Abstract
Cell-specific reverse transcriptase-polymerase chain reaction (RT-PCR), immunolocalization and microspectrofluorometry were used to identify and localize the Na+-H+ exchanger (NHE) isoforms expressed in the submandibular gland (SMG) acinar and duct cells and their regulation by basolateral and luminal P2 receptors in the duct.
The molecular and immunofluorescence analysis showed that SMG acinar and duct cells expressed NHE1 in the basolateral membrane (BLM). Duct cells also expressed NHE2 and NHE3 in the luminal membrane (LM).
Expression of NHE3 was unequivocally established by the absence of staining in SMG from NHE3 knockout mice. NHE3 was expressed in the LM and in subluminal regions of the duct.
Measurement of the inhibition of NHE activity by the amiloride analogue HOE 694 (HOE) suggested expression of NHE1-like activity in the BLM and NHE2-like activity in the LM of the SMG duct. Several acute and chronic treatments tested failed to activate NHE activity with low affinity for HOE as expected for NHE3. Hence, the physiological function and role of NHE3 in the SMG duct is not clear at present.
Activation of P2 receptors resulted in activation of an NHE-independent, luminal H+ transport pathway that markedly and rapidly acidified the cells. This pathway could be blocked by luminal but not basolateral Ba2+.
Stimulation of P2U receptors expressed in the BLM activated largely NHE1-like activity, and stimulation of P2Z receptors expressed in the LM activated largely NHE2-like activity.
The interrelation between basolateral and luminal NHE activities and their respective regulation by P2U and P2Z receptors can be used to co-ordinate membrane transport events in the LM and BLM during active Na+ reabsorption by the SMG duct.
Among all cystic fibrosis transmembrane conductance regulator (CFTR)-expressing tissues, fluid and electrolyte secretion is probably best understood in the submandibular salivary gland (SMG), which continues to be an excellent and versatile experimental system for studying the mode and regulation of transepithelial ion transport (Cook et al. 1994). The function of the major cell types in SMG is well established. Acinar cells secrete isotonic fluid containing 140 mm NaCl. The duct absorbs the NaCl and secretes KHCO3 (Cook et al. 1994). To the extent that these functions are understood in other tissues, a similar two-stage process is responsible for secretion by intestinal (Selin, 1997), nasal and airway epithelia (Boucher, 1994a, b) and the sweat gland (Quinton, 1994). Only the pancreas uses alternative mechanisms for acinar and ductal secretion (Case & Argent, 1990).
Although the physiological function of electrolyte transport by the duct (or its equivalent in other tissues) is not completely understood, it is critical for the function of the respective organ since abnormalities in ductal secretion cause cystic fibrosis (Welsh, 1995). In several species, including the rat, the bulk of Na+ absorption by the SMG duct is mediated by a Na+−H+ exchanger (NHE) activity (Cook et al. 1994). Inhibitor characterization of NHE activity in the rat parotid gland intralobular duct suggests the expression of NHE2 in these cells (Paulais et al. 1994). In previous studies we showed that NHE activity is expressed in both the basolateral (BLM) and luminal (LM) membranes of the main SMG duct of the rat (Xu et al. 1995; Zhao et al. 1995). Similar findings were reported recently in the main duct of the mouse SMG (Chaturapanich et al. 1997). However, which of the NHE isoforms are expressed and functioning in each membrane is not known.
The NHE family consists of at least five isoforms (Yun et al. 1995; Orlowski & Grinstein, 1997; Wakabayashi et al. 1997). The isoform expressed in most cells is NHE1, which is believed to have a housekeeping function in maintaining constant pHi. NHE2 and NHE3 are found mainly in the LM of epithelial cells such as the kidney proximal tubule and the epithelium of the gastrointestinal tract (Brant et al. 1995). NHE4 and NHE5 appear to have a more restricted distribution and specialized functions (Orlowski & Grinstein, 1997). A distinctive pharmacological feature of the NHE isoforms is their sensitivity to amiloride and its analogues. NHE1 is sensitive to all analogues (Yun et al. 1995); NHE2 displays moderate sensitivity to amiloride, ethylisopropyl amiloride (EIPA) and HOE 694 (HOE) (Yun et al. 1995); and NHE3 has a relatively low affinity for amiloride and very low affinity for HOE (Yun et al. 1995).
Ion transport and fluid secretion by the SMG duct is regulated by various sympathetic and parasympathetic inputs, which act on muscarinic, α-adrenergic and β-adrenergic receptors (Cook et al. 1994). Another form of regulation, which has attracted much attention recently, is through purinergic receptors. The ion transporting cells of CFTR-expressing epithelia express distinctive P2 receptors in the LM and BLM (Stutts et al. 1992, 1994; Schwiebert et al. 1995; Hwang et al. 1996). In a recent study we showed that the SMG duct expresses P2U receptors in the BLM and P2Z receptors in the LM (Lee et al. 1997). The two receptors activate different Cl− channels which probably reside in the same membrane as the respective receptor (Zeng et al. 1997). Although the regulation of Cl− channels is likely to play a central role in the regulation of ion transport by P2 receptors, it is likely that these P2 receptors (and therefore other agonists) also acutely regulate the activity of other transporters involved in fluid and electrolyte transport by the SMG duct. Since the bulk of Na+ absorption by the rat duct is mediated by NHE, regulation of NHE activity by P2 receptors may play an important role in the overall function of the SMG duct.
In the present study, we used several techniques to identify, localize and study the regulation of the NHE isoforms in the SMG duct. RT-PCR analysis of RNA prepared from single duct fragments showed that the duct expressed NHE1, NHE2 and NHE3. Immunolocalization confirmed these findings and allowed the localization of NHE1 in the BLM, and NHE2 and NHE3 in the LM. Expression of NHE3 was ascertained further by showing the absence of NHE3 protein in the LM of SMG duct from NHE3−/− mice. Based on sensitivity to amiloride and HOE, we were able to obtain functional evidence for the expression of NHE1- and NHE2-like activities in the intralobular and the perfused main duct. All pharmacological and physiological attempts to demonstrate NHE3-like activity with low sensitivity to HOE failed. In this work we also provide the first evidence for regulation of NHE activity by P2 receptors. The P2U receptor in the BLM stimulated NHE1 activity, whereas the P2Z receptor in the LM stimulated NHE2 activity. The membrane-limited stimulation of the NHE isoforms by P2 receptor can contribute to the regulation and co-ordinate Na+ absorption by the SMG duct.
METHODS
Materials and solutions
BCECF-AM (2′,7′-bis(carboxyethyl)-5-carboxyfluorescein-AM) was purchased from Molecular Probes, and collagenase CLS4 was from Worthington, Freehold, NJ, USA. EIPA was from RBI and H89 was from Alexis, San Diego, CA, USA. HOE 694 was a generous gift from Dr Hans Lang, Hoechst, Frankfurt, Germany. Antibody (Ab) recognizing NHE1 was a generous gift from Dr Sergio Grinstein, Hospital for Sick Children, Toronto, Canada and Abs specific for NHE3 were a generous gift from Dr Orson Moe, University of Texas Southwestern Medical Center, Dallas, TX, USA. All other chemicals were purchased from Sigma including ATP, UTP and benzoylbenzoyl ATP (BzATP). The standard perfusion solution (PSA) (Na+ containing) contained (mM): 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 glucose and 10 Hepes (pH 7.4 with NaOH). Na+-free solutions were prepared by replacing Na+ with NMG+. In some experiments 5 mm BaCl2 was included in the standard perfusion solution. The osmolarity of all solutions was adjusted to 310 mosmol l−1 with the major salt. Unless otherwise specified, animals were anaesthetized (40 mg kg−1) or killed (200–250 mg kg−1) by i.p. injections of sodium pentobarbital (Abbott, North Chicago, IL, USA) according to the NIH Guide for the Care and Use of Laboratory Animals.
Preparation of isolated intralobular ducts and acini
Intralobular duct fragments and acinar clusters from the SMG were prepared by a previously reported procedure (Xu et al. 1996). Female Sprague-Dawley rats (75–100 g) were anaesthetized by i.p. injection of 40 mg kg−1 sodium pentobarbital, and killed by exposure to air saturated with methoxyflurane. The submandibular glands were then removed and place in ice-cold PSA in which 10 mm NaCl was replaced with sodium pyruvate, and 0.02 % soybean trypsin inhibitor and 0.1 % bovine serum albumin were added to the standard perfusion solution. Each gland was cleaned by injection of 5–10 ml of PSA and finely minced. The minced tissue was transferred to 8 ml of PSA containing 4 mg of collagenase CLS4 (254 U mg−1), the flask was gassed with 100 % O2 and capped. After 15 min incubation at 37°C the digest was washed twice with PSA followed by a 10 s centrifugation at 100 g and resuspended in about 4 ml of PSA. For microfluorometry and cell-specific RT-PCR intralobular ducts and acinar cells from the same glands were morphologically identified by their distinctive appearance under microscopic examination (see Xu et al. 1996). For semi-quantitative RT-PCR, ducts were isolated using a density gradient centrifugation (Xu et al. 1996). Briefly, 3 ml of digest was layered on top of 3 ml Accudenz-gradient solution (20 % Accudenz in PSA; Accurate Chemical, Westbury, NY, USA) and centrifuged at 1000 g for 10 min. The ducts in the interphase were collected and washed once with PSA. To achieve higher purification the ductal fraction was centrifuged on a second similar gradient. Better than 95 % purity of duct cells was confirmed by microscopic examination before experiments.
