Abstract
Vestibular-dependent responses in leg muscles following transmastoid galvanic stimulation have been well characterized. Here we describe the properties of vestibulocollic responses evoked by transmastoid galvanic stimulation.
In twelve healthy human subjects we examined the averaged responses in unrectified sternocleidomastoid (SCM) EMG evoked by transmastoid stimulation using current pulses of 4 mA intensity and 2 ms duration. In ten subjects we also examined the effects of unilateral vestibular stimulation with the indifferent electrode at the vertex. In further experiments we studied the effects of different levels of background muscle activation, head position, current intensity and current duration. We compared these responses with click-evoked vestibulocollic responses in SCM.
A clearly defined biphasic response, beginning with a surface positivity, was recorded in the SCM ipsilateral to the side of cathode placement in all subjects. We refer to this as the p13/n23 [g] (galvanic) response, given the close similarity, in terms of waveform and latencies, to the previously described click-evoked p13/n23 vestibulocollic response. The amplitude of this response was linearly related to background muscle activation, current intensity and current duration, but independent of head position. Unilateral galvanic stimulation revealed the p13/n23 [g] response to be solely generated by the cathode.
A biphasic response beginning with a surface negativity (n12/p20 [g]) contralateral to the cathode was seen in all subjects and was generated by both the cathode contralaterally and the anode ipsilaterally.
Both the p13/n23 [g] and n12/p20 [g] potentials were abolished by selective vestibular nerve section and unaffected by severe sensorineural deafness.
We conclude that galvanic stimulation evokes short-latency vestibulocollic reflexes. These vestibulocollic reflexes have properties that are distinct from those described for galvanic-evoked vestibular reflexes in leg muscles, and which may be related to their differing physiological roles.
Galvanic (DC) stimulation has long been used as a non-mechanical means of activating the vestibular apparatus (Camis, 1930), probably by a direct action on vestibular nerve endings (Goldberg et al. 1984; Watson & Colebatch, 1997). The powerful postural effects of low current intensity stimulation have been extensively reported (e.g. Coats & Stoltz, 1969; Britton et al. 1993; Fitzpatrick et al. 1994; Day et al. 1997). A characteristic feature of the sway induced by galvanic stimulation is that its direction is determined by the orientation of the head in relation to the feet (Nashner & Wolfson, 1974; Lund & Broberg, 1983). The effects of galvanic stimulation upon leg muscle EMG have also been reported (e.g. Nashner & Wolfson, 1974; Iles & Pisini, 1992; Britton et al. 1993). These EMG responses show a similar dependence on head orientation (Nashner & Wolfson, 1974; Tokita et al. 1989) and are also strongly influenced by postural task (Britton et al. 1993; Fitzpatrick et al. 1994); in particular, responses can only be recorded in muscles that are performing a postural task. The prominence of postural effects suggests an action on otolith pathways (Walsh, 1964), but it is still uncertain whether galvanic stimulation acts selectively on a subpopulation of vestibular afferent fibres, such as those innervating the otolith organs, or on afferents arising from all vestibular end-organs. Recent studies of galvanic-evoked eye movements have provided support for a preferential action on otolith afferents by demonstrating that ocular torsion is the predominant oculomotor effect of low-intensity galvanic stimulation (Zink et al. 1997; Watson et al. 1998a).
The neck receives vestibular projections that are more potent than those to the limbs, and a specific pattern of vestibulocollic connections is present for differing vestibular end-organs (Wilson et al. 1995; Uchino et al. 1997). Vestibulocollic reflexes occurring at short latency have been recorded in man in response to clicks (Bickford et al. 1964; Colebatch et al. 1994), head taps (Halmagyi et al. 1995) and also to sudden head dropping (Ito et al. 1995). Vestibulocollic reflexes evoked by galvanic stimulation in man have not been previously described.
Our study was designed to investigate vestibulocollic reflexes induced by galvanic stimulation. Assuming such reflexes could be recorded, we wished to define their physiological properties and to compare them with vestibulocollic reflexes evoked by clicks, and also with vestibular-dependent reflexes in leg muscles.
A brief report on galvanic-evoked vestibulocollic responses in two patients before and after selective vestibular nerve section has already been published (Watson et al. 1998b).
METHODS
All subjects and patients gave informed, written consent and were studied using techniques approved by the local ethics committee. For all experiments, averaged responses to 256 stimuli delivered at 5 s−1 were recorded using both unrectified and rectified EMG of sternocleidomastoid (SCM) bilaterally. The reflex responses were measured from the unrectified average, while the rectified average was used to quantify the level of tonic activation (see ‘EMG recording and analysis’ below). Activation of SCM was achieved by head elevation from the semi-reclined position (unless specifically indicated). Clicks were delivered through calibrated headphones (TDH49, Telephonics Corp., NY, USA). Current stimulation (model DS2A, Digitimer Ltd, UK) was via 600–900 mm2 electrodes, cut from electrosurgical plating (3M, St Paul, USA), placed over the mastoid processes bilaterally and secured with adhesive tape. Due to the very short onset latency of vestibulocollic reflexes (typically 8 ms; Colebatch et al. 1994) we have used a short duration (2 ms) of galvanic stimulation. For responses in leg muscles, short-duration galvanic stimuli evoke smaller, but otherwise similar, effects to those following longer duration stimuli (Watson & Colebatch, 1997, 1998). A series of different experiments were performed.
Responses to transmastoid galvanic stimulation
In twelve normal subjects (aged 22−44 years; six females, six males) we recorded responses in SCM following transmastoid galvanic stimulation and clicks to each ear. A current of 4 mA intensity and 2 ms duration was passed between electrodes placed over the two mastoid processes (both combinations of electrode placement were used, i.e. cathode left-anode right and cathode right-anode left). Preliminary experiments showed that the use of these stimulation parameters resulted in clear EMG responses, and was well tolerated. Clicks of 100 dB (normal hearing level (NHL): referenced to the normal level of perception for clicks of this duration) intensity and 0.1 ms duration were also delivered to each ear in turn in a pseudorandom sequence; see Colebatch et al. (1994) for details.
