Abstract
The intracellular mechanisms activated by the binding of vasopressin to its receptor(s) and which result in the increase of [Ca2+]i were investigated in freshly dissociated supraoptic nucleus neurones. Various pharmacological agents were used to investigate the possible involvement of phospholipase C (PLC) and adenylate cyclase (AC) intracellular pathways in the transduction of the vasopressin action.
Both the PLC inhibitor U-73122 and the protein kinase C (PKC) inhibitor calphostin C, reduced the [Ca2+]i rise elicited by vasopressin. The cAMP analogue, 8-Br-cAMP produced an increase in [Ca2+]i and IBMX, a phosphodiesterase inhibitor, potentiated the response to vasopressin.
After pre-incubation with the AC inhibitor SQ-22536, 7 out of 18 vasopressin-sensitive neurones showed no inhibition of the vasopressin response, while the response to vasopressin was reduced by greater than 35 % in each of the other 11 neurones.
The activation of protein kinase A (PKA) with Sp-cAMPS caused an increase in [Ca2+]i which was additive to the vasopressin-elicited [Ca2+]i increase. After incubation with the PKA inhibitors Rp-cAMPS or H-89, the [Ca2+]i responses triggered by Sp-cAMPS and vasopressin were, respectively, abolished and greatly reduced.
A combined administration of SQ-22536 (AC inhibitor) followed by U-73122 (PLC inhibitor), or U-73122 followed by H-89 (PKA inhibitor), virtually abolished the response to vasopressin.
In vasopressin-responsive neurones, the pituitary adenylate cyclase-activating polypeptide (PACAP) induced a [Ca2+]i increase similar to the response to vasopressin and in both cases the increase was inhibited to the same extent by a combination of U-73122 and Rp-cAMPS.
In conclusion, we suggest that the autoregulation exerted specifically by vasopressin on vasopressin-sensitive neurones involves the activation of both PLC- and AC-linked pathways.
The rat supraoptic and paraventricular nuclei contain two populations of magnocellular neurones that synthesize either vasopressin or oxytocin. Both peptides are released from axon terminals in the neurohypophysis into the blood circulation to exert their multiple peripheral hormonal effects. The defined electrical patterns of vasopressin and oxytocin neurones, as those recorded in vivo, stimulate the release of neuropeptides from the neurohypophysis level (for review see Dyball, 1988).
In addition to their peripheral hormonal action, both peptides play various neurotransmitter-like roles in the central nervous system and interestingly, one of these is the regulation of the electrical activity of their own neurones through a somatodentritic release (for review on oxytocin, see Richard et al. 1991; and on vasopressin, see Landgraf, 1992). To date, while the facilitatory role of oxytocin on the bursting activity of oxytocin neurones is clearly established, the data concerning the autocontrol of vasopressin on vasopressin neurones remains controversial with reports of inhibitory or excitatory or no effects (Leng & Mason, 1982; Abe et al. 1983; Inenaga & Yamashita, 1986; Ludwig & Leng, 1997). In a recent study, vasopressin was reported to regularize the characteristics of the phasic firing pattern of vasopressin neurones in vivo (Gouzènes et al. 1998a). At the subcellular level, the mechanisms by which vasopressin exerts this autoregulation remain unknown. We have shown that both oxytocin and vasopressin exert a direct and specific action on freshly dissociated supraoptic nucleus by increasing the intracellular Ca2+ concentration ([Ca2+]i) (Lambert et al. 1994; Dayanithi et al. 1996). Oxytocin induces the release of Ca2+ from intracellular stores (Lambert et al. 1994) whereas the action of vasopressin mainly requires an influx of external Ca2+ via L-, N- and T-type Ca2+ channels (Sabatier et al. 1997) and also partially involves Ca2+ release from thapsigargin-sensitive stores (Dayanithi et al. 1996).
