Abstract
The response of twenty-eight human muscle spindle afferents from m. extensor carpi radialis brevis to large amplitude ramp stretch and release at the wrist joint was recorded. The dynamic index was calculated as the difference in firing rate between that just before the end of stretch and that during the subsequent static phase of stretch. The value during steady voluntary contraction was compared with that during relaxation.
In twenty-three primary afferents, the dynamic index increased in eleven and decreased in twelve afferents with a range of −8 to +25 impulses s−1. In five secondary afferents the change was less than 2 impulses s−1.
The primary afferents abruptly stopped firing when the stretch was released in the relaxed muscle. This cessation was prevented during contraction in seventeen primary afferents.
The results suggest the presence of dynamic and static fusimotor actions on the human muscle spindles during voluntary contraction.
It is known from many studies in anaesthetized animals that mammalian muscle spindle afferents respond to muscle length changes and that these responses are closely related to fusimotor activity. The natural pattern of fusimotor activity has been inferred from recordings of muscle spindle afferents in awake humans, cats and monkeys in an effort to understand the actual role of muscle spindles in motor control. However, how the mammalian central nervous system controls fusimotor action during natural movements remains the subject of controversy (Matthews, 1981; Prochazka, 1996). This paper presents a new finding in regard to the central control of fusimotor activity from experiments on human muscle spindles.
The fusimotor neurones are classified as static or dynamic, depending on their action on the dynamic responsiveness of the muscle spindle primary ending to stretches in cats (Jansen & Matthews, 1962; Crowe & Matthews, 1964a, b; Emonet-Dénand et al. 1977) and in primates (Cheney & Preston, 1976). The dynamic fusimotor neurones increase the response of the primary endings during the dynamic phase of stimuli, while the excitatory action of static neurones is dominant at a constant muscle length.
The firing rate of human muscle spindle afferents accelerates with pronounced irregularity during isometric contractions (Vallbo, 1970, 1974; Burke et al. 1978; Edin & Vallbo, 1990b; Wilson et al. 1997). The spindle discharges do not cease during the shortening phase of active slow ankle and finger movements (Burke et al. 1977; Hulliger et al. 1985). These findings indicate an excitatory action of static fusimotor neurones on muscle spindles during voluntary contraction.
The dynamic responsiveness of human muscle spindles to stretch during voluntary contraction has not been studied quantitatively, but qualitative observations suggest the presence of a dynamic fusimotor action. Primary afferents exhibit high dynamic responsiveness to stretch by extrafusal contractions during the rising phase of isometric contractions (Vallbo, 1974) and during relaxation of isometric contractions (Wilson et al. 1997).
This study was conducted to investigate the basic pattern of dynamic and static fusimotor actions on human muscle spindles. The response of muscle spindle afferents to large amplitude ramp stretch and release was recorded during steady voluntary contraction. Fusimotor excitatory action was evaluated by measuring the firing rate at a constant muscle length and during the dynamic phase of stretch and release. This approach is similar to that of Jansen & Matthews (1962), who first separated the spontaneous fusimotor action on muscle spindles into dynamic and static in decerebrated cats. The present results suggest the presence of dynamic and static fusimotor actions on human muscle spindles during voluntary contraction.
METHODS
Subjects
Fifteen experiments were performed on healthy volunteers, four males and eleven females, aged 20–37 years. All subjects gave informed consent according to the Declaration of Helsinki. The experimental plan was approved by the Human Ethical Committee of the National Rehabilitation Centre for the Disabled.
Experimental set-up
Each subject sat comfortably in a reclining chair, with the left forearm supported on a platform and clamped in the mid-position between supination and pronation. The hand was fixed to a manipulandum, which was connected to a servo-controlled torque motor, enabling measurement of joint angle, velocity and torque. On a display in front of the subjects, a horizontal cursor indicated joint torque.
An insulated tungsten electrode was percutaneously inserted into the radial nerve 5 cm proximal to the elbow joint to record nerve activity (Vallbo et al. 1979). The location of the muscle belly of m. extensor carpi radialis brevis was confirmed by palpation and surface electrical stimulation. For recording muscle activity, a pair of surface electrodes was attached near the motor point.
