Abstract
We have investigated whether vestibular information plays a role in the control of voluntary movement of the upper body. Movement consisted of a lateral tilt of the upper body in the frontal plane through an angle of about 8 deg. The influence of vestibular input was assessed from the effect of long duration (3–6 s), low-intensity (0.7 mA) galvanic vestibular stimulation (GVS) applied at different times relative to the movement.
GVS always produced a tilt of the body in the frontal plane but the response was larger and more prolonged when the onset of stimulation coincided with the cue to start moving compared with when it was applied some seconds after movement onset (i.e. while the subject was stationary in a tilted posture).
When the stimulus began 2 s before the voluntary movement the response consisted of two distinct components separated in time, one that was linked to the onset of GVS and another that was linked to onset of the voluntary movement. The large response observed when GVS onset coincided with the movement cue resembled the sum (after realignment in time) of these two separate components.
We suggest that these two components of the response to GVS relate to two different uses of vestibular information for whole-body control: first, to help maintain balance of the body, and second, to help guide and improve the accuracy of voluntary movements involving motion of the head in space.
The vestibular system provides the CNS with information concerning the orientation and motion of the head in space. It is well established that this information is used to control the eyes for the purpose of stabilizing gaze but its participation in motor mechanisms that control the whole body is not so clear. Galvanic vestibular stimulation (GVS), in which vestibular afferent input is perturbed electrically, has been used to show that the vestibular system can contribute towards whole-body control in man. For example, GVS evokes various patterns of muscle activity in the legs (and in the arms if they are being used to help support the body) of subjects attempting to stand still (Nashner & Wolfson, 1974; Britton et al. 1993; Fitzpatrick et al. 1994; Day et al. 1997). Presumably many muscles of the body are involved in the response since during stimulation the body adopts a new posture in which each body segment (from the head to the legs) tilts on the segment below it (Day et al. 1997). The tilted posture leads to a shift of the body's centre of mass. Day et al. (1997) proposed that this whole-body response obtained in stationary subjects is generated by a motor system concerned with maintaining balance of the body in the gravitational field. They suggested that the altered vestibular input produced by GVS is ‘interpreted’ centrally as though it were due to a tilt of the body arising from an apparent tilt of the support surface. They considered the response to be a counter-tilt organized to preserve balance in the face of the apparent platform tilt. According to this idea, the GVS-evoked vestibular input in stationary subjects is handled as though it were produced by a body movement caused by an external disturbance. In the present experiments we investigate how the CNS ‘interprets’ the GVS-evoked vestibular input when the stimulus is applied during active movement of the upper body. Since such movements necessarily transport the head in space, vestibular signals may carry useful information regarding the performance of the movement. This information could be used by the movement system to assess the accuracy of the movement. Distortion of vestibular signals by GVS might then be interpreted as an error in execution of the movement rather than a body movement arising from an external disturbance.
A small number of studies have reported an influence of active body movements on the response to GVS (Smetanin et al. 1987, 1988; Inglis et al. 1995). Smetanin et al. (1987) showed that the GVS-evoked body response in the lateral direction (frontal plane) was enhanced if the stimulus was given at around the time the subject performed certain voluntary movements, such as rising on tiptoes. In a later study, Smetanin et al. (1988) went on to show that a similar enhancement of the lateral response was obtained when subjects voluntarily tilted their body forwards in the sagittal plane. Furthermore, they found that this enhancement was present even if the forward tilt of the body was initiated reflexly, by vibration of tibialis anterior muscle, rather than volitionally. These authors appeared to interpret the response observed during active movement to be an augmented version of the response observed under stationary conditions.
Inglis et al. (1995) studied the interaction between GVS and the body movements produced by translation of the support surface in standing subjects. They showed that the body's response to platform translation was altered in those trials in which GVS was applied 500 ms before the translation and maintained for the duration of the trial. This effect was greater than that produced by GVS on its own in the absence of platform translation. Inglis et al. interpreted this result with a different perspective to that of Smetanin et al. (1988). Rather than active body movement (postural response) facilitating the response to GVS, they appeared to view it more as a phenomenon in which GVS causes a modification of the postural response.