Responses to lateralized galvanic stimulation
In ten of these subjects we also performed ‘unilateral’ galvanic stimulation. For these experiments the cathode and then the anode were placed over each mastoid process in turn, while the indifferent electrode (of opposite polarity) was placed at the vertex. A standard 9 mm silver electrode was used at the vertex. This montage allows separate stimulation of each vestibular apparatus (Watson & Colebatch, 1997). A current of 4 mA intensity and 2 ms duration was used.
Effects of tonic muscle activation and head position
In five subjects the transmastoid stimulation parameters were kept constant (4 mA, 2 ms; cathode left in each case), while the level of background SCM activation was systematically varied between trials. Each subject was studied with at least five levels of tonic activation while sitting upright and exerting forehead pressure against a padded support, with rectified and smoothed EMG displayed as a horizontal line on an oscilloscope. The subjects were given a target level of EMG to maintain during the data collection. In four of these subjects, at least two levels of activation were also obtained while supine, by varying the degree of head elevation. In three of these subjects the effect of head rotation was also investigated, using the same stimulus parameters, during a separate session. While sitting upright, each of these three subjects was studied with at least four levels of tonic activation with the head straight (as described above), and at least two levels of activation with the head rotated both to the left and to the right. During both left and right head rotation, activation of the left SCM (always ipsilateral to the cathode) was obtained by isometric head rotation to the right, with the subject's chin pressing against the hand of one of the experimenters.
Recovery cycle and interaction between galvanic and click stimulation
In five subjects the effect of varying interstimulus intervals on the response amplitudes was explored using a test-conditioned response paradigm. Transmastoid currents of 2 ms duration and either 3 mA (two subjects) or 4 mA (three subjects) intensity were used (adjusted to give a response similar in amplitude to 95 dB clicks in the same subject). Clicks of 95 dB (NHL) were used, rather than 100 dB clicks, to reduce the loudness of dual stimuli at short intervals. At least six interstimulus intervals (30, 40, 50, 100, 200 and 300 ms) were used in each subject. Data were collected first for unilateral clicks and then for transmastoid galvanic stimulation with the cathode placed on the same side. When interstimulus intervals of greater than 100 ms were used, the repetition rate for the pairs of stimuli was 1 s−1, while for interstimulus intervals of 100 ms or shorter, a repetition rate of 3 s−1 was used.
In five subjects the interaction between galvanic- and click-evoked responses was determined using submaximal responses of similar amplitude to each stimulus type. Responses were recorded following clicks (90 or 95 dB) to one side, transmastoid stimulation (3 or 4 mA, 2 ms) with the cathode placed on the same side as the click, or a combination of both given simultaneously. Eleven experiments were performed in the five subjects: three in one subject and two each in the remainder. The response to combined stimulation was compared with the response obtained by algebraically summing the traces of the responses to the separate stimuli.
Current intensity and duration
In ten subjects the current intensity of transmastoid stimulation was varied between runs while the duration was kept constant at 2 ms. For each subject and each polarity of stimulation, responses were obtained for between five and eleven current intensities, with the maximum current used ranging between 6 and 10 mA, depending on the level tolerated by each subject. The minimum current used was 1 mA in eight subjects and 0.5 mA and 2 mA in one subject each. In five subjects the current duration of transmastoid stimulation (one electrode polarity only) was varied (0.5−4 ms) while the current intensity was maintained constant at either 4 mA (three subjects) or 6 mA (two subjects).
Patients with vestibular neurectomy or sensorineural deafness
Four patients (age range 40−70 years; two females, two males) were studied, in each of whom unilateral vestibular nerve section (vestibulocochlear in two) had been performed between 2 and 6 years previously as treatment for vertigo due to unilateral Ménière's disease. Two additional patients (also reported by Watson et al. 1998b) were studied after vestibular nerve section and with the use of unilateral galvanic stimulation. Three patients (age range 26−66 years; one female, two males) with severe unilateral sensorineural deafness were also studied. Both transmastoid and unilateral galvanic stimulation (4 mA, 2 ms) as well as 100 dB (NHL) clicks were used in all patients.
EMG recording and analysis
The active recording electrodes (Red Dot, 3M, St Paul, USA) were placed over SCM bilaterally, 60−80 mm above reference electrodes placed over the medial portions of the clavicles. This placement of recording electrodes was slightly (20−30 mm) lower than that used in previous studies of click-evoked vestibulocollic responses (Colebatch et al. 1994) due to the placement of an earth electrode, which encircled the neck and was cut from electrosurgical plating (3M). Both unrectified and rectified EMG were amplified and bandpass filtered (8−1600 Hz). EMG was sampled (3.2 or 5.0 kHz) for 20 ms before to 100 ms after stimulus delivery, using a 1401plus analog-to-digital converter and Sigavg software (Cambridge Electronic Design, Cambridge, UK) on a PC computer.
In preliminary experiments we found stimulus artifact to be a significant problem, even with the neck-encircling earth electrode, but we were able to overcome this using a combination of two techniques, one based upon physiology and the other electronic. Click-evoked vestibulocollic responses can only be recorded in tonically active muscles, and the amplitude of responses is directly proportional to the level of background muscle activation (Colebatch et al. 1994; Lee et al. 1995). We were able to confirm that galvanic-evoked responses in SCM have the same property (see below). Thus the averaged EMG when stimulation is applied during relaxation consists of stimulus artifact only, while the average performed during tonic activation consists of stimulus artifact plus the reflex response. For each study performed during tonic muscle activation (the ‘active’ trace), we also performed a matching study with the same stimulus parameters during relaxation (the ‘relaxed’ trace). By subtracting the ‘relaxed’ trace from the ‘active’ trace, once data collection was complete, we obtained traces in which reflex responses were apparent and in which the artifact was cancelled out (Fig. 1). This method applies only if the artifact is relatively small so that the amplifiers are working within their linear range and are not saturated. Because the artifact did sometimes overload the amplifiers, an electronic device was used to minimize it around the period of galvanic stimulation (typically beginning 0.2 ms before stimulus onset and finishing 0.5 ms after stimulus termination). With the use of this device, an electronic input ‘clamp’, it was nearly always possible to obtain traces suitable for subtraction. In all studies with galvanic stimulation and most studies with clicks (the latter purely for consistency), both the electronic clamp and the technique of trace subtraction described above were used.