Immunocytochemistry
Polyclonal Ab against NHE1 was kindly provided by Dr S. Grinstein (Robertson et al. 1997). Two polyclonal Abs were used for immunolocalization of NHE2. The specificity of each Ab was verified in previous reports (Hoogerwerf et al. 1996; Bookstein et al. 1997). Two polyclonal Abs against NHE3 (antisera 1314 and 1566 in Amemiya et al. 1995) were kindly provided by Dr O. Moe, and their specificity was verified using kidney slices as a positive control. SMG slices from homozygous mutant mice with a targeted null mutation of the NHE3 gene (Schultheis et al. 1997) were used as a negative control and to obtain conclusive evidence for the expression of NHE3 in the SMG duct. SMGs from rats and mice were embedded in OCT (Miles, Eikhart, IN, USA), frozen in liquid N2 and cut into 4 μmmu;m sections. Immunostaining of frozen sections or the attachment of collagenase-digested cells to glass coverslips were as previously reported (Lee et al. 1997). The cells were fixed and permeabilized by incubation with 0.5 ml of cold methanol for 10 min at −20°C. After removal of methanol, the cells were washed with phosphate-buffered saline (PBS) and incubated in 0.5 ml PBS containing 50 mm glycine for 10 min at room temperature. This buffer was aspirated and the non-specific sites were blocked by a 1 h incubation at room temperature with a 0.25 ml PBS solution containing 5 % goat serum, 1 % bovine serum albumin and 0.1 % gelatin (blocking medium). The medium was aspirated and replaced with 50 μl of blocking medium containing control serum and 1 : 100 (tissue slices) or 1 : 500 (isolated cells) dilution of each antibody. After incubation with the primary antibody for 1.5 h at room temperature and three washes with blocking medium, the antibodies were detected with anti-rabbit goat IgG tagged with fluorescein (Jackson Laboratories, West Grove, PA, USA). Images were collected with a Bio-Rad MRC 1000 or 1024 confocal microscope.
Cell-specific RT-PCR
To avoid possible contamination by minor cells, single duct fragments or acinar clusters consisting of five to eight cells were used. The morphologically identified cells were collected by a patch pipette and ejected into an ice-cold guanidinium thiocyanate- phenol-chloroform RNA extraction solution (Trizol, Gibco BRL) immediately after completion of tissue digestion. Total RNA was isolated and purified according to a published method (Zeng et al. 1997b). The RNA was reverse transcribed using random hexa-primers and an RNase H− reverse transcriptase (Gibco BRL). The cDNA was then amplified using specific primers and AmpliTaq Gold enzyme (Perkin Elmer) and the products were separated on a 1.5 % agarose gel containing 0.1 μg ml−1 ethidium bromide (EtBr). Unless specified otherwise, after a ‘hot start’ (95°C for 10 min) forty cycles of a 3-step PCR reaction were used (usually 95°C for 30 s, 50°C for 1 min, 72°C for 45 s, with adjustments for specific primers as needed). The reactions were terminated with a long extension at 72°C for 7 min and transferred to 4°C. When the first PCR product showed faint or no bands, additional twenty-five cycles of PCR reactions were performed to confirm the findings. To verify the purity of the duct or acinar clusters, PCR reactions using primer sets for amylase 1 and kallikrein were performed before analysis of NHE isoforms. Two amylase 1 primer sets specific to acinar cells were used (amylase 1a and amylase 1b in Table 1). Kallikreins were detected using the primer sets reported by MacDonald et al. (1996). Among the ten rat kallikrein primer sets reported, we found that rKLK2 and rKLK8 primers are specific to SMG duct cells. To exclude a possibility of genomic DNA contamination, PCR reactions for β-actin containing an intron sequence were also performed (Zeng et al. 1997b). Four sets of NHE isoform-specific primers (rNHE1, rNHE2, rNHE3 and rNHE4) were chosen from the 3′ untranslated regions of each cDNA sequence of the rat NHEs. Since the annealing temperature of these primers is over 72°C, in these PCR reactions forty cycles of 2-step PCR reactions (94°C for 30 s, 70°C for 45 s) were used. Another four sets of NHE isoform-specific primers designed by Robertson et al. (1996) were also tested to detect the expressed isoforms in SMG duct and acini. Finally, four sets of NHE3-specific primers were used to confirm the existence of NHE3 mRNA in SMG duct fragments. The PCR products were designed to probe the following regions of rat NHE3 mRNA: an N-terminus region (rNHE3a), a putative amiloride-binding region suggested by Orlowski & Kandasamy (1996) (rNHE3b), a putative amiloride binding region suggested by Counillon et al. (1993a) (rNHE3c), and a C-terminus region (rNHE3d). The sequences of PCR products were confirmed by a dideoxy nucleotide sequencing.
Table 1.
PCR primers used in the present work
| Name | Sequence | Size of product (start–end: GenBank Acc. No.) |
|---|---|---|
| Amylase 1a | Sense: 5′TTA TGC GCA AGT GGG ATG GAG3′ | 421 bp |
| Anti-sense: 5′GGC TGA CCA TTG ACG ACA TTT C3′ | (932–1352: V00717) | |
| Amylase 1b | Sense: 5′GGA CAT GGT GAA CAG GTG CAA C3′ | 350 bp |
| Anti-sense: 5′CAA GTC TGA ACC CTG CTA CGC C3′ | (378–727: V00717) | |
| rKLK2 | Sense: 5′GCT GTC ATC AAT GAA TAC CTC3′ | 224 bp |
| Anti-sense: 5′TGG TCA TGC ACA GGT TGT TCA3′ | (156–379: M11565) | |
| rKLK8 | Sense: 5′TAC CAC TTT AAT GAA CCG CAA3′ | 224 bp |
| Anti-sense: 5′TAG TCA TTC CCA GGT TTT CGG3′ | (118–197: M27216, 35–178:M27217) | |
| rNHE1 | Sense: 5′CCT TTC TGG GGT TTA CAC GGG AGG GAC TGT3′ | 160 bp |
| Anti-sense: 5′GTG GAG CTC TGA CTG GCA GGG AAG ATT | CAA3′ (3850–4009: M85299) | |
| rNHE2 | Sense: 5′TGA CGG TAT TAG GGC ACA GGT TGG AAT GTA3′ | 196 bp |
| Anti-sense: 5′AAA TTG GGA CAG AGG CGG GGG TAA G3′ | (3192–3387: L11004) | |
| rNHE3 | Sense: 5′AGG GAG ATC GAG ATG GGG CTA AAG GTG GAC3′ | 243 bp |
| Anti-sense: 5′AAG CAG ATG CAG TAT GTT GGG CGG ACT TG3′ | (3845–4087: M85300) | |
| rNHE4 | Sense: 5′TCT GAG GGT AGG GAT GAT TAA TTG GTC ACA3′ | 126 bp |
| Anti-sense: 5′GCA TTG GCC TGT TTC AAC ATT TCT GA3′ | (2739–2864: M85301) | |
| rNHE3a | Sense: 5′AGA GGG CTG TGA CGA AGA G3′ | 399 bp |
| Anti-sense: 5′GTG GGT GTG AGT GTG AAG GAG3′ | (19–417: M85300) | |
| rNHE3b | Sense: 5′GTG GTG ATG CAG TGA CTG G3′ | 388 bp |
| Anti-sense: 5′TTC CAT GTC CAG ATG ACG3′ | (792–1179: M85300) | |
| rNHE3c | Sense: 5′TCA CCA GTG TTG TCC CGG AGA G3′ | 487 bp |
| Anti-sense 5′TCA CCA CCC AGC GTC ACG AAA G3′ | (312–798: M85300) | |
| rNHE3d | Sense: 5′ATG GAG AAT CTG GCA CAC AAC3′ | 400 bp |
| Anti-sense: 5′ACA TGT GTG TGG ACT CAG GG3' | (2183–2582: M85300) |
Semi-quantitative RT-PCR of SMG duct cDNA
The amount of NHE1, NHE2 and NHE3 mRNA in the SMG duct cDNA was estimated by RT-PCR using subcloned templates as standards. PCR products of rNHE1, rNHE2 and rNHE3 primers were inserted into the pCR2.1 TA cloning vector (Invitrogen, San Diego, CA, USA). The amount of each isoform-specific plasmid DNA was measured by spectrophotometry and standardized to a molar concentration. SMG ducts were purified by gradient centrifugation from SMG digests, and total RNA and cDNA were prepared by the methods described above. Between twenty and thirty cycles of PCR reactions were performed using serial dilutions of standard plasmids or cDNA samples as templates. The intensity of EtBr staining was calibrated using an image scanner (Bio-Rad Gel Doc 1000) and a standard curve was generated using the values from the PCR products of standard plasmid templates. The template concentration in cDNA samples was calculated from the linear portion of the standard curve.