The evidence appears to be in favour of a notable interaction between active body movement and GVS that is robust for different categories of movement. However, the principles of this interaction are not clear. Is it that active body movements somehow augment the whole-body response to GVS, or are active body movements themselves performed differently when GVS is applied during the movement? In the present experiments we attempt to shed some light on this question by studying the interaction between voluntary movement of the upper body and long-duration GVS with the onset of stimulation occurring at different times relative to the movement. Parts of these data have been published in abstract form (Cauquil & Day, 1996).
METHODS
With local ethics committee approval and informed consent, two groups of healthy subjects were studied using two experimental procedures (referred to as experiment 1 and experiment 2). For both experiments subjects stood facing forwards with their eyes closed and feet 16 cm apart (distance between the medial borders of the feet). This distance was chosen for two reasons. It provides a sufficiently large base of support from which voluntary movements involving upper body tilts can be made safely. It also simplifies the experiment by restricting the body tilt evoked by the stimulus to tilts only of upper body segments, with negligible tilting of the pelvis (Day et al. 1997).
General procedure
Prior to the experimental session, subjects were trained to perform the required voluntary movement with their eyes closed. This consisted of tilting the upper body laterally to the left or right through an angle of about 8 deg, and maintaining the tilted position for 6 s. During the training session angled lines were drawn on the wall in front of the subjects so that when the movement was completed they could open their eyes to see how well they were aligned with the target lines. Note that subjects were allowed to open their eyes at this time during the training session only. Subjects were told that the target lines were to be used only as rough guides concerning the amplitude of movement but that movement consistency was important.
During the experimental sessions, subjects were instructed to perform the same voluntary movement (right or left according to the instructions of the experimenter) as soon as they heard the auditory cue (1 kHz maintained until the end of the trial) to move. Subjects kept their eyes closed throughout the movement and hold phase. Eyes were opened only upon return to the normal upright posture. In some trials galvanic vestibular stimulation (GVS) was applied at fixed times relative to the cue to move. GVS consisted of a 0.7 mA direct current, which was shown previously to produce a reasonably large lateral tilt response without saturation (Day et al. 1997). The stimulating current was applied via two electrodes (3 cm diameter) made of soft dental metal that were moulded and then fixed to the mastoid processes with collodion glue. Electrical impedance was reduced using electrode gel between the electrode and skin. The polarity of the stimulating current was varied in a pseudo-random fashion across trials. The timing of stimulus onset varied between experiments but in those trials in which it was applied the stimulus always remained on until the end of the trial.
Experiment 1
In the first experiment, eleven subjects were studied (3 females, 8 males; age range 23–38 years; mean ±s.d.= 29.7 ± 4.8 years). Each trial lasted for 8 s with the auditory cue to move coming at 2 s after the beginning of the trial. The onset of GVS occurred at one of two timings, either at the same time as the cue to move or 3 s later while the subject was tilted and stationary (see Fig. 1).
Figure 1. Group mean head lateral tilt in space during voluntary movement and GVS.
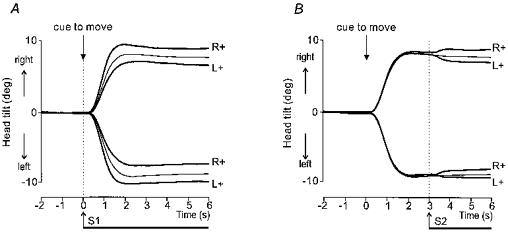
Superimposed movements to the right and left with the two polarities of stimulation, anode right (R+) or anode left (L+). Control movements without stimulation are shown by the thin traces. In A the onset of GVS coincided with the auditory cue to move. In B GVS was applied 3 s after the auditory cue when the movement was completed. Note that in both cases a given polarity of stimulation produced greater final tilt of the head in space for movements in one direction and smaller final tilt for movements in the opposite direction.
Experiment 2
In the second experiment, twelve subjects were studied (3 females, 9 males; age range 21–48 years; mean ±s.d.= 30.4 ± 7.5 years), six of whom had participated in experiment 1. Each trial lasted for 7 s with the cue to move coming at 3 s after the beginning of the trial. As in the first experiment, the onset of GVS occurred at one of two timings, either at the same time as the cue to move or 2 s earlier while the subject was upright and stationary.
For both experiments ten experimental conditions were investigated. These were made up of two movement directions (left, right), three stimulus types (no stimulus, stimulus timing 1, stimulus timing 2) and two stimulation polarities (anode right, anode left) for those trials in which a stimulus was given. Each subject underwent ten trials of each of the ten conditions, which were presented in a pseudo-random order.