Kinematic signals were sampled at 1600 Hz. Surface EMG was filtered at 1.6–800 Hz and sampled at 1600 Hz. The sampled signal was full-wave rectified and then digitally low-pass filtered (−6 dB at 13.5 Hz). Nerve signals were recorded and sampled at 12 800 Hz using a SC/ZOOM system (Department of Physiology, Umeå, Sweden). Each recorded nerve spike was inspected off-line on an expanded time scale, and when judged to be from a single unit on the basis of the regularity of firing and shape invariance of consecutive spikes, the nerve signal was converted to spike trains sampled at 1600 Hz for later analysis.
Unit identification
Slowly adapting muscle afferents from m. extensor carpi radialis brevis were identified by prodding the muscle and tendon. Care was taken to confirm the origin of each afferent as m. extensor carpi radialis brevis. Further identification procedures consisted of passive ramp stretch and release and maximal twitch contraction by surface stimulation of the motor point.
Experimental protocol
Maximal voluntary torque (MVT) of extension at the wrist joint was measured with a 180 deg joint angle. The subjects were instructed to contract wrist muscles while attempting to relax finger muscles as best as possible.
When a single muscle spindle afferent was recorded, conditioning and test movements were applied to the wrist joint in 16 s intervals (Fig. 1A). Conditioning stretches were used to reduce the effect of the previous history of muscle length and the fusimotor after-effect (Baumann et al. 1983; Morgan et al. 1984; Emonet-Dénand et al. 1985).
Figure 1. Experimental protocol and dynamic index measurement.
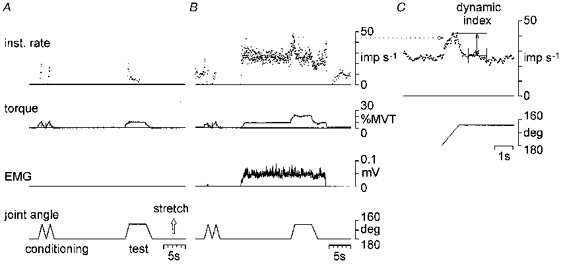
A and B, sample records that illustrate the experimental protocol used in this study (from top to bottom): the instantaneous firing rate of muscle spindle primary afferent (expressed as impulses (imp) s−1), torque at the wrist joint (expressed as % MVT), rectified EMG (expressed as mV) and joint angle (expressed as deg). Conditioning and test movements were applied to the wrist joint. The test movement consisted of stretch, hold and release with a 16 deg amplitude. It was applied during relaxation (A) and steady voluntary contraction (B). In B, the subject maintained a steady contraction, and EMG level was consistent during the test movement. C, the afferent activity from B (indicated by a dotted line) on an expanded time scale. The instantaneous firing rate in C was determined by averaging over four interspike intervals, to measure the firing rate just before the end of stretch and the dynamic index.
The test movement consisted of stretch (0.94 s), hold (3.87 s) and release (1.45 s) with an amplitude of 16 deg and an initial joint angle of 180 deg. It was applied during relaxation (Fig. 1A) and steady voluntary contraction (Fig. 1B). Initially, the subject was instructed to start a steady contraction by wrist extension. The voluntary torque was indicated on a display and was maintained at a predetermined level (1, 3, 6, 12 and 20 % MVT). Then, the subject was instructed to keep the effort of contraction, not torque level, as constant as possible during the test movement. As a result, the EMG during the test movement was almost maintained at a pre-stretch level as shown in Figs 1B, 2 and 5, although the torque changed abruptly during stretch and release.
Size of movements
The excursion of the tendon during the test movement can be roughly estimated in the following way. If the radius of the joint is approximated to 13.0 mm (Brand, 1985) and the muscle length of m. extensor carpi radialis brevis is 186 mm (Loren & Lieber, 1995), then a 16 deg flexion corresponds to 3.6 mm stretch. If it is supposed that any movement in the tendon corresponds to a stretch of the muscle fibres of the same amount, a 16 deg flexion corresponds to 2.2 % relative stretch of the resting muscle length per second. The stretch velocity is 3.8 mm s−1 and 2.2 % of the muscle length per second.