Microdissection and perfusion of the main SMG duct
The procedure used to prepare the main duct for lumen and bath perfusion was similar to that described before (Xu et al. 1996). Female rats (200–250 g) were anaesthetized (sodium pentobarbital, 40 mg kg−1, i.p.), the submandibular glands were exposed and the connective tissue was cleared in the region of the gland hilum. The SMG duct was opened and a cannula was inserted and ligated. The duct was cut, transferred to a Petri dish containing ice-cold PSA and finely dissected. After removal of the SMG, rats were killed by a high dose (200 mg kg−1) of sodium pentobarbital. The duct was then transferred to a perfusion chamber, and perfused luminally with PSA containing 5 μMmu;m BCECF-AM for 15 min. During dye loading, the chamber was continuously perfused with the standard solution to avoid possible dye binding to the connective tissue. The chamber was placed on the stage of an inverted microscope and the cannula was connected to the lumen perfusion line. The lumen was perfused at a rate of 25 μl min−1, and the bath was perfused in an anterograde direction at a rate of 6 ml min−1.
Fluorescence measurement of pHi
Intralobular ducts and the acini were loaded with BCECF by a 10 min incubation at room temperature in PSA containing 2 μMmu;m BCECF-AM. The cells were then washed once, resuspended in 5 ml PSA, and kept on ice until use. The fluorescence was recorded either by photon counting or image acquisition and analysis using the systems described before (Xu et al. 1996). For photon counting, the fluorescence was recorded from about twelve cells of a duct fragment or acinar cluster. In the case of the main duct, the fluorescence was recorded from an area equivalent to twelve cells and as close as possible to the tip of the lumen-perfusing cannula. BCECF fluorescence was recorded at excitation wavelengths of 490 and 440 nm at a resolution of 2 s−1. The fluorescence ratios of 490/440 were calibrated intracellularly by perfusing the cells with solutions containing 145 mm KCl, 10 mm Hepes and 5 μMmu;m nigericin with pH adjusted to 6.2–7.6, as described previously (Zhao et al. 1994). In the case of image acquisition, images were recorded at a resolution of 2 s−1 for each set of images, and the fluorescence of single duct fragments or acinar clusters was analysed as described previously (Zhao et al. 1994).
RESULTS
Characterization and localization of NHE isoforms in SMG
We previously reported that both the LM and BLM membranes of the SMG duct express NHE activity which is inhibited by 20 μMmu;m dimethylamiloride (Zhao et al. 1995). An initial approach to identify the NHE isoforms expressed in SMG acinar and duct cells was to analyse mRNA for NHE isoforms by RT-PCR. To circumvent the possibility of contamination by minor cells present in a SMG duct preparation (see Xu et al. 1996 for pictures of a SMG digest and isolated cells), single duct fragments and acinar clusters consisting of five to eight cells were collected by a patch pipette and ejected into an RNA extraction solution present in an Eppendorf tube. The quality and cell specificity of the RNA extracted from a single duct fragment and an acinar cluster were verified by the finding that RNA from acinar cells expressed mRNA for amylase 1 but not kallikrein and RNA from duct cells contained mRNA for kallikrein but not amylase 1 (see Methods). Figure 1 shows the RT-PCR analysis on SMG cells using type-specific primers complimentary to the 3′ untranslated regions of each mRNA. Acinar cells expressed mRNA for NHE1 and duct cells expressed mRNAs for NHE1, NHE2 and NHE3 (Fig. 1A). The identity of each of the PCR-amplified bands was confirmed by nucleotide sequencing. The same results were obtained in another set of experiments using the type-specific primers designed by Robertson et al. (1997) (data not shown).
Figure 1. Analysis of NHE isoforms in SMG cells by RT-PCR.
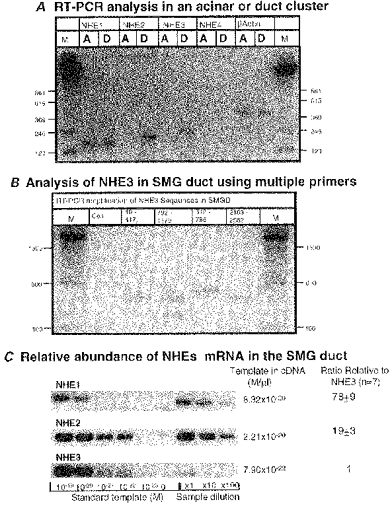
The presence and relative amounts of mRNA of each NHE isoform were analysed by RT-PCR A, the cDNA from a single acinar cluster (lanes labelled A) or a single duct fragment (lanes labelled D) was amplified using isoform-specific primer sets. B, four sets of rat NHE3 specific primers were used to amplify sequences of an N-terminus, two putative amiloride binding regions and a C-terminus of NHE3 mRNA. The lane labelled Con is a representative (N-terminus: 19-417) of negative control PCR reactions without template. C, relative amounts of each NHE mRNA in the same cDNA samples from ducts were measured by a semi-quantitative RT-PCR.
To verify further the presence of mRNA for NHE3 in SMG duct we amplified and sequenced four different regions of the NHE3 mRNA, which included an N-terminal sequence (19–417), two putative amiloride binding regions (312–798: Counillon et al. 1993a; and 792–1179: Orlowski & Kandasamy, 1996), and a C-terminal sequence (2183-2582) of the rat NHE3 mRNA (Fig. 1B). All four sets of primers amplified DNA fragments of the expected size, and the nucleotide sequences were 100 % identical to the corresponding sequences reported for NHE3 (GenBank Acc. No. M85300). To evaluate the relative abundance of each mRNA in the duct we performed a semi-quantitative RT-PCR using the pCR 2.1 subcloned templates as standards (Fig. 1C). SMG duct cells have about 80- and 20-fold greater abundance of NHE1 and NHE2 mRNA than that of NHE3, respectively.
In a second approach we used immunofluorescence in an attempt to localize the NHE isoforms in SMG acinar and duct cells. Because of potential non-specific binding of antibodies that can escape detection by appropriate controls, we consider the results with the Ab only as confirmatory of the results obtained by PCR and measurement of activity. Further, results with the Ab are considered informative only if they could be substantiated by molecular and activity measurements. The immunofluorescence results for the rat SMG are shown in Fig. 2. The Ab against NHE1 showed clear BLM localization of NHE1 in SMG duct and acinar cells and the absence of any LM staining (Fig. 2a). Based on staining intensity in the same slice, duct cells expressed at least 3-fold more NHE1 protein than acinar cells (n > 10). The two polyclonal antibodies against NHE2 stained the lumen of the rat intralobular ducts (Fig. 2b1-b3). Note that staining was confined to the LM. Staining using an Ab raised against a cytoplasmic domain of NHE3 showed that the LM and subluminal domains of the rat SMG duct may express this isoform (Fig. 2c). The luminal (Fig. 2b2, b3 and c) and nuclear (Fig. 2b1 and c) membranes of SMG acinar cells show very little, if any, staining with the NHE2 and NHE3 antibodies. We consider this staining represents non-specific binding of the Ab since expression of these isoforms could not be corroborated by PCR or measurement of activity (see below).
Figure 2. Immunolocalization of NHE isoforms in the rat SMG cells.
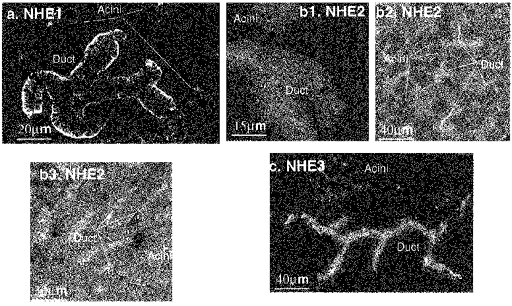
Isoform-specific polyclonal antibodies were employed for immunostaining of rat SMG cells or tissue sections. a, staining of isolated cells with Ab specific for NHE1. b, the NHE2 antibody described in Hoogerwerf et al. (1996) was used to stain isolated cells (b1) and frozen section (b2), and the antibody described in Bookstein et al. (1997) was used in panel b3 (frozen section). c, the NHE3 specific antibody (1566 in Amemiya et al. 1995) was used to stain isolated SMG cells. No staining was observed in SMG duct or acinar cells in the absence of primary Ab.
To establish unequivocally the expression of NHE3 in the SMG duct we performed similar immunoanalysis in the SMG from wild-type (WT) and NHE3−/− mice. Figure 3a–d confirms the expression of NHE1 in the BLM and NHE2 in the LM of the mouse SMG duct. Figure 3e clearly shows the staining of the LM of the SMG duct with the NHE3 Ab. At higher magnification the staining of subluminal domains is evident (Fig. 3f). Staining was completely absent from the SMG of NHE3−/− mice. Although secretory mechanisms may differ between the rat and mouse SMG (Chaturapanich et al. 1997), the findings in Fig. 3 justify the use of the Ab to conclude that there is expression of NHE3 protein in the LM of the SMG duct.