Measurement
Lateral tilts in the frontal plane of three body segments (head, trunk and pelvis) were measured using a method developed previously (Day et al. 1997). The tilt angle of each body segment was calculated from the 3-D position in space of a pair of infra-red emitting diodes (IRED) placed 25–35 cm apart. Two IREDs were mounted on a rigid helmet to measure head tilt; two were fixed to the skin at the level of the scapula to measure trunk tilt; two were mounted on a frame clamped to the iliac crests to measure pelvis tilt. The 3-D position of each IRED was measured using an opto-electronic motion measurement system (Selspot II: Selcom AB, S-433 25 Partille, Sweden). Data were collected at 400 Hz and every four consecutive data points were averaged to improve the signal-to-noise ratio, giving an effective sampling frequency of 100 Hz. The ten trials of each condition were averaged for each subject and the tilt angles were computed from these averaged records. The mean tilt angle measured over the first 1 s of each averaged record was subtracted from every data point in that record to ensure that the tilt angle of each segment always started from zero. The angular velocity of tilt was subsequently computed by digital differentiation of angular displacement.
Data analysis
The response to GVS was assessed for each subject from the averaged angular displacement and velocity records over the 2 s period following stimulus onset, after subtraction of the mean control movement (no stimulation). The absolute value (i.e. disregarding direction) of this stimulus-evoked tilt at the end of the 2 s period was measured separately for the head and trunk segments and analysed statistically for the group using a four-factor (body segment, movement direction, stimulus timing, polarity) ANOVA with repeated measures. It should be noted that for these analyses the polarity factor is not equivalent to anode right or left but has been categorized according to whether the stimulus made the movement larger (movement right with anode right, movement left with anode left) or smaller (movement right with anode left, movement left with anode right).
RESULTS
Experiment 1
Upon perception of the auditory cue to move, subjects inclined their body laterally in an attempt to bring it to an angle of about 8 deg from vertical in the frontal plane. The mean (±s.e.m.) interval between the cue and movement onset was 285 ± 17 ms (movements to the left were slightly earlier than movements to the right; 260 ± 19 and 310 ± 27 ms, respectively; P < 0.05, Student's paired t test). Movements were completed, with the body stationary and tilted, within 2.5 s of the cue to move. To perform this voluntary movement subjects did not keep their body straight but tended to tilt the trunk on the lower body and the head on the trunk. This resulted in a slightly curved posture with higher body segments adopting a more inclined attitude (mean ±s.e.m. tilt in space: pelvis, 0.70 ± 0.11 deg; trunk, 6.34 ± 0.85 deg; head, deg). Since the tilt of the pelvis was relatively small, the following analyses will focus only on changes in tilt of the trunk and the head in space.
Figure 1 shows the tilt of the head in space resulting from the voluntary movement and GVS. In this experiment the onset of the stimulus occurred either at the beginning of voluntary movement or 3 s later when the subject was attempting to maintain a static, inclined posture. In both cases the stimulus caused the subject to tilt either further or less far depending upon the polarity of the stimulus and the direction of movement. For example, with the anode behind the right ear the head tilted further for movements to the right but less far for movements to the left. The converse effect was seen when the anode was placed behind the left ear.
The change in tilt produced by GVS, over and above any changes produced by the voluntary movement, were measured over the 2 s period following stimulus onset. The analysis showed that GVS applied at the beginning of voluntary movement produced larger amplitude tilts compared with when GVS was applied during the static phase of the movement (Fig. 2; stimulus timing: F(1,10) = 26.06, P < 0.001). Larger tilts were evoked at the level of the head compared with the trunk (Fig. 2; body segment: F(1,10) = 60.93, P < 0.001), and there was a significant interaction between the factors body segment and stimulus timing (F(1,10) = 38.7, P < 0.001). This main effect and interaction arises from the fact that there was a certain amount of tilting of the head relative to the trunk both during movement and in response to GVS. Tilt of the head in space comprises the sum of tilt of the trunk in space plus the relative tilt of the head on the trunk. Thus stimulus-evoked tilting of the head on the trunk produces effects on the head in space over and above those on the trunk. All other main effects and interactions were not significant.
Figure 2. Group mean (±s.e.m.) angular displacement and velocity evoked by GVS.