Dynamic fusimotor action increases sensitivity of the primary afferents when the amplitude of stretch is large, and slightly reduces or holds the passive value when the amplitude is small (Hulliger et al. 1977). In the cat soleus muscle (50 mm), the critical amplitude is 0.2 mm at 1 Hz sinusoidal movements, corresponding to 0.4 % relative movement of the muscle length. Hence, the 2.0 % relative stretch of the resting muscle length used in the present study was five times larger than the critical amplitude in the cat soleus muscle.
Data analysis
Firing rates of human muscle spindle afferents were low compared with cat spindles and were highly irregular during voluntary contractions. For measurement of dynamic index, the instantaneous firing rate was determined by averaging over four interspike intervals (Matthews & Stein, 1969b). This procedure reduced the variability of firing rate and made it easier to pick out the response to the stretch (Fig. 1C).
The dynamic index of muscle spindle afferents was calculated by a method almost identical to those of Jansen & Matthews (1962) in decerebrated cats and Edin & Vallbo (1990a) in humans. The dynamic index was defined as the difference in firing rate between that just before the end of stretch and that during the subsequent static phase of stretch as shown in Fig. 1C. The firing rate during the static phase of stretch was assessed as the mean rate during the hold phase between 0.5 and 1.5 s after the end of stretch.
RESULTS
The discharges of thirty-four human muscle spindle afferents from m. extensor carpi radialis brevis were recorded. Twenty-three primary afferents and five secondary afferents increased or maintained their firing rate during isometric contractions. The dynamic response of these afferents to large amplitude ramp stretch and release was analysed. Six afferents decreased their firing rate during contractions, and these afferents were excluded from further analysis.
Dynamic response to stretch during voluntary contraction
Figure 2 shows the response to stretch from four muscle spindle afferents (A-D). Afferent activity is expressed as the unaveraged instantaneous firing rate. Units A-C were identified as primary afferents, and unit D as a secondary afferent from the response recorded in the relaxed muscle (left column).
Figure 2. The dynamic response of four muscle spindle afferents to ramp stretch during relaxation and voluntary contraction.
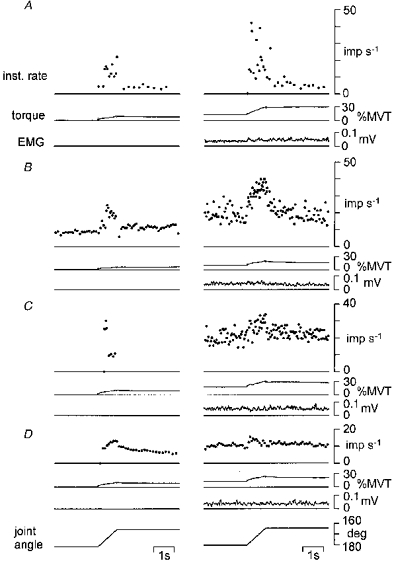
The unaveraged instantaneous firing rates (inst. rate) of three primary afferents (A-C) and a secondary afferent (D) are shown. Torque at the wrist joint and rectified EMG are also shown for each afferent. The left column is the response to stretch during relaxation. The right column is the response recorded while the subject maintained a steady contraction.
The three primary afferents increased their firing rate at a constant muscle length and during the dynamic phase of stretch while the subjects maintained a steady contraction (right column). In unit A, the increase in firing rate was dominant during the dynamic phase of stretch. In units B and C, the firing rate accelerated with pronounced irregularity at a constant muscle length. A further increase in firing rate was observed during the dynamic phase of stretch in B, but not in C. Consequently, the dynamic response to stretch was increased in A and B, and reduced in C.
The secondary afferent (D) increased its firing rate at a constant muscle length, but not during the dynamic phase of stretch. The dynamic response to stretch was reduced.
Effect of contraction strength on dynamic index
The dynamic response of spindle afferents to stretch was analysed in relation to voluntary torque. Figure 3 plots the response of the four spindle afferents to stretch against voluntary torque. The upper graphs plot the firing rate during the dynamic phase of stretch, measured just before the end of stretch (circles), and during the static phase of stretch (squares). The lower graphs plot the difference in firing rate, i.e. the dynamic index. Each point in these graphs represents the mean of two to four records.