Figure 1. Technique of trace subtraction used to minimize stimulus artifact.
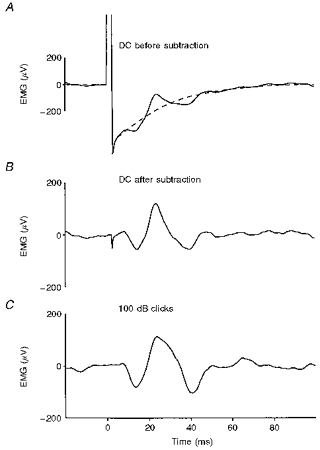
Each trace represents an average (n= 256) of unrectified EMG from the right sternocleidomastoid (SCM). In this and all subsequent figures, negative potentials at the active electrode are shown as an upward deflexion. The two superimposed traces in A and the single trace in B show responses to transmastoid stimulation (4 mA, 2 ms) with the cathode on the right mastoid. The continuous trace in A is the average with tonic SCM activation and the dashed trace is the average with SCM relaxed. Both traces show stimulus artifact, but the reflex response only occurs with activation. B, trace obtained by subtracting the relaxed average from the active one: the p13/n23 [g] response appears free of stimulus artifact. C, p13/n23 [c] response to right-sided clicks for comparison.
Analysis
Peaks in averaged unrectified EMG were described by mean latency preceded by lower case letter, e.g. ‘p13/n23′ representing a myogenic response with an initial positivity at 13 ms followed by a negativity at 23 ms (Colebatch et al. 1994). We have retained the notation p13/n23 used in previous studies, despite the latencies in the present study being slightly longer (almost certainly due to the lower placement of the recording electrodes described above). Where click and galvanic stimulation resulted in similar EMG responses occurring at a similar latency, we have used the same notation for each, using a lower case letter in square brackets to indicate the stimulus modality, e.g. for galvanic ‘p13/n23 [g]’ and for click ‘p13/n23 [c]’. Response amplitudes in unrectified EMG were measured peak-to-peak, and then divided by the mean of the rectified EMG for the 20 ms preceding the stimulus onset. This gives a value for response amplitude independent of the level of background muscle activation, since the peak-to-peak amplitude is directly proportional to the level of background muscle activation (see below). All values are given as means ±s.d. Statistical comparisons were made using Student's t tests and linear correlation analysis.
RESULTS
Transmastoid galvanic stimulation and clicks
All twelve normal subjects showed initial positive-negative biphasic p13/n23 responses unilaterally following transmastoid galvanic stimulation, as well as after click stimulation (Fig. 2). The p13/n23 response was only present in the SCM ipsilateral to the cathode (p13/n23 [g]) or ipsilateral to the side of click delivery (p13/n23 [c]). Onset latencies ranged from 6.7 to 9.8 ms (n= 12) for p13/n23 [g] and from 6.8 to 9.9 ms (n= 12) for p13/n23 [c]. The p13 and n23 peak latencies were 0.6−0.8 ms shorter following galvanic stimulation (14.0 ± 1.79 and 22.4 ± 1.88 ms, respectively) than following clicks (14.6 ± 1.79 ms, P= 0.06; 23.2 ± 1.84 ms, P < 0.05, respectively). The p13/n23 response amplitudes (expressed as a ratio of the background EMG, see above), averaged for all twelve subjects, were 1.2 for 4 mA, 2 ms transmastoid galvanic stimulation and 1.5 for 100 dB clicks (n.s.). There was a weak correlation between the amplitudes of the responses to the two modalities of stimulation (r2= 0.17, P= 0.05, n= 24). The amplitude of the p13/n23 response in the right SCM was divided by the sum of the amplitudes of the p13/n23 responses in right (R) and left (L) SCM (R/(R + L)) to obtain a measure of the symmetry of the p13/n23 response amplitude to ipsilateral cathodal or click stimulation: the range was 0.41–0.59 for p13/n23 [g], and 0.35−0.59 for p13/n23 [c], with neither being significantly different from 0.5. The p13/n23 response amplitudes were significantly correlated between the two sides for both galvanic stimulation (r2= 0.59, P < 0.01, n= 12) and for clicks (r2= 0.84, P < 0.0001, n= 12).
Figure 2. Averaged responses to transmastoid stimulation and clicks for a normal subject.
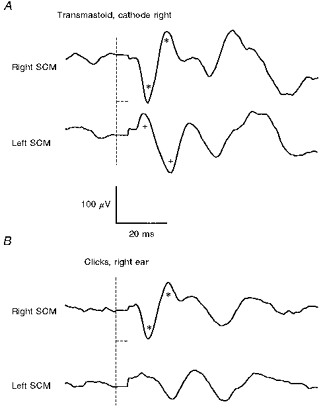
A, responses to galvanic stimulation (4 mA, 2 ms; cathode right, anode left); B, responses to right-sided clicks (100 dB). The vertical dashed lines show the time of stimulus onset, while the horizontal dashed lines show the period over which the EMG signal was clamped to minimize stimulus artifact. Note that each trace is the result of subtracting an average (n= 256) obtained with SCM relaxed (stimulus artifact only) from an average with SCM activated (reflex response plus artifact) to remove artifact (see Fig. 1). A p13/n23 [g] response is present in the top trace in A recorded from the right SCM (*), which is very similar to the p13/n23 [c] response in the top trace in B (*). A n12/p20 [g] response is present in the bottom trace of A recorded from the left SCM (+), and a very small response at the same latency may be present in the bottom trace of B.
In addition to the p13/n23 [g] response on the side of the cathode, a biphasic negative-positive response (n12/20 [g]) was observed in all subjects following transmastoid galvanic stimulation in the other SCM, i.e. ipsilateral to the anode (Fig. 2). With clicks a crossed response of similar form and latency was seen in only six of the twelve subjects. Later responses were frequently seen, which varied considerably in latency and amplitude between subjects (Fig. 2). These late responses had similar waveforms in both SCMs, independent of the side of stimulation, and our evidence suggests that these are not of vestibular origin (see below).