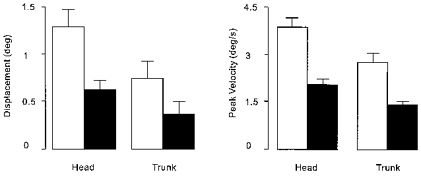
Shown are absolute values that have been calculated first by subtracting traces obtained in the control condition (no stimulation) from those obtained during stimulation. Angular displacement was measured 2 s after stimulus onset and peak angular velocity was measured over the same interval from the resulting difference traces. The contribution of each subject was taken as the mean absolute value obtained from each of the four conditions involving the two movement directions and two polarities of stimulation. Results are shown separately for the head and trunk, when GVS was applied either at the same time as the auditory cue to move (□, GVS with movement) or 3 s later (▪, GVS after movement).
In addition to tilt amplitude, the velocity of the stimulus-evoked tilt also was affected by the timing of GVS. This effect is illustrated in Fig. 3 (see figure legend for details). Statistical analysis showed that stimulus timing affected the peak angular velocity of the response (Fig. 2; stimulus timing: F(1,10) = 47.19, P < 0.001) with the early stimulus (applied at the beginning of voluntary movement) producing a larger effect. For both timings GVS produced greater angular velocity of the head in space than the trunk (Fig. 2; body segment: F(1,10) = 18.51, P < 0.002). All interactions and other main effects were not significant.
Figure 3. Group mean head lateral angular velocity during voluntary movement and GVS.
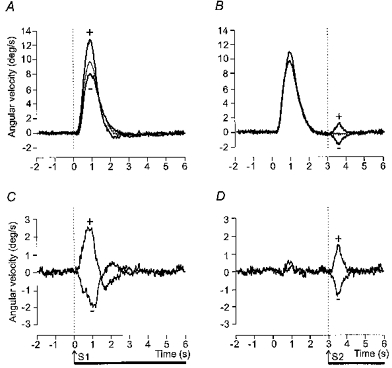
Upper panels (A and B) show superimposed the mean lateral angular velocity of the head in space for control movements (thin lines) and movements during which GVS was applied (thick lines). The movement cue was given at 0 s. Movements to the right and left have been averaged after inversion of traces from leftward movements. The trials in which GVS was applied have been sorted according to whether a given polarity of stimulation produced either greater deviation of the body in the direction of the movement (+) or less deviation (=) (see Fig. 1). Lower panels (C and D) show the same data after subtraction of the control movement (without stimulation) trace from the trials in which GVS was applied. In A and C GVS began at the same time as the movement cue. In B and D GVS began 3 s after the auditory cue when the movement was completed
The time course of the extra tilt evoked by GVS differed according to when the stimulus was applied. The peak angular velocity occurred approximately 50 % later when the stimulus was applied at the beginning of voluntary movement compared with the end (mean ±s.e.m.): trunk, 932 ± 28 vs. 645 ± 38 ms; head, 957 ± 47 vs. 581 ± 39 ms; stimulus timing: F(1,10)=684.2, P < 0.001). This effect is shown in Fig. 4 in which the stimulus-evoked angular velocity responses (after subtraction of the control movement) have been shifted in time such that the two stimuli coincide. This figure also illustrates for both the head and the trunk the striking similarity between the two responses at an early stage. At 0.5 s after stimulus onset there was no significant difference in the tilt velocity evoked by the two timings of stimulation (stimulus timing: F(1,10)=0.04, P=0.85).
Figure 4. Net effect of GVS on group mean head and trunk lateral angular velocity.
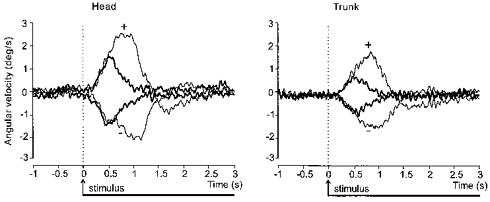
Superimposed traces showing the net effect of the two polarities (+ and −; see Fig. 3 legend) of GVS, after subtraction of the control movement as in Fig. 3C and D, on the lateral angular velocity of the head (left panel) and trunk (right panel) in space. The traces have been realigned to the onset of stimulation at 0 s. GVS began either at the same time as the movement cue (thin lines) or 3 s after the cue when the movement was completed (thick lines).