Figure 3. The effect of voluntary torque on the dynamic response to stretch of four muscle spindle afferents.
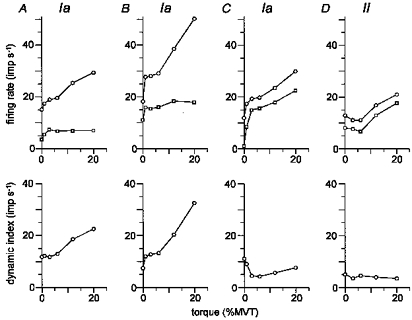
A-D are the same muscle spindle afferents as in Fig. 2. The upper graphs plot the firing rate just before the end of stretch (○) and that during the static phase of stretch (□) against the voluntary torque. The lower graphs plot the dynamic index. Each point in these graphs represents a mean of two to four records.
It is clear that the four spindle afferents increased their firing rate during the dynamic and static phases of stretch along with voluntary torque. However, the relationship between these phases varied among spindle afferents. In units A and B (Fig. 3), the firing rate during the dynamic phase of stretch steeply increased in parallel with voluntary torque, while the firing rate during the static phase reached a plateau at 3 % MVT. Hence, dynamic index increased with voluntary torque. In units C and D, the increase in firing rate during the dynamic phase of stretch was equal to or less than that during the static phase, and the dynamic index was slightly decreased.
Whether the dynamic index was increased or not was consistent in each afferent. If the dynamic index of an afferent increased with torque, it always increased, regardless of the level of torque.
Effect of voluntary contraction on dynamic index in the total sample
The effect of voluntary contraction on dynamic index was analysed in twenty-eight muscle spindle afferents. The change in dynamic index was determined by calculating the difference between a record during relaxation and that during voluntary contraction in each afferent.
Figure 4 summarizes the data recorded from twenty-one muscle spindle afferents during voluntary contractions at 6 % MVT (A) and 12 % MVT (B). The change in dynamic index is plotted against the increase in firing rate during the static phase of stretch. Of these twenty-one afferents seventeen were primary afferents (circles), and of these primary afferents the dynamic index increased in eleven (65 %) and decreased in six (35 %). The range was −7.6 to +17.0 impulses s−1 at 6 % MVT and −6.9 to +19.0 impulses s−1 at 12 % MVT. In all eleven afferents with increased dynamic index, the change was larger at 12 % MVT than that at 6 % MVT (Wilcoxon signed-rank test at P < 0.05). Six primary afferents from Fig. 4 were further tested at 20 % MVT, showing a slightly larger change in dynamic index.
Figure 4. The effect of voluntary contraction on the dynamic index in twenty-one muscle spindle afferents.
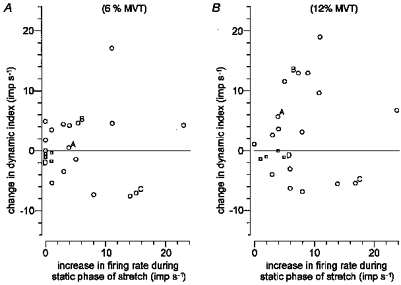
The change in dynamic index was calculated as the difference between the dynamic index in trials in the relaxed muscle and that during voluntary contraction at 6 (A) and 12 % MVT (B). The change in dynamic index is plotted against the increase in firing rate during the static phase of stretch. Each point in these plots represents an individual afferent: ○, primary afferents (n=17); □, secondary afferents (n=4). The afferents from Figs 2 and 3 are indicated (A-D).
A further six primary afferents and one secondary afferent were tested at 1–3 % MVT only and are not shown in Fig. 4. In total twenty-three primary afferents were tested, the dynamic index increased in eleven afferents (48 %) and decreased in twelve afferents (52 %) with a range of −7.6 to +25.1 impulses s−1. In all five secondary afferents tested the change in dynamic index was less than 2.0 impulses s−1.
