects a time course of the effect of TMS on the soleus H reflex was obtained in all four situations. In the remaining six subjects a time course was not obtained during dynamic plantar flexion and standing in each of three subjects. In all subjects TMS was adjusted to an intensity of 0.95 × MEP threshold in each situation. Measurements during walking were made in mid-stance 300 ms after the heel trigger and during dynamic plantar flexion at a comparable time after EMG onset. We ensured that the background EMG activities measured during walking, dynamic plantar flexion and tonic plantar flexion were of similar size. When comparing data from the eleven subjects in whom a time course was obtained in all situations, the short-latency facilitation (measured 1 ms after its onset; conditioning-test intervals −4 to −1 ms) was on average larger during walking (average size of facilitation: 141 ± 19 %) and dynamic plantar flexion (132 ± 17 %) than during tonic plantar flexion (122 ± 15 %) and standing (108 ± 14 %). These data are shown as open columns in Fig. 2E. The difference between walking and dynamic plantar flexion was not statistically significant (P > 0.1), but the other differences were (walking vs. tonic plantar flexion: P < 0.05, walking vs. standing: P < 0.001). These differences were seen although a lower intensity of TMS was used during walking (and dynamic plantar flexion) than during tonic plantar flexion and quiet standing, since the threshold of the MEP during walking (and dynamic plantar flexion) was generally slightly lower than during tonic plantar flexion (45 % compared with 47 % of maximal stimulator output) and quiet standing (45 % compared with 50 % of maximal stimulator output).
Whereas a short-latency facilitation was seen in 16 of 17 subjects during walking and in all thirteen investigated subjects during dynamic plantar flexion when the TMS stimulation intensity was adjusted to 0.95 × MEP threshold, it was only the case in 10 of 17 subjects during tonic plantar flexion and 8 of 14 subjects during quiet standing.
In Fig. 2E, the black columns show the population mean (from the eleven subjects in whom all four situations were investigated) of the size of the conditioned reflex (as a percentage of the control reflex size) measured at conditioning-test intervals of 10–12 ms. There was a significant difference (P < 0.05) between the inhibition/facilitation observed at these intervals during walking and dynamic plantar flexion (82.9 ± 4.1 % and 85.8 ± 3.3 % respectively) as compared with quiet standing/tonic plantar flexion (118.4 ± 5.0 % and 119.5 ± 6.1 %, respectively).
In all seventeen subjects the long-latency facilitation was seen during either standing or tonic plantar flexion. A similar long-latency facilitation was not seen in any of the subjects during walking or in any of the thirteen investigated subjects during dynamic plantar flexion. Instead, an inhibition was seen at similar conditioning- test intervals in 16 out of 17 subjects during walking and in 11 out of 13 subjects during dynamic plantar flexion.
The effect of changing the intensity of TMS
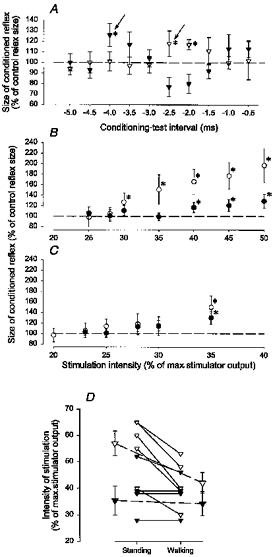
In Fig. 3 the intensity of TMS has been systematically varied in a single subject up to the intensity at which an MEP was evoked during quiet standing, tonic plantar flexion and in the mid-stance of walking. The conditioning-test interval was adjusted to evoke either a short-latency facilitation (○) or a long-latency facilitation/inhibition (•). As in Nielsen & Petersen (1995) a time course was made in the beginning of each experiment while the subject performed a strong tonic plantar flexion and the onset of the short-latency facilitation was determined. An interval within the initial 0.5 ms after this onset was used for the actual experiment. In the subject used for the illustration in Fig. 3 the short-latency facilitation had an onset at a conditioning-test interval of −2.5 ms and an interval of −2.0 ms was therefore used for the experiment. The long-latency effects were measured at a conditioning-test interval of 10 ms. In this subject a short-latency facilitation was never seen during quiet standing, regardless of the intensity of stimulation (Fig. 3A). During walking the threshold of the short-latency facilitation was lower than during tonic plantar flexion (34 %vs. 40 % of maximal stimulator output, respectively).