Lateralized galvanic stimulation
Unilateral cathodal stimulation (ten subjects) evoked responses similar to those following transmastoid stimulation with the cathode on the same mastoid process, with a p13/n23 [g] response in the SCM ipsilateral to the cathode and a n12/p20 [g] response in the contralateral SCM (Fig. 3). The p13/n23 [g] response following unilateral cathodal stimulation did not have significantly different latency or amplitude to that following transmastoid galvanic stimulation, and no p13/n23 [g] responses were observed after unilateral anodal stimulation, implying that the cathodal electrode is exclusively responsible for the p13/n23 [g] response to transmastoid stimulation. The (ipsilateral) p13/n23 [g] response following unilateral cathodal stimulation was significantly larger than the corresponding (contralateral) n12/p20 [g] response (mean 1.1 vs. 0.64, respectively; P < 0.00001, n= 20). Although no p13/n23 [g] responses were observed in either SCM after unilateral anodal stimulation, a n12/p20 response was recorded in the SCM ipsilateral to the side of anode placement (Fig. 3). The amplitude of the crossed n12/p20 [g] response following unilateral cathodal stimulation was significantly larger than that of the uncrossed n12/p20 [g] response following unilateral anodal stimulation (mean 0.64 vs. 0.29, respectively; P < 0.01, n= 20). These findings imply that the n12/p20 [g] response to transmastoid stimulation is generated by both the cathode and the anode. Consistent with this conclusion, the sum of the amplitudes of the crossed n12/p20 [g] response and the uncrossed n12/p20 [g] response in the one SCM (0.93) was not significantly different from the amplitude of the n12/p20 [g] response recorded following transmastoid stimulation (0.92, P= 0.9, n= 20). Onset latencies for the n12/p20 response were generally difficult to determine, due to overlap with stimulus artifact, but values ranged from 6.1 to 11.4 ms (n= 7) for cathodal stimulation, and from 8.0 to 8.4 ms (n= 2) for anodal stimulation. The mean peak latencies for the n12/p20 [g] response following unilateral cathodal stimulation of 12.8 ± 2.8 and 20.5 ± 1.9 ms were not significantly different from those following unilateral anodal stimulation of 13.1 ± 1.7 and 19.1 ± 1.0 ms. Although the latency of the negative peak is closer to 13 ms than 12 ms, we used the notation ‘n12′ because in this study its latency is approximately 1 ms shorter than the p13 [g] latency.
Figure 3. Responses to unilateral galvanic stimulation in a normal subject.
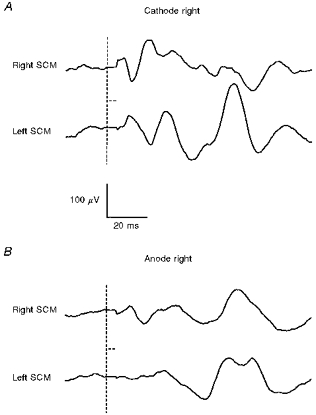
Same subject as in Fig. 2: averaged responses (n= 256) in unrectified EMG from right and left SCMs to right-sided unilateral galvanic stimulation (4 mA, 2 ms). A, responses to unilateral cathodal stimulation (anode at vertex); B, responses to unilateral anodal stimulation (cathode at vertex). The vertical dashed lines show the time of stimulus onset, while the horizontal dashed lines show the period over which the EMG signal was clamped to minimize stimulus artifact. A p13/n23 [g] response is seen in the ipsilateral SCM with cathodal stimulation, with a crossed n12/p20 [g] response in the contralateral SCM. With right-sided anodal stimulation, a small n12/p20 [g] is seen ipsilaterally, which resembles the crossed response to unilateral cathodal stimulation. No short-latency response is seen in the left SCM with right-sided anodal stimulation.
Effects of background muscle activation and head position
In all five subjects there was a strong linear correlation between background muscle activation and p13/n23 [g] response amplitude (r2= 0.90–0.97, P < 0.005 in every case), without any evidence of saturation at the higher activation levels (Fig. 4). In no instance was the y-intercept significantly different from zero. There were no significant differences between the amplitudes of p13/n23 [g] responses in the different head positions when allowance was made for tonic activation levels (Fig. 4).
Figure 4. Relationship between p13/n23 [g] response amplitude and background SCM activation.
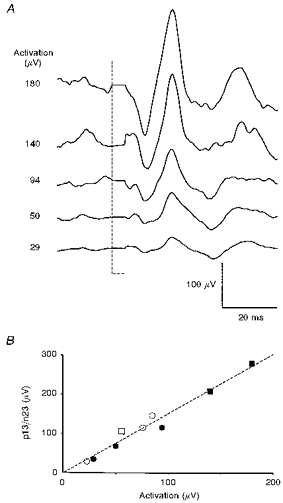
A, selection of averaged responses (n= 256) of the unrectified EMG of left SCM to 4 mA, 2 ms current (cathode left, anode right) in a single subject, with the level of prestimulus mean rectified EMG for the same muscle shown on the left. B, graph of response amplitude (peak-to-peak amplitude, uncorrected for different levels of activation) versus the mean rectified EMG for the same subject. The circles represent the averages performed with the subject sitting, and the squares represent those with the subject reclined; filled symbols indicate averages shown in A. The linear relationship shown here was typical and similar results were obtained with head rotation.
Recovery cycle and interaction between galvanic and click stimulation
No significant change in p13/n23 amplitude was found with interstimulus intervals varying between 30 and 300 ms, for either galvanic or click stimulation (Fig. 5).
Figure 5. Recovery cycle for a normal subject, for galvanic stimulation and clicks.
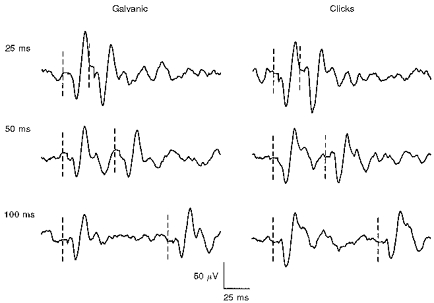
Averaged responses (n= 256) for unrectified EMG of right SCM. Interstimulus interval is shown above each average. The vertical dashed lines show the time of stimulus delivery. No significant effect of interstimulus interval on the amplitude of the second response was found across the range of intervals studied, up to 300 ms, for either type of stimulus. The galvanic stimulus was a transmastoid current of 3 mA, duration 2 ms, with the cathode right. The click intensity was 95 dB, given to the right ear.