Intrasubject variations were analysed to ascertain whether the varying effects of voluntary contraction on dynamic index in the whole sample were due to intersubject differences. Recordings from two to five primary afferents in six subjects were available (16 afferents). It was found that primary afferents from the same subject displayed as much variation as those noted in the whole sample from thirteen subjects, that is, for the same subject, the dynamic index increased in some primary afferents, while in others it decreased. Hence, the widely varying response of dynamic index to voluntary contraction seen in the whole sample could not be attributed to subject-specific variation.
Response to release during voluntary contraction
The discharges of the primary afferents abruptly stopped when the stretch was released in the relaxed muscle. This sudden cessation was prevented during voluntary contraction. Figure 5 shows the records of two primary afferents that exhibit a smooth decrease in firing rate upon a release of stretch during steady contraction. This effect was recognized in seventeen (74 %) of twenty-three primary afferents by qualitative analysis. The presence of this effect was independent of any change in dynamic index. It was observed in eight of eleven primary afferents in which dynamic index increased (A) and in nine of twelve afferents in which dynamic index decreased (B).
Figure 5. The response of muscle spindle primary afferents to stretch and release during voluntary contraction.
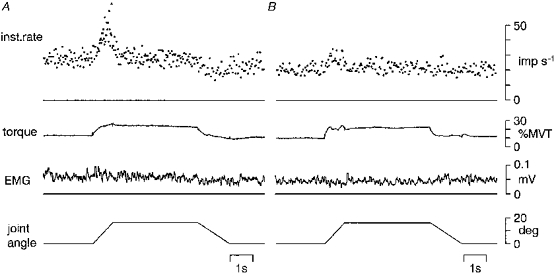
The unaveraged instantaneous firing rate of primary afferents, torque at the wrist joint, rectified EMG and joint angle are shown during stretch, hold and release. The subjects maintained a steady contraction. Unit A exhibits a high dynamic response to stretch, and unit B shows reduced dynamic response to stretch. Both afferents show maintained firing during muscle shortening.
DISCUSSION
Dynamic and static fusimotor activity in humans
The purpose of this study was to explore the basic pattern of dynamic and static fusimotor activity in humans. The approach was to measure the dynamic responsiveness of muscle spindles to large amplitude ramp stretch and release during steady voluntary contraction. Analysis was done on the twenty-eight spindle afferents that increased their firing rate at a constant muscle length due to an increase in overall fusimotor action during contraction. The results give the first convincing evidence of the existence of dynamic and static fusimotor effects in humans.
First, fusimotor actions on the dynamic index of muscle spindle primary afferents were subdivided into two groups. The dynamic index was increased in 48 % of the primary afferents and decreased in the others, compared with the dynamic index in the relaxed muscle. As the size of the muscle stretch was large, an increase in dynamic index is clear evidence of dynamic fusimotor action and a decrease in dynamic index suggests static fusimotor action (Crowe & Matthews, 1964a, b; Emonet-Dénand et al. 1977; Cheney & Preston, 1976; Hulliger et al. 1977).
Second, an abrupt cessation of spindle primary firing upon release of stretch during relaxation was replaced by a smooth decrease in firing rate during contraction in a high proportion (74 %), independently of the existence of dynamic fusimotor action. It is brought about by a fast, powerful excitatory action on the spindles and strongly indicates static fusimotor action (Crowe & Matthews, 1964b; Lennerstand & Thoden, 1968). Dynamic fusimotor action can also maintain firing of primary afferents during muscle shortening, although it is not as powerful, nor as consistent, as static action (Appenteng et al. 1982; Morgan et al. 1985).
Firing rates of spindle afferents in the present sample rarely exceeded 50 impulses s−1. The fusimotor effects during voluntary contraction, the increase in firing rate at a constant muscle length and the change in dynamic index, were less than 30 impulses s−1. By comparison, in cat gait, Ia firing rates of 50–200 impulses s−1 were typical with the maximal stretch velocity of 200 % of the resting muscle length per second. The low firing rate and the small fusimotor effects in human muscle spindles might be due to the size and velocity of stretch used during experiments (Prochazka & Gorassini, 1998) and the unloading effects by the extrafusal contractions (Vallbo, 1970), although there has been discussion of a basic species difference in this regard.