Figure 3. Comparison of the threshold for the short-latency facilitation and the long-latency effects during quiet standing (A), tonic plantar flexion (B) and in the mid-stance of walking (C).
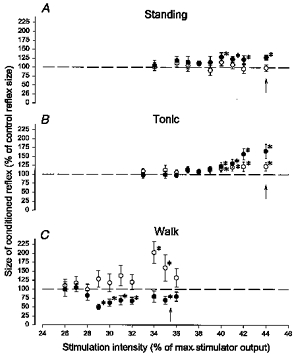
The data shown are from a single subject. The short-latency facilitation (○) was measured at a conditioning-test interval of −2.0 ms and the long-latency effect (•) at a conditioning-test interval of 10 ms. The vertical arrows in the figure indicate the MEP threshold. A one-way ANOVA test was performed to test for statistically significant effects as a function of the stimulus intensity. This was significant in all cases except for the open circles in A. Subsequently, a Student's t test was performed for the individual data points. The asterisks in the figure indicate when the conditioned reflex was significantly different from the control reflex size (P < 0.05). Other details are the same as in Fig. 1.
In all of the thirteen investigated subjects the short-latency facilitation similarly had a lower threshold in the mid-stance of walking (300 ms after heel trigger) than during quiet standing (Fig. 4A). This difference was statistically significant (P < 0.05). Notice that in ten of the subjects a short-latency facilitation was never observed, regardless of the stimulation intensity (indicated by the arrows in Fig. 4). The threshold of the short-latency facilitation was slightly lower during walking than during tonic plantar flexion in 10 of 12 subjects in whom these two situations were compared, but this difference was less clear and the population average did not reach a statistically significant level (Fig. 4B; P > 0.1). Nevertheless, it should be noticed that in four of the subjects in whom a short-latency facilitation was easily evoked during walking, it was not seen during tonic plantar flexion, regardless of the stimulation intensity. There was no difference in the threshold of the short-latency facilitation between walking and matched dynamic plantar flexion in the six subjects in whom these two situations were compared (Fig. 4C; P > 0.1).
Figure 4. Difference in threshold for the short-latency facilitation in the mid-stance of walking, during quiet standing (A), during tonic plantar flexion (B) and during dynamic plantar flexion (C).
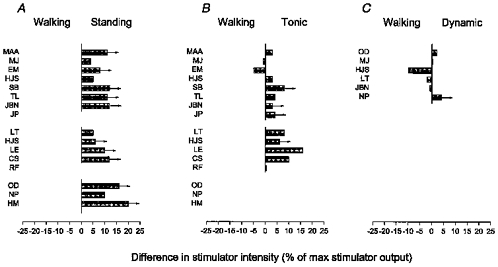
In all subjects the EMG profile during dynamic plantar flexion was matched with that recorded during walking and it was ensured that the measurements were made at the same level of background soleus EMG activity during walking, tonic plantar flexion and dynamic plantar flexion. In all subjects and in all situations the short-latency facilitation was measured at a conditioning-test interval within 0.5 ms after the onset of the short-latency facilitation (from −5 to −1 ms). The threshold of the facilitation was determined as the intensity at which the conditioned reflex was significantly (P < 0.05) larger than the test reflex. The arrows indicate that the MEP threshold was reached without the appearance of the short-latency facilitation. In all subjects the short-latency facilitation was compared between mid-stance of walking and quiet standing. The subjects’ initials are shown to the left of the histograms.
In the subject used for the illustration in Fig. 3, as for all twelve investigated subjects, the long-latency effects (conditioning-test interval: 10 ms; in six subjects an interval of 12 ms was used because of a longer latency of the long-latency effects) were strikingly different during walking and dynamic plantar flexion compared with standing and tonic plantar flexion. The long-latency facilitation was only seen during standing (Fig. 3A) and tonic plantar flexion (Fig. 3B), but never during walking (Fig. 3C), regardless of the TMS intensity. There was in general a tendency for the long-latency inhibition measured during walking or dynamic plantar flexion to have a lower threshold than the short-latency facilitation as was the case for the subject illustrated in Fig. 3, but this difference did not reach a statistically significant level.