Figure 3. Immunolocalization of NHE isoforms in the SMG of wild-type and NHE3−/− mice.
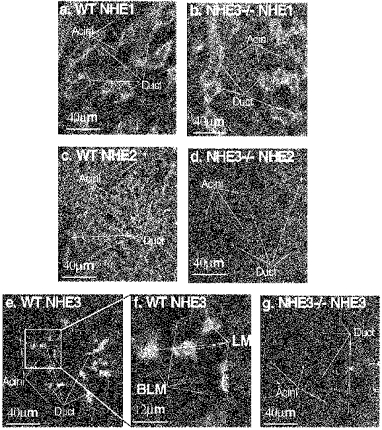
Frozen sections from the SMG of WT (a, c, e and f) and NHE3−/− (b, d and g) were stained with Ab against NHE1 (a and b), NHE2 (c and d) and NHE3 (e-g). Note the high magnification in f. Staining was eliminated in the absence of primary Ab.
The final approach was to characterize the NHE activity in SMG duct and acinar cells by their sensitivity to amiloride and its analogues EIPA and HOE. Essentially the same results were obtained with all inhibitors. In Fig. 4 we present the results with HOE, since it distinguishes best between the NHE isoforms. The Ki values for inhibition of NHE1, NHE2, and NHE3 by HOE are 0.16, 5 and 650 μMmu;m, respectively (Counillon et al. 1993b). To verify the specificity of our measurements, NHE activity of SMG acinar and duct cells present in the same recording field was measured simultaneously by imaging BCECF fluorescence. The cytosol of acinar and intralobular duct cells was acidified by an NH4+ pulse and subsequent perfusion with a Na+-free solution. Under control conditions duct cells recovered pHi at a rate of 0.72 ± 0.06 pH units min−1 (n= 8) and acinar cells at a rate of 0.31 ± 0.03 pH units min−1 (n= 9). After a second acidification protocol the cells were treated with 1.5 μMmu;m HOE before and during the perfusion with the Na+-containing solutions. At this inhibitor concentration only duct cells showed definitive NHE activity (dpH/d t for duct cells = 0.32 ± 0.01, n= 8 and for acinar cells = 0.04 ± 0.01, n= 9; Fig. 4). Finally, the cells were re-acidified and treated with 50 μMmu;m HOE. Since this concentration is 10 times higher than the Ki for NHE2 but 13 times lower than that for NHE3 (Counillon et al. 1993b), we expected only partial inhibition of NHE activity by 50 μMmu;m HOE. Surprisingly, 50 μMmu;m HOE abolished NHE activity in duct cells (dpH/d t = 0.04 ± 0.00, n= 8; Fig. 4). These results suggest that NHE1- and NHE2-like exchangers are the only functioning exchangers in SMG duct cells and NHE1 is the only exchanger in SMG acinar cells.
Figure 4. NHE1- and NHE2-like, but not NHE3-like Na+-H+ exchange activity can be found in SMG duct cells.
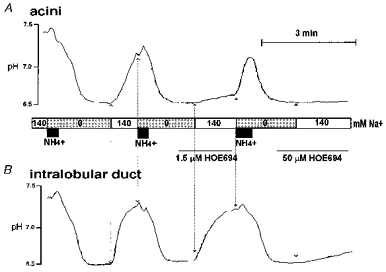
BCECF-loaded SMG acini (A) and intralobular ducts (B) were used to measure the differential sensitivity of NHE activity to HOE 694 in the same recording field. After each cytosolic acidification by an NH4+ pulse and Na+-free solution the cells were sequentially challenged with a Na+-containing solution in the presence of 0, 1.5 and 50 μMmu;m HOE. Note that 1.5 μMmu;m HOE nearly abolished NHE activity in acini and 50 μMmu;m HOE nearly abolished NHE activity in ducts.
The presence of NHE3 mRNA (Fig. 1) and probably protein (Fig. 2 and Fig. 3) but not activity (Fig. 4) may have resulted from tonic inhibition of NHE3 in the isolated duct cells. Hence, we used several known protocols to upregulate and/or activate NHE3 activity. NHE3 is strongly inhibited by protein kinase A (PKA) (Cabado et al. 1996). Hence, we attempted to activate the protein by modification of this activity. Treatment of SMG duct with a high concentration of H89 to inhibit PKA activity (n= 7) did not recover any NHE3-like activity. NHE3 expression and activity are upregulated by low Na+ diet and treatment with corticosteroids (Cho et al. 1994). Maintaining rats on a low Na+ diet for 7–14 days (n= 6) or treatment with 0.6 mg (100 g of body weight)−1 dexamethasone for 3 days (n= 6) clearly induced the expression of the γ-subunit of the epithelial Na+ channel (ENaC) in the SMG duct, but did not uncover NHE3 activity. Stimulation of duct cells with adrenaline (not shown) or ATP (see below), which is likely to induce exocytosis, also failed to uncover NHE3-like activity. Hence, at present we are unable to identify conditions under which NHE3-like activity can be measured in the SMG duct. In addition, it is possible that the expression and activity of NHE3 in the LM is very low and difficult to detect in the background of the potent NHE1 and NHE2 activities.
Using the perfused main SMG duct system described before (Xu et al. 1996), we determined the functional localization of the active NHE isoforms. Figure 5 confirms our previous findings of NHE activity in both the LM and BLM of the rat SMG duct (Zhao et al. 1995). Including 15 μMmu;m HOE in the luminal perfusate inhibited the luminal NHE activity by about 90 %. Removal of HOE resulted in slow recovery of pHi. At a higher concentration of HOE the inhibition could not be readily reversed by washing away the inhibitor. After re-acidification of the cytosol, the BLM was exposed to 1.5 μMmu;m HOE before and during the exposure to Na+. NHE activity in the BLM was nearly abolished by this concentration of HOE. To obtain a range for the sensitivity to HOE, in addition to the experiment in Fig. 5 we determined the effect of four concentrations between 1 and 30 μMmu;m (1 experiment) and the effect of 6 and 30 μMmu;m (2 additional experiments) of HOE on luminal NHE activity. The percentage inhibition at each concentration was calculated and the values were used in the program PHARM/PCS version 4.0 (Tallarida & Murray, 1986) to calculate the Ki. The Ki for luminal NHE was estimated to be 3.43 μMmu;m (ranges of 95 % confidence level: 1.98 ≤Ki≤ 5.97 μm). This range is close to that reported for NHE2 and very different from that reported for NHE1 and NHE3 (Counillon et al. 1993b). The Ki for inhibition of BLM NHE activity could not be determined with sufficient accuracy, probably due to impaired access of HOE to the BLM through the extensive connective tissue. However, in all experiments (n= 4) 1 μMmu;m HOE blocked the BLM NHE activity by more than 85 %. This value is compatible with the expression of NHE1.
Figure 5. Membrane localization of the NHE1- and NHE2-like activities in the main SMG duct.
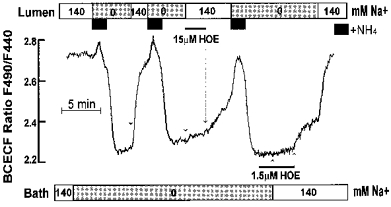
The HOE sensitivity of basolateral and luminal NHEs was evaluated in the perfused main SMG duct. Note that 15 μMmu;m HOE inhibited most of the luminal and 1.5 μMmu;m HOE the basolateral NHE activities.
Regulation of pHi by purinergic agonists
Recently we reported the expression of P2U receptors in BLM and P2Z receptors in the LM of SMG duct cells (Lee et al. 1997b). Hence, it was of interest to determine whether P2 receptors regulate NHE activity, and which NHE isoform is modulated by each P2 receptor. Initially we tested the effect of the various P2 agonists on pHi (Fig. 6). Stimulation with 1 mm ATP, which activates both receptors, induced cellular acidification reducing pHi to about 7.2 over a 2 min period (Fig. 6A). BzATP, which activates only the P2Z receptors, was as effective as ATP in acidifying the cells (Fig. 6B). The effect of a high concentration of UTP tended to be more complicated (Fig. 6C), although in most experiments it caused a small acidification. Low concentration of UTP had little effect on pHi (Fig. 6D). Hence, P2 receptors probably modulate several H+ transporters to cause cytosolic acidification, which mask the effect of the receptors on NHE activity.
Figure 6. Purinergic agonists acidify the SMG duct.