Relation between fusimotor and skeletomotor activity
The data have a bearing on another issue: whether the fusimotor activity, particularly dynamic activity, is modified independently of the skeletomotor output. In this regard, dynamic fusimotor action was graded with voluntary contraction and was partly linked to skeletomotor activity in the present sample. This pattern fits with skeleto-fusimotor coactivation (Vallbo, 1970, 1974). The association between fusimotor and skeletomotor activity could be partly attributed to skeletofusimotor (beta) action (Prochazka, 1996). Recent studies have suggested that beta-innervation is common in forearm muscles in cats (Scott et al. 1995) and in humans (Kakuda et al. 1998).
It is worth noting that, whereas considerable variability was observed within a subject, individual afferents showed consistent types of fusimotor drive during repeated tests. Hence, it is unlikely that the dynamic fusimotor effects seen in this study were the results of anxiety or discomfort during experiments. This finding does not preclude additional fusimotor activation in more difficult tasks (Prochazka et al. 1987).
Functional implications of the fusimotor system in humans
The high sensitivity of muscle spindle primary afferents to small stretches (Matthews & Stein, 1969a) and the fusimotor control of the spindle sensitivity to stretches of a wide range of amplitude (Goodwin et al. 1975; Hulliger et al. 1977) are important physiological properties of muscle spindles. The demonstration of the presence of a dual fusimotor action in this study indicates that static fusimotor action prevents the spindles from falling silent, and that dynamic action maintains sensitivity to small stretch, so that muscle spindles continue to signal information on the mechanical state of the muscle (Matthews, 1981).
This view would be supported by the recent statistical analyses in humans. It has been shown that human muscle spindles code the small mechanical events that occur during slow finger movements (Wessberg & Vallbo, 1995) and during isometric contractions (Halliday et al. 1995).
It should be pointed out that some muscle spindle afferents showed predominantly static, and others predominantly dynamic, fusimotor effects, and that the differences in fusimotor effects were seen in the same subject. The intrasubject variability in response profiles of human spindle afferents to isometric contractions was observed in previous studies, and it was probably due to different types of fusimotor drive (Vallbo, 1970; Edin & Vallbo, 1990b). It seems reasonable to conclude that the heterogeneity of fusimotor effects between different spindles is not specific to the present sample. It remains possible that consistent fusimotor effects come about at higher forces.
Two different explanations could be given in regard to the heterogenous effects. With simultaneous recordings from several muscle spindle afferents in anaesthetized cats, there were differences in types of fusimotor drive during reflex activation (Johansson et al. 1991). The ensembles of muscle spindle afferents can discriminate better between different muscle stretches than individual afferents, with the existence of fusimotor activity (Bergenheim et al. 1995).
On the other hand, it would be possible to postulate that the types and amount of fusimotor drive are finely controlled, dependent on the location of each muscle spindle. The length changes imposed on muscle spindles would not simply be related to the parent muscle length changes (Hoffer et al. 1989). Muscle spindles monitor changes in local fibre length and muscle spindle feedback is greatest in the reflex regulation of nearby muscle fibres (Eng & Hoffer, 1997).
In conclusion, this study demonstrates the existence of separate static and dynamic fusimotor actions on the human muscle spindles. It provides a basis for understanding the functional role of the fusimotor system during natural movements.
Acknowledgments
This work was supported by the Ministry of Health and Welfare of Japan.