Experiment 2
It is not clear from the superimposed traces of Fig. 4 whether the large response obtained when GVS onset coincided with the movement cue was simply an exaggerated version of the stationary response or whether it contained a separate, additional component. To explore these two possibilities we measured the effect of starting GVS some seconds before a voluntary movement and sustaining it throughout the movement.
The control movements (without stimulation) and the initial tilt response to GVS were similar to those described in experiment 1. The grand average stimulus-evoked tilt responses to the two timings of stimulation are shown for the head and trunk tilt velocities separately in Fig. 5. In this figure the left and right movements have been combined (after inversion of traces obtained from movements to the left) and the control movements have been subtracted (as in Fig. 3). In keeping with the results from experiment 1, when the onset of stimulation coincided with the cue to move, the peak velocity response was greater than when stimulation began 2 s earlier while the body was stationary (Table 1; stimulus timing: F(1,11)=72.81, P < 0.001). Again, the stimulus-evoked head angular velocity was greater than that of the trunk (Table 1; body segment: F(1,11)=48.17, P < 0.001) and there was a significant interaction between the factors body segment and stimulus timing (F(1,11)=7.82, P < 0.05).
Figure 5. Effect of starting GVS before the movement on the net GVS-evoked response.
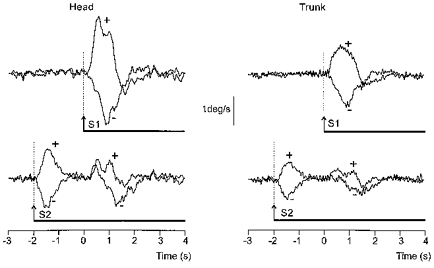
Superimposed traces showing the group mean net effect of the two polarities (+ and −; see Fig. 3 legend) of GVS, after subtraction of the control movement as in Fig. 3C and D, on the lateral angular velocity of the head (left traces) and trunk (right traces) in space. The movement cue was given at 0 s. GVS began either at the same time as the movement cue (upper traces) or 2 s before the cue when the body was stationary (lower traces). Note the two distinct components of the response when GVS began 2 s before the movement cue.
Table 1.
Group mean (±s.e.m.) peak angular velocity (deg s−1)
| Time of GVS onset relative to movement cue | 0 s | −2 s (component 1) | −2 s (components 1 + 2)* |
|---|---|---|---|
| Head | 3.35 ± 0.27 | 1.74 ± 0.13 | 2.82 ± 0.28 |
| Trunk | 2.34 ± 0.14 | 1.43 ± 0.10 | 2.19 ± 0.18 |
Measured from the sum (after realignment in time) of response waveforms obtained during the first and second components of the response (see Fig. 6).
Figure 6. Artificial summation of the two components of the response obtained when GVS started before movement onset.
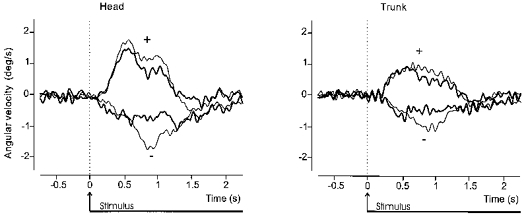
Superimposed traces derived from data illustrated in Fig. 5 for the net GVS-evoked (after subtraction of control movement) lateral angular velocity of the head (left panel) and trunk (right panel) in space. The thin traces show the response when GVS began at the same time as the movement cue at 0 s. The thick traces have been artificially constructed from the two components of the response obtained when GVS began 2 s before the movement cue (see Fig. 5, lower traces). The first component of the response was shifted forward in time by 2 s and then summed with the second component of the response.
This protocol showed that when stimulus onset occurred 2 s before the cue to move, a second response was observed around the time of the dynamic phase of the voluntary movement. Although the grand average response hints at some complex features (see Fig. 5), the second response was largely in the same direction as the initial response. For example, with the anode behind the right ear the early stimulus caused the trunk and head to tilt to the right. When this was followed by a voluntary tilt of the body further to the right then the body segments were observed to tilt more rapidly than in control movements. Conversely, these voluntary movements to the right were performed more slowly when the anode was on the left causing an initial leftward tilt of the body.