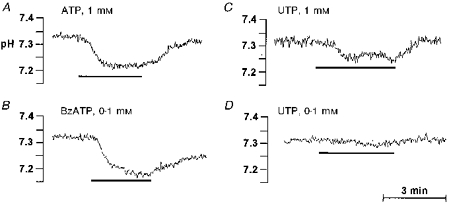
Stimulation with 1 mm ATP (A) or 0.1 mm BzATP (B) reduces pHi to about 7.2. High concentration of UTP (1 mM) caused small acidification (C), whereas low concentration of UTP (0.1 mM) had little effect on pHi (D). All of the panels shown are representative of at least six similar experiments.
To determine the membrane from which the agonist modulated pHi we measured the effect of the three agonists in the perfused main SMG duct. Early micropuncture and microperfusion studies indicated that transcellular ion transport by the main and intralobular ducts is similar (Cook et al. 1994). Stimulation with 1 mm ATP through the LM caused an acidification similar to that observed with the intralobular duct. However, when the BLM was stimulated with the same concentration of ATP the cytosol alkalinized in 50 % (n= 5/10) of experiments. This alkalization was most consistently observed when the cells were somewhat acidic (Fig. 7A). The removal of ATP resulted in recovery of pHi. BzATP evoked a prominent acidification when applied to the LM, but in all experiments tested (n= 5) it had no effect on pHi when applied to the bath (Fig. 7B). Stimulation of the BLM with a high concentration of UTP increased pHi in 2/3 experiments, whereas a high concentration of luminal UTP, which can interact with the P2Z receptor (Lee et al. 1997), caused a modest cytosolic acidification (Fig. 7C).
Figure 7. Membrane-specific response of the SMG main duct to purinergic agonists.
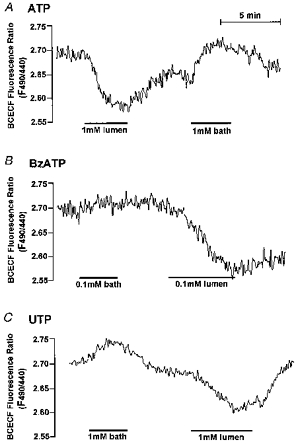
A, stimulation of the LM with 1 mm ATP reduced the pHi of the main SMG duct, whereas the same concentration of ATP in the bath increased pHi in 50 % of experiments (n= 10). B, in all experiments (n= 5) 0.1 mm BzATP had no effect on pHi when applied to the bath side but decreased the pHi when applied to the luminal side. C, stimulation of the BLM with 1 mm UTP increased pHi in 2 of 3 experiments, whereas luminal UTP caused a modest cytosolic acidification.
ATP activates basolateral and luminal NHE activities
Considering the complicated changes in pHi caused by the nucleotides, their effect on NHE activity could be dissected only by measuring the effect of NHE inhibitors on pHi of resting and stimulated cells. Figure 8 shows that the NHE1- and NHE2-like activities of the intralobular SMG duct are activated during stimulation with ATP. Under resting conditions 2 μMmu;m HOE had no measurable effect on pHi and 50 μMmu;m HOE decreased pHi by only 0.050 ± 0.006 pH units (n= 3) over a 3 min period (Fig. 8A). After stimulation with 1 mm ATP, 2 μMmu;m HOE decreased pHi by 0.043 ± 0.002 pH units (n= 6). This protocol underestimates the activity of NHE1 due to the continued activity of NHE2 in the same cells. Addition of 50 μMmu;m HOE reduced the pHi by 0.135 ± 0.009 pH units (n= 6) (Fig. 8B).
Figure 8. ATP activated NHE1- and NHE2-like activities in the intralobular SMG ducts.
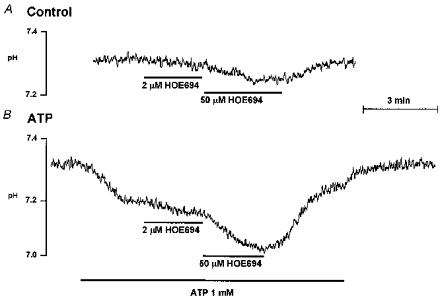
Resting ducts (A) or ducts stimulated with 1 mm ATP (B) were exposed to 2 μMmu;m and then 50 μMmu;m HOE as indicated.
To exclude the possibility that the increased NHE activity in Fig. 8 was exclusively due to the ATP-induced acidification, we tested the effect of ATP on ducts acidified to the same extent. The experimental protocol and the results are illustrated in Fig. 9. In control experiments the ducts were acidified twice and the ratio of the Na+-dependent recovery from acidification during the two periods was calculated as 0.86 ± 0.06 (n= 5) (Fig. 9A, control). In the second set of experiments the ducts were stimulated with ATP about 30 s before and during the second recovery from acidification. In these experiments the ratio of the rates of pHi recovery was 1.09 ± 0.02 (n= 14) (Fig. 9A, ATP). This indicates that ATP increased the rate of pHi recovery by about 26 % (P < 0.05) relative to control ducts. The same protocol was repeated after inhibition of NHE1 activity with 1.5 μMmu;m HOE. Under these conditions the control ratio was 0.79 ± 0.05 (n= 6) and it was increased by ATP stimulation to 0.97 ± 0.06 (n= 5), a 23 % increase (P < 0.05). The absolute increase in dpH/d t by ATP was 0.20 ± 0.03 pH units min−1 under control conditions and 0.09 ± 0.01 pH units min−1 in the presence of 1.5 μMmu;m HOE, indicating that multiple NHE isoforms were stimulated by ATP. The effect of ATP was independent of buffering capacity, which was the same in acidified resting and stimulated cells (44.56 ± 3.78 and 43.79 ± 3.76 mM (pH unit)−1 (n= 3), respectively, at pHi 6.76 ± 0.03).
Figure 9. ATP activates NHE1- and NHE2-like activities in acidified intralobular ducts.
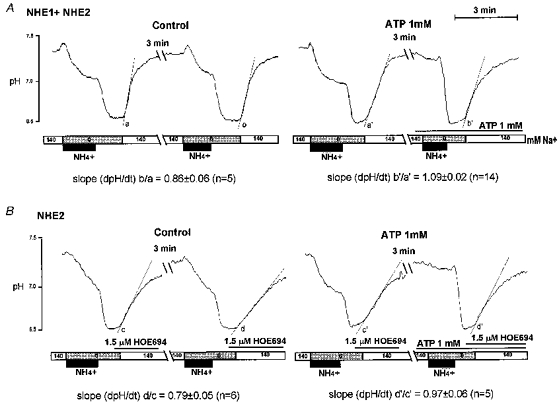
In all experiments the ducts were acidified twice and the ratio of each Na+-dependent recovery (slope b/a) was calculated. Experiments were performed under resting (left panels) and stimulated condition (right panels) and in the absence (upper panels) or presence (lower panels) of 1.5 μMmu;m HOE.
The effect of luminal ATP on NHE2-like activity in the main SMG duct is shown in Fig. 10. Stimulation of the duct with 1 mm luminal ATP caused the typical acidification. Addition of 1.5 μMmu;m HOE to the bath solution had no effect under these conditions (not shown). On the other hand 50 μMmu;m HOE doubled the decrease in pHi (Fig. 10A). When the duct was first incubated with 50 μMmu;m luminal HOE, there was a slow and small reduction in pHi. Stimulation of this duct with 1 mm luminal ATP was followed by a fast and large reduction in pHi. This reduction in pHi is probably responsible for the increased NHE2-like activity.
Figure 10. Luminal ATP activates NHE2 in the main SMG duct.
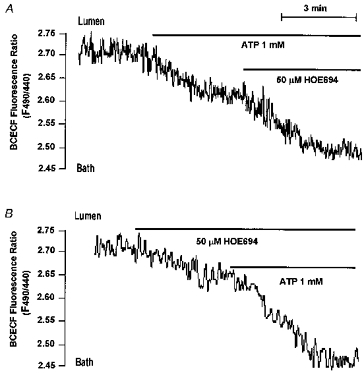
Perfused ducts were stimulated with 1 mm luminal ATP before (A) or after (B) exposure to 50 μMmu;m luminal HOE. Both panels are each representative of at least 3 experiments with similar results.
The acidification induced by luminal ATP made it difficult to isolate the effect of basolateral ATP on pHi. To overcome this difficulty, we attempted to block the acidification prior to ATP stimulation. In a previous work we described the presence of a Na+- and H+-permeable pathway that could be inhibited by a high concentration of Ba2+ (Xu et al. 1995; Zhao et al. 1995). Figure 11 shows the localization of the pathway to the LM and that the acidification induced by ATP is probably mediated by this pathway. Thus, Fig. 11A shows that addition of 5 mm Ba2+ to the bath had no effect on pHi, whereas the same concentration of luminal Ba2+ reversibly increased pHi. The effect of Ba2+ was faster and larger in SMG ducts stimulated with luminal ATP (Fig. 11B). Additional experiments showed that preincubation of the duct with Ba2+ strongly reduced the acidification induced by luminal ATP (not shown). Finally, 50 μMmu;m HOE completely blocked the alkalization induced by luminal Ba2+ in ducts stimulated with ATP (Fig. 11C), demonstrating further the activation of an NHE2-like activity by luminal ATP.