The p13/n23 response following simultaneous galvanic and click stimulation was always smaller than the algebraic sum of the traces for the separate p13/n23 [g] and p13/n23 [c] responses, in all subjects (overall 1.5 vs. 1.9, respectively; P < 0.0001).
Effects of varying current intensity and duration
In all ten subjects (twenty sides), the p13/n23 [g] response amplitude increased with increasing current intensity, although there was apparent saturation at the highest current intensities in some. Over the linear range, which included between five and eleven data points, correlation coefficients (r2) were between 0.72 and 0.99 (P < 0.05 in each case), with fifteen out of the twenty responses showing a correlation coefficient of greater than 0.9 (P < 0.001 for each of these). The gradients of the lines of best fit varied considerably, between 0.10 and 0.54, but in no case was the y-intercept significantly different from zero. In 8 out of 20 responses the p13/n23 [g] response amplitude showed saturation, with current intensities between 4 and 7 mA. The p13/n23 [g] response saturated at an amplitude between 1.2 and 2.3, amplitudes that were the same or larger than that for the same subject's p13/n23 [c] response to 100 dB clicks. The n12/p20 [g] response amplitudes showed similar changes with increasing current intensity, but with lower correlation coefficients between intensity and amplitude in most subjects than for the p13/n23 [g] response. In two subjects who showed saturation of the p13/n23 response at a current intensity of 4 mA, the n12/p20 response in the opposite SCM continued to increase linearly with increasing the current intensity to 6 mA, the highest current used in these subjects.
All five subjects showed an increase in p13/n23 [g] response amplitude with increasing current duration from 0.5 ms. In two subjects, p13/n23 [g] response amplitude saturation occurred at a current duration of 2.5 ms, while in three subjects no saturation was evident with increasing current duration to 4 ms, the maximum used. Linear correlation coefficients ranged between 0.89 and 0.98 (P < 0.01 in each case) for the data points prior to apparent saturation. The y-intercept was not significantly different from zero in any of these subjects.
Patients with vestibular neurectomy or sensorineural deafness
None of the six patients studied after unilateral vestibular nerve section showed p13/n23 [g] or n12/p20 [g] responses with unilateral cathodal or anodal stimulation to the side of nerve section (Fig. 6). All six patients showed well-formed p13/n23 responses following ‘cathodal’ or click stimulation to the side of the intact nerve (Fig. 6). Most of the patients also showed n12/p20 [g] responses with either unilateral cathodal (five patients) or unilateral anodal (four patients) stimulation of the intact nerve; as in normal subjects, cathodal stimulation produced a crossed n12/p20 [g] response and anodal stimulation produced an uncrossed n12/p20 [g] response. Prominent late responses were seen in SCM bilaterally following galvanic stimulation (transmastoid or unilateral directed to either side) in three of the patients, including one of the patients with unilateral vestibulocochlear nerve section. This patient showed prominent later responses with unipolar galvanic stimulation, but no cochlear-dependent ‘n34/p44′ responses with click stimulation given to the side of nerve section (Colebatch et al. 1994). The patients with severe sensorineural deafness all showed normal p13/n23 and n12/p20 responses following galvanic and click stimulation.
Figure 6. Galvanic stimulation after selective right vestibular nerve section.
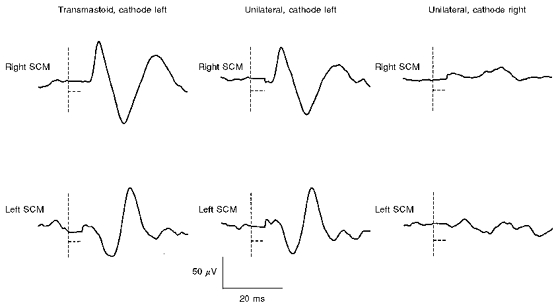
The patient was studied 2 months after selective vestibular nerve section: averaged responses (n= 256) to galvanic stimulation (5 mA, 2 ms) in unrectified EMG of both SCM muscles. The traces on the left show responses to transmastoid stimulation with the cathode on the intact (left) side. The middle traces show responses to unilateral stimulation with the cathode on the intact (left) side, and the traces on the right show responses to unilateral stimulation with the cathode on the sectioned (right) side. The vertical dashed lines show the time of stimulus onset, while the horizontal dashed lines show the period over which the EMG signal was clamped to minimize stimulus artifact. The response to transmastoid stimulation (both p13/n23 [g] in left SCM and n12/p20 [g] in right SCM) is the result of the action of the cathode on the intact (left) side. No responses were observed with unilateral stimulation on the sectioned (right) side.
DISCUSSION
The earliest responses in SCM induced by galvanic stimulation over the mastoid processes are abolished by selective vestibular nerve section (see also Watson et al. 1998b) and are unaffected by profound sensorineural deafness. While the stimulus would have also activated cutaneous receptors, which are known to evoke short-latency reflexes in SCM (Di Lazzaro et al. 1995), such responses differ fundamentally from the responses we have described both in beginning later (onset latency, 13 ms) and in having an identical waveform bilaterally following unilateral (trigeminal) stimulation. We conclude that the short-latency responses we have described following galvanic stimulation represent vestibulocollic reflexes.