DISCUSSION
The results from both experiments confirm the observations of Smetanin et al. (1987, 1988) that a larger response occurs when GVS is applied during the execution of a voluntary body movement compared with when stationary. The symmetry of the effects produced by opposite polarities of stimulation suggests that movement-related load and inertial factors do not explain these results. Furthermore, the results from the second experiment suggest that the larger response is not simply an augmented version of that obtained when the subject is still. Our interpretation is that the response appears to be larger when a subject is moving because it contains an additional component. The essence of our model is that the response consists of two separate components with one component linked to the onset of GVS, irrespective of whether the subject is moving or stationary, and the other linked to performance of voluntary movement.
We were able to dissociate the two components of the response by beginning the period of stimulation 2 s prior to the voluntary movement and maintaining it until after the movement was completed (experiment 2). Under this condition there was an initial response consisting of a small lateral tilt of the trunk on the pelvis together with a tilt of the head on the trunk. This was as described previously (Day et al. 1997). By the time the movement cue was given 2 s later this early response had developed fully and the body was again static having adopted the new posture. During the subsequent execution of the voluntary tilt the continued presence of GVS produced a second effect. It acted largely to speed up or slow down the voluntary movement culminating in a more or less tilted posture depending upon the polarity of stimulation and movement direction. It is unlikely that this second effect was a compensation for starting the movement from the slightly tilted position produced by the initial response. If this were the case one might expect the voluntary movement to be attenuated when the initial response had already tilted the body towards the desired final position, but the converse was observed.
As stated above, our interpretation of the results is that the large response obtained when GVS is applied at the beginning of voluntary movement represents the combined action of two components. At its simplest, the two proposed components (one linked to GVS onset and the other linked to movement onset) would summate linearly to produce the large combined response. We tested this idea by artificially summing (after realignment in time) the two sequential components obtained when the stimulus was started some seconds before movement onset. The shape and duration of the artificially summed waveform was similar to the actual response waveform thus providing some support for the two-component hypothesis. However, the match was not perfect with a weak tendency for the summed waveform to be smaller than the actual response. On its own this discrepancy does not allow us to reject the two-component hypothesis. It may simply mean that the two components summate in a more complex, non-linear fashion. One other possible explanation for the discrepancy arises from the implicit assumption that the movement-related component would remain the same regardless of how long before movement onset the stimulus commenced. This assumption in turn relies upon GVS-evoked vestibular afferent discharge rates remaining constant over time, which is unlikely to be the case. A number of published records show examples of adaptation of firing rate during maintained galvanic stimulation (Lowenstein, 1955; Lifschitz, 1973; Goldberg et al. 1984). Courjon et al. (1987) observed this adapting response pattern to a step change of stimulating current in 7 % of a sample of Scarpa's ganglion units in the rat. Similar partial adaptation of vestibular afferent firing in the present experiments would contribute to the observed discrepancy between the summed and actual waveforms. Overall, therefore, the two-component hypothesis provides a parsimonious explanation of the results.
The component of the response linked to the onset of GVS has previously been suggested to fulfil a balance function (Day et al. 1997). The component of the response linked to the onset of voluntary movement is likely to serve a different function. The fact that this latter response is linked to the onset of movement suggests strongly that it is related to the function of movement control. Thus the change in vestibular afferent input evoked by GVS causes the movement system to alter the metrics of the intended movement. It is not possible to say from the present data whether GVS acts to modify the movement by altering the motor program at a preparatory stage or by altering the movement at a later stage via feedback mechanisms. However, the possibility we favour is similar to the notion put forward by Inglis et al. (1995) to explain their observed effect of GVS on the body response to translation of the support surface. They raised the possibility that the vestibular system may play some role in interpreting sensory reafference during the body response to the translation. In line with this idea we suggest the following: voluntary tilt of the upper body and head would evoke a natural change in vestibular afferent firing. Presumably, both the rate and extent of the voluntary tilt would influence this firing pattern, which therefore would contain valuable information regarding movement performance. This movement-related vestibular afferent firing pattern would be distorted by GVS. Animal experiments have shown that the effect of GVS on vestibular afferent firing is to produce predominantly a step change with cathodal stimulation producing an increase and anodal stimulation a decrease in firing rate (Lowenstein, 1955; Goldberg et al. 1984; Courjon et al. 1987; Minor & Goldberg, 1991). Even without precise knowledge of the effect of GVS on vestibular afferent firing during movement it seems clear that left-sided and right-sided afferents would experience different distortions of their normal firing pattern. The firing rates of vestibular afferents on the side of the cathode would have been largely increased and those on the side of the anode largely decreased. It is feasible for such an effect to be interpreted centrally as an error in the rate and/or extent of the voluntary movement leading to a correction of the movement. For example, with the anode on the right and cathode on the left GVS would produce a decrease in firing of right-sided afferents and an increase of left-sided afferents. We shall assume that for voluntary movements to the right this gives rise to the interpretation that the movement is being performed too slowly or through too small an angle. The increase in tilt velocity and amplitude of the movement observed under this condition could then be thought of as a compensatory adjustment to the movement. This would mean, however, that voluntary movements to the left would appear to be going too fast or too far leading to a compensatory decrease in tilt velocity and amplitude, as was observed. Similarly, changing the polarity of stimulation would, and did, reverse the sign of these effects. We suggest that such an adjustment of voluntary movement based on vestibular information forms the basis of the second component of the response to GVS.