Figure 11. Effects of Ba2+ on luminal ATP-induced acidification in the SMG main duct.
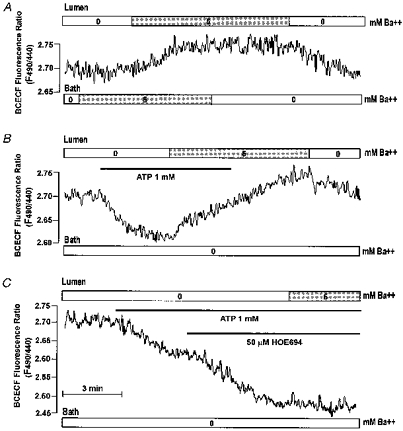
Perfused SMG ducts were sequentially exposed to 5 mm Ba2+ at the BLM and then the LM (A). Ducts stimulated with 1 mm luminal ATP (B and C) were exposed to 5 mm luminal Ba2+ before (B) or after (C) treatment with 50 μMmu;m HOE. All panels are representative of at least 3 similar experiments.
Inhibition of the acidification by pretreatment with luminal Ba2+ allowed us to demonstrate the stimulation of NHE1-like activity by basolateral ATP. In the presence of luminal Ba2+ stimulation of the duct with 1 mm basolateral ATP increased pHi (Fig. 12A). Pretreatment of the cells with 2 μMmu;m basolateral HOE inhibited the effect of basolateral ATP, which was restored upon removal of HOE (Fig. 12C). As expected, basolateral BzATP had no effect on pHi (Fig. 12B).
Figure 12. Basolateral ATP activates NHE1-like activity in the main SMG duct.
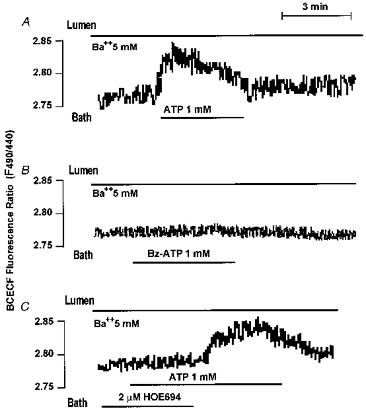
Ducts were treated with 5 mm luminal Ba2+ before and during stimulation of the BLM with 1 mm ATP (A, n= 5), 0.1 mm BzATP (B, n= 3) or 2 μMmu;m HOE and then 1 mm ATP (C, n= 3). Where indicated HOE was removed from the bath solution (C).
DISCUSSION
The bulk of Na+ absorption by CFTR-expressing and other epithelia is electroneutral and mediated by Na+−H+ exchange activity in the LM (Reuss, 1996). Two of the best studied systems are the kidney (Yun et al. 1995) and the SMG (Cook et al. 1994). NHE3 has been firmly identified as the transporter in the kidney proximal tubule responsible for electroneutral Na+ absorption. Similarly, expression of NHE3 in the gastrointestinal tract correlated with the capacity of electroneutral Na+ absorption (Yun et al. 1993). The present study indicates that NHE2-like activity is the predominant activity in the LM of the SMG duct with potential involvement of NHE3 under yet unidentified conditions. NHE1-like activity resides in the BLM of SMG duct and acinar cells.
The identity of the NHE isoforms expressed in SMG cells and their membrane localization have not been conclusively identified up to now. Following our work in the rat excretory duct demonstrating NHE activity in the LM and BLM (Xu et al. 1995; Zhao et al. 1995), it was found that the mouse SMG excretory duct displays similar activities (Chaturapanich et al. 1997). Based on sensitivity to EIPA it was suggested that NHE2 mediates most of the NHE activity in the LM of the mouse SMG excretory duct. In a recent immunofluorescence (IF) study He et al. (1997) concluded that both SMG duct and acinar cells express NHE1 in the BLM and NHE2 and NHE3 in the LM. In the present study we could not obtain either molecular or functional evidence for the expression of NHE2 or NHE3 in SMG acinar cells. Because of that we consider the faint immunostaining of acinar cells as non-specific. In fact, one of the NHE2 antibodies used in the present work (Hoogerwerf et al. 1996) was also used by He et al. (1997) to demonstrate the presence of NHE2 in the LM of acinar cells. We can reproduce their results in frozen sections, although isolated SMG acinar cells (after collagenase digestion) showed almost no staining with this Ab, but the LM of the SMG duct is still labelled (compare Fig. 2b1 and 2b2). Therefore, when possible, it is important to verify findings made by immunostaining with additional independent techniques such as RT-PCR analysis and measurement of activity, as reported here. Hence, based on our combined findings we conclude that SMG acinar cells express only NHE1 in the BLM, which probably mediates the acetylcholine- and cell shrinkage-mediated, amiloride-sensitive alkalization in these cells (Seo et al. 1995).
NHE1 is also expressed in the BLM of the rat and mouse SMG intralobular and excretory duct. Measurement of maximal NHE activity in acidified rat SMG intralobular or perfused excretory ducts, in the presence and absence of 1.5–2 μMmu;m HOE to block NHE1, showed that the maximal rate of H+ transport at pHo 7.4 mediated by the two active exchangers is about equal. The high activity of NHE1 in the rat SMG duct may relate to the high metabolic rate of these cells. The intralobular duct has a high number of vertically oriented, rod-shaped mitochondria along the enfolded basal and lateral membranes (Magee & Dalley, 1986). Na+ efflux across the BLM is mediated mainly by the Na+,K+-ATPase pump, which is expressed at very high levels in this membrane (Cook et al. 1994). Energy consumption by the pump probably generates a high concentration of H+ in the proximity of the BLM, which can affect many cellular activities. These H+ ions must be disposed off before they will diffuse through the cytosol towards the LM. Hence, during periods of high Na+ absorption (stimulated secretion) a high capacity of H+ efflux across the BLM is probably needed to maintain constant pHi. In addition, high NHE activity in the BLM will generate cytosolic HCO3− for secretion across the LM. This, however, may be of secondary importance in the SMG duct since these cells express Na+−HCO3− cotransporter in the BLM (M. G. Lee and S. Muallem, unpublished observation) and this cotransporter was shown to mediate most of the HCO3− secretion in the pancreatic duct (Ishiguro et al. 1996).
The evidence for expression of functional NHE2-like activity in the LM of SMG is concluded from the sensitivity of pHi recovery to HOE. Our results show that NHE2 is expressed in the excretory duct and in the intralobular duct, which mediates the bulk of electroneutral Na+ absorption and most of the HCO3− secretion (Cook et al. 1994). In this respect, of particular interest is the unique dependence of NHE2 on pHo. NHE2 is active at alkaline pHo and has a pKa for H+o of about 7.9 (Yu et al. 1993). This makes NHE2 particularly suitable for operation in the LM of HCO3−-secreting cells. During active HCO3− secretion the pHo of rat SMG duct lumen can exceed 8.0 (Cook et al. 1994). This will facilitates NHE2 activity and Na+ absorption. Another benefit to the cell is control of local pHi next to the LM during active HCO3− secretion. When secretion by acinar cells is copious and the resident time of the secreted fluid in the duct lumen is low, HCO3− secretion is reduced and the salivary fluid has a pH of about 7.4 (Cook et al. 1994). Under these conditions NHE2 activity is expected to be reduced to about 30 % of maximum (Yu et al. 1993) to effectively prevent Na+ absorption and excess H+ secretion to the duct lumen. Hence, NHE2 has a built-in mechanism to respond to the rate of HCO3− secretion by adjusting Na+ absorption. It, therefore, may not be a coincidence that the highest level of NHE2 expression is found in tissues and cells that secrete large amounts of HCO3−. NHE2 is also expressed at high levels in cells of the gastric gland (Wang et al. 1993). It was suggested that in these cells NHE2 may be expressed in the BLM, which experiences an alkaline environment during active acid secretion (Schultheis et al. 1998). This NHE2 can protect gastric cells from H+ back leak from the gland lumen.
A puzzling observation is our inability to find an NHE3-like activity in the SMG duct. The evidence for luminal expression of this protein in the SMG duct is quite strong. NHE3 mRNA was detected by RT-PCR. Collection of morphologically identifiable cells with a patch pipette and verification of the presence of ductal markers (the kallikrein genes) ensured that the PCR products are indeed of ductal origin. The immunolocalization results become quite convincing when staining disappeared in SMG from NHE3−/− mice. Hence, we can conclude with reasonable certainty that the SMG duct expresses the NHE3 isoform. A clue to why NHE3 activity could not be found may be provided by the finding that a large portion of the NHE3 staining was in subluminal, intracellular regions. It is now increasingly appreciated that NHE3 is stored in subplasmalemmal regions in cell lines and native tissues (Orlowski & Grinstein, 1997). This can result in a very low number of copies of NHE3 protein in the LM of the SMG duct and thus low NHE3 activity. The background of the potent NHE1- and NHE2-like activities would exacerbate the problem of detecting the low NHE3 activity. Localization of NHE3 in subluminal vesicles raises the possibility that its activity and localization in the LM are regulated by exocytosis. Unfortunately, we were unable to find any acute or chronic treatment that would lead to an increased NHE3-like activity in the LM of the SMG duct. Another possibility is that rat NHE3 is more sensitive to amiloride and its analogues than the human isoforms and 50 μMmu;m HOE was sufficient to inhibit its activity. Although this cannot be excluded at the present time, sequencing the two regions of the rat SMG NHE3 that were proposed to interact with amiloride showed complete identity with the rat kidney isoform. In the rat kidney NHE3 shows low affinity for amiloride (Hoogerwerf et al. 1996). Therefore, at present, it is not clear when NHE3-like activity becomes operative in the SMG duct or what role it might play in ductal function. The answer to these important questions may become approachable in a future study when the function of the SMG gland in the NHE3−/− mice is analysed.