Modulation of the short-latency facilitation during walking
In six subjects, the modulation of the short-latency facilitation of the H reflex was investigated at different times in the stance phase of the walking cycle. It was not possible in any of the subjects to evoke an H reflex in the soleus muscle in the swing phase and at the very beginning of the stance phase. In the subject used for the illustration in Fig. 5, TMS evoked a short-latency facilitation of the H reflex at a conditioning-test interval of −3.0 ms, and a conditioning-test interval of −2.5 ms was therefore used for the experiment. The intensity of TMS was adjusted to 0.95 × MEP threshold at an interval 300 ms after the heel trigger. As can be seen, the short-latency facilitation (○) was large just after the onset of the EMG in the soleus muscle (150 ms after the heel trigger), but then decreased abruptly within the following 50–100 ms. This pattern is very similar to that seen at the onset of voluntary ramp-and-hold plantar flexion in sitting subjects (Nielsen & Petersen, 1995). After the initial decrease, the facilitation increased steadily more or less in parallel with the EMG activity in the soleus muscle until its maximum around 450 ms after the heel trigger. It then decreased abruptly again and was no longer present 600 ms after the heel trigger just before the onset of the swing phase.
Figure 5. The modulation of the short-latency facilitation and long-latency inhibition through the stance phase.
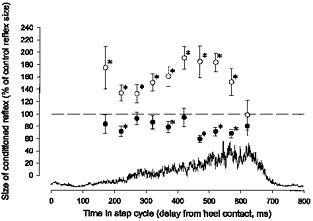
The data are from a single subject. TMS was adjusted to an intensity of 0.95 × MEP threshold at 300 ms after heel contact during walking. In all parts of the stance phase the control reflex was adjusted to 15 % of Mmax. The short-latency facilitation was measured at a conditioning-test interval of −2.5 ms (○) and the long-latency inhibition was measured at a conditioning-test interval of +12 ms (•). The rectified soleus EMG is shown by the trace. Other details are the same as in Fig. 1.
In all six subjects, it was similarly found that the facilitation increased in parallel with the soleus EMG until a maximum shortly before onset of swing at which time it abruptly disappeared. In 4 of the 6 subjects the large facilitation at the very beginning of the soleus EMG was observed.
Measurements were also made at a conditioning-test interval of 12 ms (•). In 2 of the 6 subjects an inhibition of the H reflex of more or less equal size was seen throughout the stance phase. In the remaining four subjects the inhibition was clearer in mid- and late stance.
Comparison of magnetic and electrical cortical stimulation
The lower threshold and larger size of the short-latency facilitation during walking than during quiet standing may reflect an increased excitability of the corticomotoneuronal cells responsible for the facilitation. To investigate this possibility the effect of magnetic and electrical stimulation of the motor cortex was compared in five experiments on four subjects during walking and quiet standing. Figure 6A-C shows data from one of these subjects. Initially, a time course of the effect of the two types of stimulation on the soleus H reflex was obtained during tonic plantar flexion (Fig. 6A). In both cases a short-latency facilitation was observed, but the latency of this facilitation was 1.5 ms shorter for the electrical (conditioning-test interval: −4.0 ms) than for the magnetic stimulation (conditioning- test interval: −2.5 ms), probably reflecting the fact that the electrical stimulation activates the axons of the corticospinal cells deep in the cortex, whereas the magnetic stimulation activates the cells either indirectly (trans-synaptically) or directly at a site close to the soma (Day et al. 1987; Edgley et al. 1990, 1997; Nielsen et al. 1995). In the rest of the study the size of the facilitation was consequently measured at these two conditioning-test intervals (marked by arrows in Fig. 6A).
Figure 6. The effect of transcranial magnetic (TMS) and transcranial electrical stimulation (TES) on the soleus H reflex.
The data shown in A-C are from the same subject. A, time course of the effect of TMS (▿) and TES (▾) on the soleus H reflex during tonic plantar flexion. A conditioning-test interval within 0.5 ms after the onset of the short-latency facilitation (−2.5 ms for TMS and −4.0 ms for TES) was chosen for the rest of the experiment. In B and C, the threshold of the short-latency facilitation during walking (○) and during quiet standing (•) following TMS (B) and TES (C) is shown. The stimuli were applied 300 ms after heel contact during walking. The vertical bars designate 1 s.e.m. The asterisks designate a statistically significant facilitation (P < 0.05). In D, the threshold for the short-latency facilitation (measured 0.5 ms after its onset) induced by TMS (▿) and TES (▾) during standing (left) and mid-stance of walking (right) is shown for all four subjects. The larger triangles represent the population mean of the data.