The site of action and afferents excited by galvanic stimulation
Galvanic stimulation has been shown to act upon the most distal part of the vestibular nerve, the ‘spike trigger zone’, in animal studies, with cathodal stimulation increasing and anodal stimulation decreasing resting discharge levels in several animal species, including the thornback ray (Lowenstein, 1955) and the squirrel monkey (Goldberg et al. 1984). Our finding of opposing effects for cathodal and anodal stimulation (see also Watson & Colebatch, 1997), as well as the finding of a linear relationship between current intensity and response amplitude, with a very low threshold, supports an action at the same site in humans. As a result of the high resting discharge rate of the vestibular afferent fibres, small current changes at the spike trigger zone are potentially able to modulate firing rate, in contrast to the threshold current level that would be required to initiate an action potential in a myelinated nerve fibre. While no selectivity for afferents arising from a specific vestibular end-organ has been shown in direct recordings in the squirrel monkey (Goldberg et al. 1984), both the potent effects on posture in man and the effects on ocular torsion (Zink et al. 1997; Watson et al. 1998a) support a preferential action upon otolith afferents, at least at relatively low current intensity levels. Given that clicks are likely to specifically activate saccular afferents (Townsend & Cody, 1971; Didier & Cazals, 1989; McCue & Guinan, 1994; Murofushi et al. 1995, 1996), the close similarities between responses evoked by galvanic stimulation and by clicks in both SCM and the soleus muscle (Watson & Colebatch, 1998) also supports a degree of selectivity of low-intensity galvanic stimulation for otolith, including saccular, afferents. It should be noted, however, that prominent horizontal nystagmus has been described in humans when higher current intensities were used, suggesting that more intense galvanic stimulation may additionally activate canal afferents (Pfaltz, 1970).
Pathways mediating galvanic-evoked vestibulocollic reflexes
Using unilateral stimulation we have shown that the short-latency EMG response in SCM evoked by transmastoid galvanic stimulation can be resolved into three components. First, the cathode produces a p13/n23 [g] response in the SCM ipsilateral to the side of stimulation. Second, the cathode also produces a ‘crossed’ n12/p20 [g] response in the SCM contralateral to the side of stimulation. Third, the anode produces an ‘uncrossed’ n12/p20 response in the SCM ipsilateral to the side of stimulation. The latter two effects summate to produce the n12/p20 [g] response to transmastoid stimulation. Figure 7 shows a diagram of the pathways that we propose to account for each of these responses. The majority of vestibulocollic projections lie in the medial vestibulospinal tract (Wilson & Maeda, 1974; Sato et al. 1996), but at least some, such as ipsilateral saccular projections to extensor muscles in the cat (Uchino et al. 1997), lie in the lateral vestibulospinal tract. The p13/n23 [c] response has been shown to correspond to a brief period of motor unit inhibition in single unit histograms (Colebatch & Rothwell, 1993), and its onset latency is consistent with a disynaptic vestibulocollic reflex pathway (Colebatch et al. 1994). These conclusions are likely to apply equally to the p13/n23 [g] response. Considering that the cathode has an excitatory effect upon primary afferent discharge rate, and also that primary afferents are predominantly excitatory on the vestibular nucleus (e.g. DeVito et al. 1956), the projection from the vestibular nucleus to the ipsilateral SCM motoneurones must be inhibitory. In addition, our observations indicate that excitability recovers within 30 ms at both central synapses. On the other hand, given that the n12/p20 responses are of opposite type to the p13/n23 response, they are likely to reflect excitation of SCM motoneurones. Because the crossed n12/p20 [g] response following cathodal stimulation has an onset latency similar to the p13/n23 [g] response, it is likely to be mediated by a disynaptic crossed excitatory pathway. The uncrossed n12/p20 [g] response due to the action of the anode is also likely to be mediated by a disynaptic excitatory pathway. Considering that the anode has an inhibitory effect on primary afferent discharge, the excitation of SCM motoneurones could be explained by the inhibition of a tonically active inhibitory projection, i.e. by disinhibition. DeVito et al. (1956) showed in cats that if the resting discharge rate of a neurone in Deiter's nucleus was altered by cathodal stimulation of the vestibular nerve, then anodal stimulation of the same nerve would almost always produce an opposite change in that neurone's discharge rate. Thus the simplest explanation for the uncrossed n12/p20 [g] response is disinhibition via the same (inhibitory) projection that mediates the p13/n23 [g] response. The smaller amplitude of the uncrossed n12/p20 [g] response evoked by the anode compared with the p13/n23 [g] response evoked by the cathode is consistent with previous observations in animals (Goldberg et al. 1984) and humans (Halmagyi et al. 1990; Watson & Colebatch, 1997) that larger responses follow excitation than follow inhibition of vestibular nerve discharge. We failed to observe a crossed inhibitory (p13/n23) response after anodal stimulation, which might have been predicted from these putative pathways, as a result of disfacilitation of the crossed excitatory pathway. This probably indicates that the crossed pathway is less potent than the ipsilateral one. Crossed vestibulocollic responses in normal subjects to click stimulation are generally small or absent, only becoming prominent when there is hypersensitivity of the vestibular apparatus to sound (Colebatch et al. 1998).
Figure 7. Proposed reflex pathways subserving short-latency responses following galvanic stimulation.
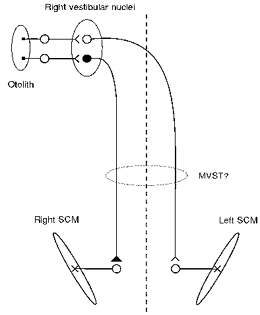
Excitatory pathways are shown unfilled and inhibitory pathways are shown filled. Vestibular primary afferent neurones have a high resting discharge rate that is increased by the action of the cathode and decreased by the action of the anode. The p13/n23 [g] response is generated by an ipsilateral inhibitory projection. Inhibition of the same projection (due to the action of the anode on the same side) generates the uncrossed n12/p20 response. The crossed n12/p20 [g] response (evoked by the cathode) is generated by a crossed excitatory pathway. These pathways are most likely to lie in the medial vestibulospinal tract (MVST).
The physiological role of galvanic-evoked vestibulocollic reflexes
Descending volleys in vestibulospinal pathways have more potent effects upon neck motoneurones than upon the muscles of the limbs in the cat, with strong monosynaptic projections to neck motoneurones and largely polysynaptic projections to hindlimb motoneurones (Wilson & Yoshida, 1969). In the cat, stimulation of each individual canal produces a pattern of muscle excitation that is generally consistent with a role in resisting imposed head displacements (Wilson & Maeda, 1974). Loss of vestibular function causes instability of the head and abolishes the normal head and body righting responses to falling (Money & Scott, 1962). Although vestibulocollic reflexes evoked by head displacements have been described in humans (e.g. Ito et al. 1995; Keshner et al. 1995), the contribution of such reflexes to head stabilization in man appears to be relatively limited (Bronstein, 1988; Corna et al. 1996).