In conclusion, we propose that vestibular information is used by the central nervous system for two separate functions related to whole-body control; first, to help maintain balance of the body in the gravitational field, and second, to help guide and improve the accuracy of voluntary (and possibly non-voluntary) movements of the body involving motion of the head in space. To achieve this, one possibility would be that vestibular information is routed in parallel to two distinct systems where it is processed separately for these two functions of balance and movement.
Acknowledgments
We would like to thank Mr R. Bedlington, Mr D. Buckwell and Mr W. Cameron for technical assistance. A. S. C. was supported by the European Space Agency and from CEC grant access to large scale facilities.
References
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic response in the arm and the leg following galvanic vestibular stimulation in man. Experimental Brain Research. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Cauquil AS, Day BL. Influence of voluntary movement on the lateral tilt of body segments induced by galvanic vestibular stimulation in man. The Journal of Physiology. 1996;494.P:66P. [Google Scholar]
- Courjon JH, Precht W, Sirkin DW. Vestibular nerve and nuclei unit responses and eye movement responses to repetitive galvanic stimulation of the labyrinth in the rat. Experimental Brain Research. 1987;66:41–48. doi: 10.1007/BF00236200. [DOI] [PubMed] [Google Scholar]
- Day BL, Cauquil AS, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. The Journal of Physiology. 1997;500:661–672. doi: 10.1113/jphysiol.1997.sp022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. The Journal of Physiology. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. Journal of Neurophysiology. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Shupert CL, Hlavacka F, Horack FB. Effect of galvanic vestibular stimulation on human postural responses during support surface translations. Journal of Neurophysiology. 1995;73:896–901. doi: 10.1152/jn.1995.73.2.896. [DOI] [PubMed] [Google Scholar]
- Lifschitz WS. Responses from the first order neurons of the horizontal semicircular canal in the pigeon. Brain Research. 1973;63:43–57. doi: 10.1016/0006-8993(73)90075-9. 10.1016/0006-8993(73)90075-9. [DOI] [PubMed] [Google Scholar]
- Lowenstein O. The effect of galvanic polarization on the impulse discharge from sense endings in the isolated labyrinth of the thornback ray (Raja clavata) The Journal of Physiology. 1955;127:104–117. doi: 10.1113/jphysiol.1955.sp005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiologica Scandinavica. 1983;117:307–309. doi: 10.1111/j.1748-1716.1983.tb07212.x. [DOI] [PubMed] [Google Scholar]
- Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. Journal of Neuroscience. 1991;11:1636–1648. doi: 10.1523/JNEUROSCI.11-06-01636.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner LM, Wolfson P. Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of the human labyrinth. Brain Research. 1974;67:255–268. doi: 10.1016/0006-8993(74)90276-5. 10.1016/0006-8993(74)90276-5. [DOI] [PubMed] [Google Scholar]
- Smetanin BN, Popov KE, Gurfinkel VS, Shlykov VY. Effect of movement and illusion of movement on human vestibulo-motor response. Neirofiziologiya. 1988;20:192–196. [Google Scholar]
- Smetanin BN, Shlykov VY, Kudinova MP. Facilitation of vestibulo-motor response by voluntary movements in man. In: Gantchev GN, Dimitrov B, Gatev P, editors. Motor Control. New York: Plenum Press; 1987. [Google Scholar]