In Fig. 6B-C, the intensity of the two stimuli was varied systematically up to the threshold of the MEP during either quiet standing (•) or in the mid-stance phase of walking (○). As already described, the short-latency facilitation evoked by the magnetic stimulation had a lower threshold and a larger size during walking than during quiet standing (Fig. 6B). In contrast, the short-latency facilitation evoked by electrical stimulation (Fig. 6C) had the same threshold and the same size in the two situations.
In none of the five experiments (Fig. 6D, smaller triangles) on four subjects was a difference found in the threshold for the short-latency facilitation evoked by electrical stimulation (▾) between the two situations, whereas this was the case for all subjects when the facilitation was evoked by magnetic stimulation (▿). When looking at the mean of the data from the five experiments (Fig. 6D, larger triangles), the threshold of the facilitation evoked by magnetic stimulation was significantly lower during walking than during standing (x= 42.0± 4.0 compared with x= 57.0 ± 4.6; P < 0.05), whereas this was not the case for the facilitation evoked by electrical stimulation x= 34.2 ± 4.6 compared with x= 35.4 ± 5.4; P= 0.37).
DISCUSSION
We have demonstrated that the effect of TMS on the soleus H reflex is strongly modulated according to the voluntary motor task performed by the subject. In the stance phase of walking and during a matched dynamic plantar flexion TMS evoked a pronounced short-latency, short-lasting facilitation, which was often followed by a late and long-lasting inhibition. In contrast during tonic plantar flexion the short-latency facilitation was generally small and was often followed by a late facilitation.
Stability of stimulation
An initial consideration is whether these observations could simply be explained by changes in the position of the magnetic coil relative to the head of the subject in the different situations. At first, it does seem difficult to believe that reliable stimuli may be applied to the human head during vigorous movements such as walking. However, we obtained a remarkable stability of the position of the coil by using the harness introduced by Schubert et al. (1997), and the stability of this position was further checked throughout the study using a grid of markers on the scalp of the subject as described by Lavoie et al. (1995). With these two procedures consistently reproducible results could be obtained for the whole duration of an experiment judged from the near constant threshold of the soleus MEP during quiet standing in repeated tests throughout each experiment. The influence of possible changes in the position of the coil was, finally, minimized by randomly alternating measurements in the different situations.
Since test and conditioned stimuli were randomly alternated within each trial and in each situation it is also unlikely that changes in the position of the stimulating electrode in relation to the tibial nerve could explain our observations.
For at least two other important reasons we feel very confident that our data are not explained by the stimulation conditions. Firstly, such changes are highly unlikely to explain the qualitative differences in the time course of the effect of TMS on the H reflex that we observed during walking and dynamic plantar flexion compared with standing and tonic plantar flexion. Secondly, the observation of a larger short-latency facilitation in all investigated subjects during walking compared with quiet standing is difficult to reconcile with the possibility that the stimulation conditions changed systematically between the tasks in all subjects. Our failure to demonstrate the short-latency facilitation when increasing the stimulation as much as possible (i.e. without the occurrence of an MEP in the soleus muscle) during tonic plantar flexion and standing in several subjects, in whom facilitation was readily demonstrated during walking, also argues against a simple change in the position of the coil as an explanation for the larger short-latency facilitation during walking compared with these two tasks.
In the following section we will first discuss our interpretation of the short-latency facilitation and the significance of its increase during walking compared with standing. We will then discuss our interpretation of the long-latency facilitation and inhibition.
Evidence for increased cortical excitability during walking
We took special care to measure the size of the short-latency facilitation within the initial 0.5–1.0 ms after its onset. This is because only at such short intervals can the facilitation be attributed to activation of the direct corticomotoneuronal (CM) cells in the motor cortex (Cowan et al. 1986; Day et al. 1987; Edgley et al. 1990; Nielsen et al. 1993). At longer intervals non-monosynaptic pathways are likely to contribute (Nielsen et al. 1993; Gracies et al. 1994; Burke et al. 1994). Focusing only on the initial part of the facilitation has the advantage that the observed changes in the facilitation may, firstly, be ascribed to one specific pathway (i.e. the fastest conducting CM pathway) and, secondly, that possible changes in the excitability of neurones in indirect pathways from the cortex to the spinal motoneurones may be disregarded.