In the cat, afferents arising from both types of otolith receptor, the sacculus and utriculus, project to neck motoneurones by disynaptic and trisynaptic pathways in the cat (Sato et al. 1995; Uchino et al. 1997). The otolith organs are sensitive to linear acceleration, including gravity, and thus can signal head orientation with respect to gravity (Fernandez & Goldberg, 1976). Their discharge may therefore contribute not only to head stabilization in response to perturbations, but also to determining head orientation with respect to the vertical, i.e. orienting or righting reflexes.
Like click-evoked vestibulocollic reflexes, the size of the galvanic-evoked vestibulocollic reflex was directly related to the level of tonic motoneuronal discharge. In the present study the effects of head position were explicable purely by changes in tonic muscle activity, with no significant change in latency or waveform between the different head postures studied. This result clearly differs from previous findings for vestibular-dependent leg muscle responses. In leg muscles, the EMG response evoked by galvanic stimulation has two components, the ‘short-latency’ response beginning at around 50 ms and the ‘medium-latency’ response beginning at around 100 ms (Britton et al. 1993; Fitzpatrick et al. 1994). Both of these components reverse in type (an excitatory response becoming an inhibitory one, and vice versa) when the head is rotated from one side to the other. Click-evoked vestibular reflexes in leg muscles are very similar to the short-latency response to galvanic stimulation and show similar modulation with change in head position (Watson & Colebatch, 1998). It has been estimated that a delay of approximately 30 ms occurs between vestibular nerve activation by galvanic stimulation and the onset of the descending volley in the spinal cord that mediates the short-latency response (Britton et al. 1993), suggesting a polysynaptic pathway in contrast to the disynaptic nature of the vestibulocollic reflex, and similar to findings in the cat (Wilson & Yoshida, 1969). The contrasting properties of vestibular reflexes recorded in the neck and leg muscles are likely to relate to the differing roles of vestibular signals in physiological control in these muscle groups. The modulation of vestibular responses by head position is consistent with their playing a role in stabilizing the body (Nashner & Wolfson, 1974). Vestibulocollic reflexes are thought to stabilize the head in space (Wilson et al. 1995) and this differing role may explain their relative independence of the position of the head in relation to the body.
Acknowledgments
This research was supported by a grant from the National Health and Medical Research Council of Australia. S. R. D. W. received a Medical Research Scholarship from the Garnett Passe and Rodney Williams Memorial Foundation during the period of this study.
References
- Bickford RG, Jacobson JL, Cody DT. Nature of average evoked potentials to sound and other stimuli. Annals of the New York Academy of Sciences. 1964;112:204–223. doi: 10.1111/j.1749-6632.1964.tb26749.x. [DOI] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Experimental Brain Research. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Bronstein AM. Evidence for vestibular input contributing to dynamic head stabilization in man. Acta Otolaryngologica. 1988;105:1–6. doi: 10.3109/00016488809119438. [DOI] [PubMed] [Google Scholar]
- Camis M. The Physiology of the Vestibular Apparatus. London, UK: Oxford University Press; 1930. pp. 209–212. [Google Scholar]
- Coats AC, Stoltz MS. The recorded body-sway response to galvanic stimulation of the labyrinth: a preliminary study. Laryngoscope. 1969;79:85–103. doi: 10.1288/00005537-196901000-00004. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Day BL, Bronstein AM, Davies RA, Gresty MA, Luxon LM, Rothwell JC. Vestibular hypersensitivity to clicks is characteristic of the Tullio phenomenon. Journal of Neurology, Neurosurgery and Psychiatry. 1998 doi: 10.1136/jnnp.65.5.670. (in the Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Rothwell JC. Vestibular-evoked EMG responses in human neck muscles. The Journal of Physiology. 1993;473:18P. [Google Scholar]
- Corna S, Ito Y, von Brevern M, Bronstein A, Gresty M. Reflex ‘unloading’ and ‘defence capitulation’ responses in human neck muscle. The Journal of Physiology. 1996;496:589–596. doi: 10.1113/jphysiol.1996.sp021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Severac Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. The Journal of Physiology. 1997;500:661–672. doi: 10.1113/jphysiol.1997.sp022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vito RV, Brusa A, Arduini A. Cerebellar and vestibular influences on deitersian units. Journal of Neurophysiology. 1956;19:241–253. doi: 10.1152/jn.1956.19.3.241. [DOI] [PubMed] [Google Scholar]
- Didier A, Cazals Y. Acoustic responses recorded from the saccular bundle on the eighth nerve of the guinea pig. Hearing Research. 1989;37:123–128. doi: 10.1016/0378-5955(89)90034-8. 10.1016/0378-5955(89)90034-8. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Quartone A, Higuchi K, Rothwell JC. Short-latency trigemino-cervical reflexes in man. Experimental Brain Research. 1995;102:474–482. doi: 10.1007/BF00230651. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long duration centrifugal force. Journal of Neurophysiology. 1976;39:970–984. doi: 10.1152/jn.1976.39.5.970. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. The Journal of Physiology. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. Journal of Neurophysiology. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Curthoys IS, Cremer PD, Henderson CJ, Todd MJ, Staples MJ, D'Cruz DM. The human horizontal vestibulo-ocular reflex in response to high-acceleration stimulation before and after unilateral vestibular neurectomy. Experimental Brain Research. 1990;81:479–490. doi: 10.1007/BF02423496. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Yavor RA, Colebatch JG. Tapping of the head activates the vestibular system: a new use for the clinical reflex hammer. Neurology. 1995;45:1927–1929. doi: 10.1212/wnl.45.10.1927. [DOI] [PubMed] [Google Scholar]