An interesting finding of the present study was the regulation of pHi and the NHE isoforms by P2 receptors. Stimulation of the luminal P2Z receptors caused profound cytosolic acidification. Ca2+-mobilizing agonists in several cell types have been shown to cause similar acidification that was attributable to metabolic acid production and/or Ca2+−H+ exchanges (Niggli et al. 1982). These mechanisms are not likely to account for most of the P2Z-induced acidification in the SMG duct since the acidification was faster and more pronounced than that evoked by Ca2+-mobilizing agonists. More important, the mechanism responsible for the acidification was inhibited by Ba2+ and restricted to the LM. We have previously described the expression of a Na+ and H+ permeable pathway that was inhibited by Ba2+ in the rat SMG duct (Zhao et al. 1995). This pathway was partially active in resting cells.
A possible mechanism by which activation of P2Z receptors caused cytosolic acidification is H+ influx through the P2Z receptor. The ionotropic P2Z receptors function as non-selective cation channels (Nuttle et al. 1993). Indeed, it was reported that ATP increased the rate of Na+-independent H+ efflux from SMG cells (Lachish et al. 1996). However, the previous (Lachish et al. 1996) as well as our results are not sufficient to determine whether H+ fluxes are directly mediated by the P2Z receptor or by another H+-permeable pathway that is regulated by the P2Z receptor. We favour the second possibility since α-adrenergic stimulation of the SMG duct through the BLM caused acidification similar to that observed with ATP (not shown) and luminal Ba2+ increased pHi in unstimulated SMG duct cells. Further functional characterization of this pathway is in progress.
Characterization of the effect of NHE inhibitors and the use of the perfused excretory duct showed that basolateral and luminal NHE activities can be stimulated in a membrane-specific fashion by the P2 receptors. Activation of the P2U receptors resulted in activation of basolateral and activation of the P2Z receptors activated luminal NHE activities. Thus, as was found for Cl− channel (Lee et al. 1997b; Zeng et al. 1997a), the P2 receptors activated only the NHE isoform expressed in the same membrane as the respective receptors. The discussion above on the possible link between the activities of the basolateral and luminal NHEs leads us to consider the regulation by P2 receptors as an additional mechanism to co-ordinate the activities of the two exchangers. The main source of extracellular ATP in the basolateral side of the duct is nerve endings. Several mechanisms can increase the concentration of luminal ATP, which include ATP stored in secretory vesicles of acinar and duct cells and transport of ATP by CFTR and/or other ATP transporting proteins of the ATP binding cassette family (Pasyk & Foskette, 1997). Thus, supply of ATP to the lumen is likely to be controlled by the secretory state of the cells which, in turn, is determined, at least in part, by P2 receptors in the BLM. In this manner luminal NHE activity will be activated by luminal ATP only when the cells are stimulated to secrete by basolateral ATP that activates basolateral NHE to control pHi of the stimulated cells.
In summary, the present work provides what we believe is convincing evidence for the expression of NHE1 in the BLM of SMG duct and acinar cells and NHE2 and NHE3 in the LM of SMG duct cells. The physiological role of NHE3 is not clear at present. NHE2-like activity mediates most of the electroneutral Na+ absorption in the LM whereas NHE1 probably regulates pHi in the BLM region of the cell during Na+ absorption. The activities of basolateral and luminal NHEs appear to be regulated by P2 receptors in a membrane-specific manner, which may play an important role in co-ordinating the overall process of Na+ absorption.
Acknowledgments
We thank Dr Sergio Grinstein (Hospital for Sick Children, Toronto, Canada) and Dr Orson Moe (University of Texas Southwestern Medical Center) for the generous gift of NHE1 and NHE3 antibodies, respectively. This work was supported by NIH grants DK38938, DK46591 and DE12309.
References
- Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney International. 1995;48:1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- Bookstein C, Xie Y, Rabenau K, Musch MW, McSwine RL, Rao MC, Chang EB. Tissue distribution of Na+/H+ exchanger isoforms NHE2 and NHE4 in rat intestine and kidney. American Journal of Physiology. 1997;273:C1496–1505. doi: 10.1152/ajpcell.1997.273.5.C1496. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Human airway ion transport. Part one. American Journal of Respiratory and Critical Care Medicine. 1994a;150:271–281. doi: 10.1164/ajrccm.150.1.8025763. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Human airway ion transport. Part two. American Journal of Respiratory and Critical Care Medicine. 1994b;150:581–593. doi: 10.1164/ajrccm.150.2.8049852. [DOI] [PubMed] [Google Scholar]
- Brant SR, Yun CHC, Donowitz M, Tse C-M. Molecular cloning, functional analysis and unique tissue distribution of a human, amiloride resistant Na+/H+ exchanger isoform, NHE3. American Journal of Physiology. 1995;269:C198–206. doi: 10.1152/ajpcell.1995.269.1.C198. [DOI] [PubMed] [Google Scholar]
- Cabado AG, Yu FH, Kapus A, Lukacs G, Grinstein S, Orlowski J. Distinct structural domains confer cAMP sensitivity and ATP dependence to the Na+/H+ exchanger NHE3 isoform. Journal of Biological Chemistry. 1996;271:3590–3599. doi: 10.1074/jbc.271.7.3590. 10.1074/jbc.271.7.3590. [DOI] [PubMed] [Google Scholar]
- Case RM, Argent BE. Pancreatic secretion: in vivo, perfused gland, and isolated duct studies. Methods in Enzymology. 1990;192:256–271. doi: 10.1016/0076-6879(90)92075-o. [DOI] [PubMed] [Google Scholar]
- Chaturapanich G, Ishibashi H, Dinudom A, Young JA, Cook DI. H+ transporters in the main excretory duct of the mouse mandibular salivary gland. The Journal of Physiology. 1997;503:583–598. doi: 10.1111/j.1469-7793.1997.583bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Musch MW, DePaoli AM, Bookstein CM, Xie Y, Burant CF, Rao MC, Chang EB. Glucocorticoids regulate Na+/H+ exchange expression and activity in region- and tissue-specific manner. American Journal of Physiology. 1994;267:C796–803. doi: 10.1152/ajpcell.1994.267.3.C796. [DOI] [PubMed] [Google Scholar]
- Cook DI, van Lennep EW, Roberts ML, Young JA. Secretion by the major salivary glands. In: Johnson L, Christensen J, Jackson M, Jacobson E, Walsh J, editors. Physiology of the Gastrointestinal Tract. 3. Vol. 2. New York: Raven Press; 1994. pp. 1061–1117. [Google Scholar]
- Counillon L, Franchi A, Pouyssegur J. A point mutation of the Na+/H+ exchanger gene (NHE1) and amplification of the mutated allele confer amiloride resistance upon chronic acidosis. Proceedings of the National Academy of Sciences of the USA. 1993a;90:4508–4512. doi: 10.1073/pnas.90.10.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counillon L, Scholz W, Lang HJ, Pouyssegur J. Pharmacological characterization of stably transfected Na+/H+ antiporter isoforms using amiloride analogs and a new inhibitor exhibiting anti-ischemic properties. Molecular Pharmacology. 1993b;44:1041–1045. [PubMed] [Google Scholar]
- He X, Tse C-M, Donowitz M, Alper SL, Gabriel SE, Baum BJ. Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflügers Archiv. 1997;433:260–268. doi: 10.1007/s004240050276. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf WA, Tsao SC, Levine SA, Montgomery JLM, Yun CHC, Yip JW, Cohen ME, Lazenby AJ, Tse C-M, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush border proteins. American Journal of Physiology. 1996;270:G29–41. doi: 10.1152/ajpgi.1996.270.1.G29. [DOI] [PubMed] [Google Scholar]
- Hwang T-H, Schwiebert EM, Guggino WB. Apical and basolateral ATP stimulates tracheal epithelial chloride secretion via multiple purinergic receptors. American Journal of Physiology. 1996;270:C1611–1623. doi: 10.1152/ajpcell.1996.270.6.C1611. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Wilson RW, Case RM. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. The Journal of Physiology. 1996;495:179–191. doi: 10.1113/jphysiol.1996.sp021583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachishi M, Alzola E, Chaib N, Metioui M, Grosfils K, Kabre E, Moran A, Marino A, Dehaye JP. Study of nonspecific cation channel coupled to P2Z purinergic receptors using an acid load technique. American Journal of Physiology. 1996;271:C1920–1926. doi: 10.1152/ajpcell.1996.271.6.C1920. [DOI] [PubMed] [Google Scholar]
- Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJH, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. Journal of Biological Chemistry. 1997a;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- Lee MG, Zeng W, Muallem S. Characterization and localization of P2 receptors in rat submandibular gland acinar and duct cells. Journal of Biological Chemistry. 1997b;272:32951–32955. doi: 10.1074/jbc.272.52.32951. [DOI] [PubMed] [Google Scholar]
- Macdonald RJ, Southard-Smith EM, Kroon E. Disparate tissue-specific expression of members of the tissue kallikrein multigene family of the rat. Journal of Biological Chemistry. 1996;271:13684–13690. doi: 10.1074/jbc.271.23.13684. [DOI] [PubMed] [Google Scholar]
- Magee DF, Dalley AF. Digestion and the Structure and Function of the Gut. Basel: Karger Press; 1986. Mouth and saliva; pp. 25–54. [Google Scholar]
- Niggli V, Sigel E, Carafoli E. The purified Ca2+ pump of human erythrocyte membranes catalyzes an electroneutral Ca2+-H+ exchange in reconstituted liposomal systems. Journal of Biological Chemistry. 1982;257:2350–2356. [PubMed] [Google Scholar]
- Nuttle LC, El-Moatassim C, Dubyak GR. Expression of the pore-forming P2Z purinoceptor in Xenopus oocytes injected with poly(A)+ RNA from murine macrophages. Molecular Pharmacology. 1993;44:93–101. [PubMed] [Google Scholar]
- Orlowski J, Grinstein S. Na+/H+ exchangers of mammalian cells. Journal of Biological Chemistry. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- Orlowski J, Kandasamy RA. Delineation of transmembrane domains of the Na+/H+ exchanger that confer sensitivity to pharmacological antagonists. Journal of Biological Chemistry. 1996;271:19922–19927. doi: 10.1074/jbc.271.33.19922. [DOI] [PubMed] [Google Scholar]
- Pasyk EA, Foskette JK. Cystic fibrosis transmembrane conductance regulator-associated ATP and adenosine 3′-phosphate 5′-phosphosulphate channels in endoplasmic reticulum and plasma membranes. Journal of Biological Chemistry. 1997;272:7746–7751. doi: 10.1074/jbc.272.12.7746. [DOI] [PubMed] [Google Scholar]
- Paulais M, Cragoe EJ, Turner RJ. Ion transport mechanisms in rat parotid intralobular striated ducts. American Journal of Physiology. 1994;266:C1594–1062. doi: 10.1152/ajpcell.1994.266.6.C1594. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Human genetics. What is good about cystic fibrosis? Current Biology. 1994;4:742–743. doi: 10.1016/s0960-9822(00)00166-4. [DOI] [PubMed] [Google Scholar]
- Reuss L. Epithelial transport. In: Hoffman J, Jamieson J, editors. Handbook of Physiology, section 14, Cell Physiology. New York: Oxford University Press; 1996. pp. 310–387. [Google Scholar]
- Robertson MA, Woodside M, Foskette JK, Orlowski J, Grinstein S. Muscarinic agonists induce phosphorylation-independent activation of the NHE-1 isoform of the Na+/H+ antiporter in salivary acinar cells. Journal of Biological Chemistry. 1997;272:287–294. [PubMed] [Google Scholar]
- Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Disruption of NHE2 Na+/H+ exchanger gene leads to loss of gastric parietal cells. Journal of Clinical Investigation. 1998;101:1243–125. doi: 10.1172/JCI1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheis PJ, Clarke LL, Meneton P, Miller LL, Harline M, Soleimani M, Riddle T, Duffy J, Doetshman T, Lorenz J, Shull GE. Disruption of NHE3 Na+/H+ exchanger gene leads to absorptive defects in intestine and a mild acid-base disturbance. Journal of the American Society of Nephrology. 1997;8:A0208. [Google Scholar]
- Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Selin JH. Intestinal electrolyte absorption and secretion. In: Feldman M, Scharschmidt B, Sleisenger MH, editors. Textbook of Gastrointestinal Disease, section 10. 6. Philadelphia, Pennsylvania: W. B. Saunders; 1997. pp. 1451–1471. [Google Scholar]
- Seo JT, Larcombe-McDouall JB, Case RM, Steward MC. Modulation of Na+-H+ exchange by altered cell volume in perfused rat mandibular salivary gland. The Journal of Physiology. 1995;487:185–195. doi: 10.1113/jphysiol.1995.sp020870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutts MJ, Chinet TC, Mason SJ, Fullton JM, Clarke LL, Boucher RC. Regulation of Cl− channels in normal and cystic fibrosis airway epithelial cells by extracellular ATP. Proceedings of the National Academy of Sciences of the USA. 1992;89:1621–1625. doi: 10.1073/pnas.89.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutts MJ, Fitz JG, Paradiso AM, Boucher RC. Multiple modes of regulation of airway epithelial chloride secretion by extracellular ATP. American Journal of Physiology. 1994;267:C1442–1451. doi: 10.1152/ajpcell.1994.267.5.C1442. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations with Computer Programs. 2. New York: Springer-Verlag; 1986. [Google Scholar]
- Tse CM, Levine SA, Yun CHC, Brant SR, Pouyssegur J, Montrose MH, Donowitz M. Functional characteristics of a cloned epithelial Na+/H+ exchanger (NHE3): resistance to amiloride and inhibition by protein kinase C. Proceedings of the National Academy of Sciences of the USA. 1993;90:9110–9114. doi: 10.1073/pnas.90.19.9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi S, Shigekawa M, Pouyssegur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiological Reviews. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- Wang Z, Orlowski J, Shull GE. Primary structure and functional expression of a novel gastrointestinal isoform of the rat Na+/H+ exchanger. Journal of Biological Chemistry. 1993;268:11925–11928. [PubMed] [Google Scholar]
- Watts BA, Good DW. Apical membrane Na+/H+ exchange in rat medullary thick ascending limb. pHi-dependence and inhibition by hyperosmolarity. Journal of Biological Chemistry. 1994;269:20250–20255. [PubMed] [Google Scholar]
- Welsh MJ. Cystic fibrosis. Scientific American. 1995;273:52–59. doi: 10.1038/scientificamerican1295-52. [DOI] [PubMed] [Google Scholar]
- Xu X, Diaz J, Zhao H, Muallem S. Characterization, localization and axial distribution of Ca2+ signaling receptors in the rat submandibular gland ducts. The Journal of Physiology. 1996;491:647–662. doi: 10.1113/jphysiol.1996.sp021246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhao H, Diaz J, Muallem S. Regulation of [Na +]i in resting and stimulated submandibular salivary ducts. Journal of Biological Chemistry 1995. 1995:19606–19612. [PubMed] [Google Scholar]
- Yu FH, Shull GE, Orlowski J. Functional properties of the rat Na/H exchanger NHE-2 isoform expressed in Na/H exchanger-deficient Chinese hamster ovary cells. Journal of Biological Chemistry. 1993;268:25536–25541. [PubMed] [Google Scholar]
- Yun CHC, Gurubhagavatula S, Levine SA, Montgomery JLM, Cohen ME, Cragoe EJ, Pouyssegur J, Tse C-M, Donowitz M. Glucocorticoid stimulation of ileal Na+ absorptive cell brush border Na+/H+ exchange and association with an increase in message for NHE-3, an epithelial Na+/H+ exchanger isoform (NHE-2) Journal of Biological Chemistry. 1993;268:206–211. [PubMed] [Google Scholar]
- Yun CHC, Tse C-M, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. American Journal of Physiology. 1995;269:G1–11. doi: 10.1152/ajpgi.1995.269.1.G1. [DOI] [PubMed] [Google Scholar]
- Zeng W, Lee MG, Muallem S. Membrane-specific regulation of Cl− channels by purinergic receptors in rat submandibular gland acinar and duct cells. Journal of Biological Chemistry. 1997a;272:32956–32965. doi: 10.1074/jbc.272.52.32956. [DOI] [PubMed] [Google Scholar]
- Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, Marino CR, Kopito R, Freedman S, Cotton C, Muallem S, Thomas P. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. American Journal of Physiology. 1997b;273:C442–455. doi: 10.1152/ajpcell.1997.273.2.C442. [DOI] [PubMed] [Google Scholar]
- Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3− transporters in the rat pancreatic duct. Journal of General Physiology. 1994;104:57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Xu X, Diaz J, Muallem S. Na +, K+, and H+/HCO3− transport in submandibular salivary ducts. Membrane localization of transporters. Journal of Biological Chemistry 1995. 1995:19599–19605. [PubMed] [Google Scholar]