TMS has been shown to activate corticospinal cells either indirectly (trans-synaptically) or directly (close to the cell soma; Edgley et al. 1990, 1997). The descending volley evoked by the magnetic stimulus may therefore be influenced by changes in the excitability of the cortical cells, as documented by recording of the pyramidal tract volley evoked by magnetic stimulation during a functional motor task in monkeys (Baker et al. 1995) and more recently in man (Lazzaro et al. 1998), or - more indirectly - by an increase of the short-latency facilitation of the H reflex during contraction of the muscle in which the H reflex is evoked (Nielsen et al. 1993, 1995; Mazzocchio et al. 1994; Nielsen & Petersen, 1995). From this it might be argued that the increase of the short-latency facilitation during walking compared with quiet standing is sufficient evidence to suggest an increased CM cell excitability during walking. This is, however, not the case. If the ease with which successive motoneurones are recruited in the pool by TMS is increased during walking (i.e. increased recruitment gain; cf. Kernell & Hultborn, 1990) this would also be seen as an increased facilitation. The lack of an increased short-latency facilitation during walking compared with quiet standing following transcranial electrical stimulation is therefore a key finding in the present study. In contrast to transcranial magnetic stimulation, electrical stimulation penetrates to a deeper site and activates the axons of the cortical cells in the white matter (Burke et al. 1990, 1994; Edgley et al. 1990, 1997; Nielsen et al. 1995). The short-latency facilitation evoked by transcranial electrical stimulation, therefore, not only has a shorter latency than the facilitation evoked by magnetic stimulation, but is also not influenced by changes in cortical excitability (Nielsen et al. 1993, 1995; Nielsen & Petersen, 1995). The observation of a lower threshold and larger size of the short-latency facilitation evoked by magnetic stimulation, but not by electrical stimulation, during walking compared with quiet standing thus suggests that CM cells which project to the soleus motoneurones increase their excitability in relation to the stance phase of walking.
Although the short-latency facilitation was found to be significantly larger during walking than during tonic plantar flexion and in some subjects was present only during walking, we do not want to put too much emphasis on this part of our data, since the effect of transcranial electrical stimulation was not compared in these two tasks. However, we do think that it is possible to suggest, based on this data, that the excitability of the CM cells during walking is at least of the same order of magnitude as during tonic plantar flexion. As for the comparison between dynamic plantar flexion and walking, we found no difference, suggesting that the CM cells are equally susceptible to TMS in these two tasks.
Comparison with animal studies
In the cat it has been suggested that corticospinal cells are rhythmically active during walking and contribute to the EMG in the appropriate phases of the walking cycle (Armstrong, 1988). It does not surprise us to find evidence that the direct CM pathway in human subjects may be similarly active and contribute to the generation of EMG activity during walking. Given the increased demand for an accurate control of the different limb segments in the bipedal, upright human subject compared with the quadrupedal cat, the opposite would have been more surprising. It should, nevertheless, be emphasized that we do not wish to suggest that corticospinal activity is obligatory for the basic rhythmic motor activity during walking. There is ample evidence which demonstrates that humans can at least learn to walk without an intact corticospinal tract (Wernig & Müller, 1992; Nathan, 1994), and the recent demonstration of a spinal central pattern generator (CPG) for locomotion in monkey (Fedirchuk et al. 1998) and the accumulating evidence suggesting a similar CPG in man (Calancie et al. 1994; Bussel et al. 1996; Gerasimenko et al. 1996) make it likely that the role of the motor cortex in the basic rhythm of walking is of a facultative nature in humans as was suggested by Armstrong and Drew for the cat (see Armstrong, 1988).