Email: j90016@simail.ne.jp
References
- Appenteng K, Prochazka A, Proske U, Wand P. Effect of fusimotor stimulation of Ia discharge during shortening of cat soleus muscles at different speeds. The Journal of Physiology. 1982;329:509–526. doi: 10.1113/jphysiol.1982.sp014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann TK, Emonet-Dénand F, Hulliger M. Temporal characteristics of the sensitivity-enhancing after-effects of fusimotor activity on spindle Ia afferents. Brain Research. 1983;258:139–143. doi: 10.1016/0006-8993(83)91239-8. [DOI] [PubMed] [Google Scholar]
- Bergenheim M, Johansson H, Pedersen J. The role of the gamma-system for improving information transmission in populations of Ia afferents. Neuroscience Research. 1995;23:207–215. doi: 10.1016/0168-0102(95)00941-l. [DOI] [PubMed] [Google Scholar]
- Brand PW. Clinical Mechanics of the Hand. St Louis, Toronto, Princeton: The C.V. Mosby Company; 1985. [Google Scholar]
- Burke D, Hagbarth K-E, Löfstedt L. Muscle spindle activity in man during shortening and lengthening contractions. The Journal of Physiology. 1978;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hagbarth K-E, Skuse NF. Recruitment order of human spindle endings in isometric voluntary contractions. The Journal of Physiology. 1978;285:101–112. doi: 10.1113/jphysiol.1978.sp012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Preston JB. Classification of fusimotor fiber in the primate. Journal of Neurophysiology. 1976;39:9–19. doi: 10.1152/jn.1976.39.1.9. [DOI] [PubMed] [Google Scholar]
- Crowe A, Matthews PBC. The effects of stimulation of static and dynamic fusimotor fibres on the response to stretching of the primary endings of muscle spindles. The Journal of Physiology. 1964a;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe A, Matthews PBC. Further studies of static and dynamic fusimotor fibres. The Journal of Physiology. 1964b;174:132–151. doi: 10.1113/jphysiol.1964.sp007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, VallboÅ B. Dynamic response of human muscle spindle afferents to stretch. Journal of Neurophysiology. 1990a;63:1297–1306. doi: 10.1152/jn.1990.63.6.1297. [DOI] [PubMed] [Google Scholar]
- Edin BB, VallboÅ B. Muscle afferent responses to isometric contractions and relaxations in humans. Journal of Neurophysiology. 1990b;63:1307–1313. doi: 10.1152/jn.1990.63.6.1307. [DOI] [PubMed] [Google Scholar]
- Emonet-Dénand F, Hunt CC, Laporte Y. Effects of stretch on dynamic fusimotor after-effects in cat muscle spindles. The Journal of Physiology. 1985;360:201–213. doi: 10.1113/jphysiol.1985.sp015612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F, Laporte Y, Matthews PBC, Petit J. On the subdivision of static and dynamic fusimotor actions on the primary ending of the cat muscle spindle. The Journal of Physiology. 1977;268:827–861. doi: 10.1113/jphysiol.1977.sp011884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng JJ, Hoffer JA. Regional variability of stretch reflex amplitude in the cat medial gastrocnemius muscle during a postural task. Journal of Neurophysiology. 1997;78:1150–1154. doi: 10.1152/jn.1997.78.2.1150. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Hulliger M, Matthews PBC. The effects of fusimotor stimulation during small amplitude stretching on the frequency-response of the primary endings of the mammalian muscle spindle. The Journal of Physiology. 1975;253:175–206. doi: 10.1113/jphysiol.1975.sp011186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Kakuda N, Wessberg J, Vallbo ÅB, Conway BA, Rosenberg JR. Correlation between Ia afferent discharges, EMG and torque during steady isometric contraction of human finger muscles. In: Taylor A, Gladden MH, Durbara R, editors. Alpha and Gamma Motor Systems. New York: Plenum Press; 1995. pp. 547–549. [Google Scholar]
- Hoffer JA, Caputi AA, Pose IE, Griffiths RI. Roles of muscle activity and load on the relationship between muscle spindle length and whole muscle length in the freely walking cat. Progress in Brain Research. 1989;80:75–85. doi: 10.1016/s0079-6123(08)62201-3. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Matthews PBC, Noth J. Static and dynamic fusimotor action on the response of Ia fibres to low frequency sinusoidal stretching of widely ranging amplitude. The Journal of Physiology. 1977;267:811–838. doi: 10.1113/jphysiol.1977.sp011839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, VallboÅ B. Discharge in muscle spindle afferents related to direction of slow precision finger movements in man. The Journal of Physiology. 1985;362:437–453. doi: 10.1113/jphysiol.1985.sp015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JKS, Matthews PBC. The central control of the dynamic response of muscle spindle receptors. The Journal of Physiology. 1962;161:357–378. doi: 10.1113/jphysiol.1962.sp006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H, Sjölander P, Sojka P. Fusimotor reflex profiles of individual triceps surae primary muscle afferents assessed with multi-afferent recording technique. Journal de Physiologie. 1991;85:6–19. [PubMed] [Google Scholar]