- Iles JF, Pisini JV. Vestibular-evoked postural reactions in man and modulation of transmission in spinal reflex pathways. The Journal of Physiology. 1992;455:407–424. doi: 10.1113/jphysiol.1992.sp019308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Corna S, von Brevern M, Bronstein A, Rothwell J, Gresty M. Neck muscle responses to abrupt free fall of the head: comparison of normal with labyrinthine-defective human subjects. The Journal of Physiology. 1995;489:911–916. doi: 10.1113/jphysiol.1995.sp021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshner EA, Cromwell RL, Peterson BW. Mechanisms controlling human head stabilisation. II. Head-neck characteristics during random rotations in the vertical plane. Journal of Neurophysiology. 1995;73:2302–2312. doi: 10.1152/jn.1995.73.6.2302. [DOI] [PubMed] [Google Scholar]
- Lee CL, Clouston P, Sheean G, Yiannikas C. The influence of voluntary EMG activity and click intensity on the vestibular click evoked myogenic potential. Muscle and Nerve. 1995;18:1210–1213. doi: 10.1002/mus.880181021. [DOI] [PubMed] [Google Scholar]
- Lowenstein O. The effect of galvanic polarization on the impulse discharge from sense endings in the isolated labyrinth of the thornback ray (Raja clavata) The Journal of Physiology. 1955;127:104–117. doi: 10.1113/jphysiol.1955.sp005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiologica Scandinavica. 1983;117:307–309. doi: 10.1111/j.1748-1716.1983.tb07212.x. [DOI] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ. Acoustically responsive fibres in the vestibular nerve of the cat. Journal of Neuroscience. 1994;14:6058–6070. doi: 10.1523/JNEUROSCI.14-10-06058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money KE, Scott JW. Functions of separate sensory receptors of non-auditory labyrinth of the cat. American Journal of Physiology. 1962;202:1211–1220. doi: 10.1152/ajplegacy.1962.202.6.1211. [DOI] [PubMed] [Google Scholar]
- Murofushi T, Curthoys IS, Topple AN, Colebatch JG, Halmagyi GM. Responses of guinea pig neurons to clicks. Experimental Brain Research. 1995;103:174–178. doi: 10.1007/BF00241975. [DOI] [PubMed] [Google Scholar]
- Murofushi T, Halmagyi GM, Yavor RA, Colebatch JG. Absent vestibular evoked myogenic potentials in vestibular neurolabyrinthitis. Archives of Otolaryngology, Head and Neck Surgery. 1996;122:845–848. doi: 10.1001/archotol.1996.01890200035008. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Wolfson P. Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of the human labyrinth. Brain Research. 1974;67:255–268. doi: 10.1016/0006-8993(74)90276-5. 10.1016/0006-8993(74)90276-5. [DOI] [PubMed] [Google Scholar]
- Pfaltz CRP. The diagnostic importance of nystagmography in the galvanic test. In: Stahle J, editor. Vestibular Function on Earth and in Space. Oxford, UK: Pergamon Press; 1970. pp. 187–199. [Google Scholar]
- Sato H, Endo K, Ikegami H, Imagawa M, Sasaki M, Uchino Y. Properties of utricular nerve-activated vestibulospinal neurons in cats. Experimental Brain Research. 1996;112:197–202. doi: 10.1007/BF00227638. [DOI] [PubMed] [Google Scholar]
- Tokita T, Ito Y, Takagi K. Modulation by head and trunk positions of the vestibulo-spinal reflexes evoked by galvanic stimulation of the labyrinth. Acta Otolaryngologica. 1989;107:327–332. doi: 10.3109/00016488909127516. [DOI] [PubMed] [Google Scholar]
- Townsend GL, Cody DT. The averaged inion response evoked by acoustic stimulation: its relation to the saccule. Annals of Otology, Rhinology and Laryngology. 1971;80:121–131. doi: 10.1177/000348947108000116. [DOI] [PubMed] [Google Scholar]
- Uchino Y, Sato H, Sasaki M, Imagawa M, Ikegami H, Isu N, Graf W. Sacculocollic reflex arcs in cats. Journal of Neurophysiology. 1997;77:3003–3012. doi: 10.1152/jn.1997.77.6.3003. [DOI] [PubMed] [Google Scholar]
- Walsh EG. Physiology of the Nervous System. London, UK: Longmans; 1964. pp. 171–178. [Google Scholar]
- Watson SRD, Brizuela AE, Curthoys IS, Colebatch JG, MacDougall HG, Halmagyi GM. Maintained ocular torsion produced by bilateral and unilateral galvanic (DC) vestibular stimulation in humans. Experimental Brain Research. 1998a doi: 10.1007/s002210050533. (in the Press) [DOI] [PubMed] [Google Scholar]
- Watson SRD, Colebatch JG. EMG responses in the soleus muscles evoked by unipolar galvanic vestibular stimulation. Electroencephalography and Clinical Neurophysiology. 1997;105:476–483. doi: 10.1016/s0924-980x(97)00044-1. 10.1016/S0924-980X(97)00044-1. [DOI] [PubMed] [Google Scholar]
- Watson SRD, Colebatch JG. Vestibular-evoked EMG responses in soleus: a comparison between click and galvanic stimulation. Experimental Brain Research. 1998;119:504–510. doi: 10.1007/s002210050366. 10.1007/s002210050366. [DOI] [PubMed] [Google Scholar]
- Watson SRD, Fagan P, Colebatch JG. Short latency EMG responses to galvanic stimulation in the sternocleidomastoid muscle are abolished by selective vestibular nerve section. Electroencephalography and Clinical Neurophysiology. 1998b doi: 10.1016/s0924-980x(98)00033-2. (in the Press) [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Boyle R, Fukushima K, Rose PK, Shinoda Y, Sugiuchi Y, Uchino Y. The vestibulocollic reflex. Journal of Vestibular Research. 1995;5:147–170. 10.1016/0957-4271(94)00035-Z. [PubMed] [Google Scholar]
- Wilson VJ, Maeda M. Connections between semicircular canals and neck motoneurons in the cat. Journal of Neurophysiology. 1974;37:346–357. doi: 10.1152/jn.1974.37.2.346. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Comparison of effects of stimulation of Deiter's nucleus and medial longitudinal fasciculus on neck, forelimb and hindlimb motoneurons. Journal of Neurophysiology. 1969;32:743–758. doi: 10.1152/jn.1969.32.5.743. [DOI] [PubMed] [Google Scholar]
- Zink R, Steddin S, Weiss A, Brandt T, Dieterich M. Galvanic vestibular stimulation in humans: effects on otolith function in roll. Neuroscience Letters. 1997;232:171–174. doi: 10.1016/s0304-3940(97)00610-1. 10.1016/S0304-3940(97)00610-1. [DOI] [PubMed] [Google Scholar]