In the cat the modulation of the activity of corticospinal neurones does not seem to reflect feedback from rhythmically active sensory pathways. Many neurones which were not influenced by stimulation of sensory afferents were thus rhythmically active during walking, and those neurones that did receive a cutaneous input discharged at times when their receptive fields were not activated during movement (Armstrong, 1988). We cannot provide similar evidence from our study, and we therefore accept the possibility that the observed changes in CM cell excitability reflect changes in the input to the cells from sensory afferents activated during the stance phase. It may indeed be that one function of the increased CM cell excitability is to keep possible transcortical reflexes open for corrections of sudden perturbations in ongoing walking movements (cf. Petersen et al. 1998). In any case one of our future objectives based on the findings in this study will be to clarify the role of the CM pathway in the adaptation of human gait to environmental and motivational influences. This has been suggested to be the major role of the corticospinal tract in the cat (Armstrong, 1988; Drew, 1991).
Comparison with other human studies
Two other studies have investigated the effect of TMS on ankle muscles during walking (Capaday et al. 1996; Schubert et al. 1997). In both of these studies the size of motor potentials (MEPs) evoked by TMS rather than (as in the present study) the effect of subliminal TMS on the H reflex was investigated. In both studies it was concluded that the activity of the corticospinal pathway to ankle dorsiflexors was of a similar size during walking compared with other tasks involving the dorsiflexors. Schubert et al. (1997) also found that MEPs in the medial gastrocnemius muscle had a similar size during walking as during voluntary plantar flexion. These findings are entirely in agreement with the findings of the present study, although it should be emphasized that the MEPs do not reflect activity in the direct CM pathway exclusively. Indeed, Capaday et al. (1996; and C. Capaday personal communication) found that soleus MEPs were smaller in the stance phase of walking than during tonic plantar flexion. This is at first glance in conflict with our finding of a larger (or similar-sized) short-latency facilitation during walking than during tonic plantar flexion. However, the main difference between walking and tonic plantar flexion observed in the present study was the long-latency effects. During walking, the cortical stimulus evoked a long-latency inhibition at the same conditioning-test intervals at which a facilitation was observed during tonic plantar flexion. As both the inhibition and the facilitation are likely to influence the size of the MEP measured in the study by Capaday et al. (1996), we suggest that it is this change from a long-latency facilitation during tonic plantar flexion to a long-latency inhibition during walking that explains the decrease in the MEP observed by them.
The point that we want to stress in this regard is that it is necessary to consider individual identified pathways when evaluating the role of the motor cortex in a specific task.
What explains the long-latency inhibition and facilitation?
The late facilitation during tonic plantar flexion has previously been observed by Cowan et al. (1986; see also Nielsen & Petersen, 1995) and was suggested by them to be caused by a polysynaptic corticospinal pathway. The inhibition which was observed at similar latencies during walking and during dynamic plantar flexion may have a similar origin. One possibility would be activation of I a interneurones conveying reciprocal inhibition between ankle dorsi- and plantar flexors (Iles & Pisini, 1992; Nielsen et al. 1993), but these interneurones are not likely to be more easily activated during the stance phase of walking than during tonic plantar flexion (Capaday et al. 1990; N. Petersen & J. Nielsen, unpublished observations). Other possibilities are Renshaw neurones and interneurones mediating presynaptic inhibition of I a afferents. It is difficult to obtain evidence regarding recurrent inhibition during walking, but the available evidence suggests that recurrent inhibition of soleus motoneurones may be decreased in the stance phase of walking (Faist et al. 1996a). Presynaptic inhibition of soleus I a afferents has been shown to be decreased by TMS at rest and during plantar flexion in sitting subjects (Iles, 1996; Meunier & Pierrot-Deseilligny, 1998). Whether this is also the case during walking is unclear, but the observation of increased presynaptic inhibition of I a afferents during walking compared with standing (Capaday & Stein, 1986; Faist et al. 1996b) may suggest that TMS would have a different effect on presynaptic inhibition during this task.
A less likely possibility is that the inhibition occurs at a cortical level. It has been demonstrated that TMS activates not only direct excitatory corticospinal pathways (Burke et al. 1990), but also inhibitory intracortical connections (Ziemann et al. 1996). The silent period in the voluntary EMG following TMS is thus probably caused at least partly by inhibition of the motor output from the motor cortex due to activation of such intracortical inhibitory connections (Ziemann et al. 1993; Brasil-Neto et al. 1995). Rothwell and colleagues have also provided strong evidence that TMS may activate inhibitory connections that traverse the two hemispheres (Ziemann et al. 1996). If such inhibitory intracortical pathways are more excitable during walking and dynamic contraction it might explain the occurrence of the inhibition (or maybe rather disfacilitation) of the H reflex during these two tasks. Further experiments are clearly necessary to investigate the exact mechanism for the observed long-latency effects during walking.