- Kakuda N, Miwa T, Nagaoka M. Coupling between single muscle spindle afferent and EMG in human wrist extensor muscles: physiological evidence of skeletofusimotor (beta) innervation. Electroencephalography and Clinical Neurophysiology. 1998;109:360–363. doi: 10.1016/s0924-980x(98)00030-7. 10.1016/S0924-980X(98)00030-7. [DOI] [PubMed] [Google Scholar]
- Lennerstand G, Thoden U. Muscle spindle responses to concomitant variation in length and in fusimotor activation. Acta Physiologica Scandinavica. 1968;74:153–165. doi: 10.1111/j.1748-1716.1968.tb04224.x. [DOI] [PubMed] [Google Scholar]
- Loren GJ, Lieber RL. Tendon biomechanical properties enhance human wrist muscle specialization. Journal of Biomechanics. 1995;28:791–799. doi: 10.1016/0021-9290(94)00137-s. 10.1016/0021-9290(94)00137-S. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Muscle spindles: their messages and their fusimotor supply. In: Brookes VB, editor. Handbook of Physiology, section 1, The Nervous System, Motor Control. part 2. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 189–228. [Google Scholar]
- Matthews PBC, Stein RB. The sensitivity of muscle spindle afferents to sinusoidal stretching. The Journal of Physiology. 1969a;200:723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC, Stein RB. The regularity of primary and secondary muscle spindle discharges. The Journal of Physiology. 1969b;202:59–82. doi: 10.1113/jphysiol.1969.sp008795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Prochazka A, Proske U. The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. The Journal of Physiology. 1984;356:465–477. doi: 10.1113/jphysiol.1984.sp015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Prochazka A, Proske U. Action of single dynamic fusimotor neurones on cat soleus Ia afferents during muscle shortening. Experimental Brain Research. 1985;58:56–61. doi: 10.1007/BF00238953. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell L, Sheperd T, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. part I. New York: American Physiological Society; 1996. pp. 89–127. [Google Scholar]
- Prochazka A, Gorassini M. Models of ensemble firing of muscle spindle afferents recorded during normal locomotion in cats. Journal of Neurophysiology. 1998;507:277–291. doi: 10.1111/j.1469-7793.1998.277bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Hulliger M, Trend P, Dürmüller N. Dynamic and static fusimotor set in various behavioral contexts. In: Hník P, Soukup T, Vejsada R, Zelená J, editors. Mechanoreceptors. New York, London: Plenum Press; 1987. pp. 417–430. [Google Scholar]
- Scott A, Kümmel H, Illert M. Skeletofusimotor (beta) innervation of proximal and distal forelimb muscles of the cat. Neuroscience Letters. 1995;190:1–4. doi: 10.1016/0304-3940(95)11485-f. 10.1016/0304-3940(95)11485-F. [DOI] [PubMed] [Google Scholar]
- Vallbo Å B. Discharge patterns in human muscle spindle afferents during isometric voluntary contractions. Acta Physiologica Scandinavica. 1970;80:552–566. doi: 10.1111/j.1748-1716.1970.tb04823.x. [DOI] [PubMed] [Google Scholar]
- Vallbo Å B. Human muscle spindle discharges during isometric voluntary contractions. Amplitude relations between spindle frequency and torque. Acta Physiologica Scandinavica. 1974;90:319–336. doi: 10.1111/j.1748-1716.1974.tb05594.x. [DOI] [PubMed] [Google Scholar]
- Vallbo ÅB, Hagbarth K-E, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiological Reviews. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Wessberg J, VallboÅ B. Coding of pulsatile motor output by human muscle spindle afferents during slow finger movements. The Journal of Physiology. 1995;488:833–840. doi: 10.1113/jphysiol.1995.sp020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LR, Gandevia SC, Burke D. Discharge of human muscle spindle afferents innervating ankle dorsiflexors during target isometric contractions. The Journal of Physiology. 1997;504:221–232. doi: 10.1111/j.1469-7793.1997.221bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