References
- Armstrong DM. The supraspinal control of mammalian locomotion. The Journal of Physiology. 1988;405:1–37. doi: 10.1113/jphysiol.1988.sp017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Marple-Horvat DE. Role of the cerebellum and motor cortex in the regulation of visually controlled locomotion. Canadian The Journal of Physiology and Pharmacology. 1996;74:443–455. 10.1139/cjpp-74-4-443. [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Task-related variation in corticospinal output evoked by transcranial magnetic stimulation in the macaque monkey. The Journal of Physiology. 1995;488:795–801. doi: 10.1113/jphysiol.1995.sp021011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cammarota A, Valls-Sole J, Pascual-Leone A, Hallett M, Cohen LG. Role of intracortical mechanisms in the late part of the silent period to transcranial stimulation of the human motor cortex. Acta Neurologica Scandinavica. 1995;92:383–386. doi: 10.1111/j.1600-0404.1995.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. Journal of Neurophysiology. 1984;52:435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E. Non-monosynaptic transmission of the cortical command for voluntary movement in man. The Journal of Physiology. 1994;480:191–202. doi: 10.1113/jphysiol.1994.sp020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hicks RG, Stephen JP. Corticospinal volleys evoked by anodal and cathodal stimulation of the human motor cortex. The Journal of Physiology. 1990;425:283–299. doi: 10.1113/jphysiol.1990.sp018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussel B, Roby-Brami A, Neris OR, Yakovleff A. Evidence for a spinal stepping generator in man. Paraplegia. 1996;34:91–92. doi: 10.1038/sc.1996.15. [DOI] [PubMed] [Google Scholar]
- Calancie B, Needham-Shropshire B, Jacobs P, Willer K, Zych G, Green BA. Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain. 1994;117:1143–1159. doi: 10.1093/brain/117.5.1143. [DOI] [PubMed] [Google Scholar]
- Capaday C. Neurophysiological methods for studies of the motor system in freely moving human subjects. Journal of Neuroscience Methods. 1997;74:201–218. doi: 10.1016/s0165-0270(97)02250-4. [DOI] [PubMed] [Google Scholar]
- Capaday C, Barbeau H, Bonnard M, Lavoie BA. Cortical control during the swing phase of human walking. Society for Neuroscience Abstracts. 1996;22:1850. [Google Scholar]
- Capaday C, Cody FW, Stein RB. Reciprocal inhibition of soleus motor output in humans during walking and voluntary tonic activity. Journal of Neurophysiology. 1990;64:607–616. doi: 10.1152/jn.1990.64.2.607. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. Journal of Neuroscience. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan JM, Day BL, Marsden C, Rothwell JC. The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and leg in intact man. The Journal of Physiology. 1986;377:333–347. doi: 10.1113/jphysiol.1986.sp016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Experimental Brain Research. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Dick JP, Cowan JM, Berardelli A, Marsden CD. Motor cortex stimulation in intact man. 2. Multiple descending volleys. Brain. 1987;110:1191–1209. doi: 10.1093/brain/110.5.1191. [DOI] [PubMed] [Google Scholar]
- Drew T. Visuomotor coordination in locomotion. Current Opinion in Neurobiology. 1991;1:652–657. doi: 10.1016/s0959-4388(05)80044-3. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. The Journal of Physiology. 1990;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Comparison of activation of corticospinal neurons and spinal motor neurons by magnetic and electrical transcranial stimulation in the lumbosacral cord of the anaesthetized monkey. Brain. 1997;120:839–853. doi: 10.1093/brain/120.5.839. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Walden JG, Nguyen LH. Locomotor control in macaque monkeys. Brain. 1981;104:647–663. doi: 10.1093/brain/104.4.647-a. [DOI] [PubMed] [Google Scholar]
- Faist M, Blahak C, Berger W. Modulation of recurrent inhibition in soleus and tibialis anterior muscle during human gait. Electroencephalographic Clinical Neurophysiology. 1996a;99:367. (abstract) [Google Scholar]
- Faist M, Dietz V, Pierrot-Deseilligny E. Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Experimental Brain Research. 1996b;109:441–449. doi: 10.1007/BF00229628. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Nielsen J, Petersen N, Hultborn H. Pharmacologically evoked fictive motor patterns in the acutely spinalized Marmoset monkey (Callithrix jacchus) Experimental Brain Research. 1998 doi: 10.1007/s002210050523. (in the Press) [DOI] [PubMed] [Google Scholar]
- Gerasimenko Y, McKay WB, Pollo FB, Dimitrijevic MR. Stepping movements in paraplegic patients induced by epidural spinal cord stimulation. Society for Neuroscience Abstracts. 1996;22:1372. [Google Scholar]
- Gracies JM, Meunier S, Pierrot-Deseilligny E. Evidence for corticospinal excitation of presumed propriospinal neurones in man. The Journal of Physiology. 1994;475:509–518. doi: 10.1113/jphysiol.1994.sp020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapeds and fish. In: Brooks VB, editor. Handbook of Physiology, section 1, The Nervous System, vol. II, Motor Control. Bethesda: American Physiological Society; 1981. pp. 1179–1236. chap. 26. [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. The Journal of Physiology. 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Pisini JV. Cortical modulation of transmission in spinal reflex pathways of man. The Journal of Physiology. 1992;455:425–446. doi: 10.1113/jphysiol.1992.sp019309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D, Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Research. 1990;507:176–179. doi: 10.1016/0006-8993(90)90542-j. 10.1016/0006-8993(90)90542-J. [DOI] [PubMed] [Google Scholar]
- Lavoie BA, Cody FWJ, Capaday C. Cortical control of human soleus muscle during volitional and postural activities studied using focal magnetic stimulation. Experimental Brain Research. 1995;103:97–107. doi: 10.1007/BF00241968. [DOI] [PubMed] [Google Scholar]
- Lazzaro VD, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. The Journal of Physiology. 1998;508:625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocchio R, Rothwell JC, Day BL, Thompson PD. Effect of tonic voluntary activity on the excitability of human motor cortex. The Journal of Physiology. 1994;474:261–267. doi: 10.1113/jphysiol.1994.sp020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Cortical control of presynaptic inhibition of Ia afferents in humans. Experimental Brain Research. 1998;119:415–426. doi: 10.1007/s002210050357. 10.1007/s002210050357. [DOI] [PubMed] [Google Scholar]
- Nathan PW. Effects on movement of surgical incisions into the human spinal cord. Brain. 1994;117:337–346. doi: 10.1093/brain/117.2.337. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Evidence favouring different descending pathways to soleus motoneurones activated by magnetic brain stimulation in man. The Journal of Physiology. 1995;486:779–788. doi: 10.1113/jphysiol.1995.sp020853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Ballegaard M. Latency of effects evoked by electrical and magnetic brain stimulation in lower limb motoneurones in man. The Journal of Physiology. 1995;484:791–802. doi: 10.1113/jphysiol.1995.sp020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Deuschl G, Ballegaard M. Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. The Journal of Physiology. 1993;471:223–243. doi: 10.1113/jphysiol.1993.sp019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Christensen LOD, Nielsen J. Transcranial magnetic stimulation of the corticospinal tract during walking in man. Society for Neuroscience Abstracts. 1997;23:770. [Google Scholar]
- Petersen N, Christensen LOD, Morita H, Sinkjaer T, Nielsen J. Evidence that a transcortical pathway contributes to stretch reflexes in the tibialis anterior muscle in man. The Journal of Physiology. 1998;512:267–276. doi: 10.1111/j.1469-7793.1998.267bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Progress in Neurobiology. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Schubert M, Curt A, Jensen L, Dietz V. Corticospinal input in human gait: modulation of magnetically evoked motor responses. Experimental Brain Research. 1997;115:234–246. doi: 10.1007/pl00005693. [DOI] [PubMed] [Google Scholar]
- Wernig A, Müller S. Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia. 1992;30:229–238. doi: 10.1038/sc.1992.61. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Netz J, Szelenyi A, Homberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neuroscience Letters. 1993;156:167–171. doi: 10.1016/0304-3940(93)90464-v. 10.1016/0304-3940(93)90464-V. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. The Journal of Physiology. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]