In peripheral targets, distinction is made between oxytocin receptors and V1a-, V1b-, and V2-type vasopressin receptors. Oxytocin and V1-type vasopressin receptors are associated with hydrolysis of phosphatidylinositols and induce a rise in [Ca2+]i while V2-type vasopressin receptors increase intracellular cAMP levels. In the central nervous system, pharmacological studies have demonstrated that vasopressin receptors resemble the peripheral V1a-subtype and expression of V1b- or V2-subtypes has never been clearly reported (Barberis & Tribollet, 1996). Our microspectrofluorimetry studies have revealed that in supraoptic vasopressin neurones, a V1a-type receptor antagonist, SR 49059, inhibits the vasopressin-induced [Ca2+]i rise (Dayanithi et al. 1996) but interestingly, we have also found that a V2-type agonist is able to increase the [Ca2+]i in vasopressin neurones (Gouzènes et al. 1998b).
Little is known about vasopressin-induced intracellular transduction pathways in the brain. It has been reported that vasopressin increases the production of inositol phosphates in the hippocampus (Diaz-Brinton et al. 1994; Stephens & Logan, 1986), in septum (Shewey & Dorsa, 1988; Lebrun et al. 1990; Poulin & Pittman, 1993) and in dorsomedial medulla oblongata (Moratalla et al. 1988). In other studies, the vasopressin failed to activate adenylate cyclase (AC) in hippocampus and septum (Barberis, 1983; Dorsa et al. 1983; Audigier & Barberis, 1985; Brinton & McEwen, 1989). In hippocampal cultures from rat fetuses, vasopressin-induced cAMP increases occurred only during the first few days in culture (Diaz-Brinton & Brownson, 1993). Interestingly, it has been demonstrated that dibutyryl cAMP could mimic the effects of vasopressin on the electrical activity of supraoptic neurones and that the amount of cAMP in supraoptic tissues incubated with vasopressin was significantly higher compared with control conditions (Abe et al. 1983).
Here, we have characterized the intracellular messengers involved in mediating the actions of vasopressin using fura-2 microspectrofluorimetry on single magnocellular vasopressin-sensitive neurones freshly dissociated from the rat supraoptic nucleus. Finally, the vasopressin action was compared with that of the pituitary adenylate cyclase-activating polypeptide (PACAP) action. As this peptide is known to bind to receptors coupled to both phospholipase C (PLC) and AC (Spengler et al. 1993) and to induce [Ca2+]i increases in supraoptic neurones as well as somatodendritic release of vasopressin (Shibuya et al. 1998). Preliminary accounts of this work have appeared in abstract form (Sabatier et al. 1998).
METHODS
Supraoptic nuclei dissection and cell dissociation
The supraoptic tissues from two adult male Wistar rats (100–250 g body weight) were used for each cell dissociation procedure. The animals were killed by decapitation with a guillotine following the guidelines laid down by the French/European ethical committee. The dissection of the supraoptic nuclei and the cell dissociation were performed as previously described (Lambert et al. 1994; Dayanithi et al. 1996; Sabatier et al. 1997) with modifications. After dissection, the tissue pieces were transferred to Locke buffer containing (mm): NaCl, 140; KCl, 5; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 1.8; glucose, 10; Hepes, 10; pH 7.2 adjusted with NaOH. The osmolarity of all the solutions ranged between 298 and 303 mosmol l−1. The tissue pieces were enzymatically dissociated by incubation in oxygenated Locke buffer supplemented with 0.5 mg ml−1 deoxyribonuclease I, 1 mg ml−1 protease X (both from France Biochem, Meudon, France) and 1 mg ml−1 protease XIV (Boehringer Mannheim) for 45 min. After incubation, tissues were washed with Locke buffer and left in Locke buffer for 15 min under O2 before undergoing gentle mechanical trituration. In contrast to Lambert et al. (1994) or Dayanithi et al. (1996), in the current procedure using different enzymes from different sources, we harvested a greater number of cells possessing long dendritic processes. Under these conditions, the dissociated fura-2-loaded cells survive for several hours (8–10 h) and the responses to the test substances are more consistent. The cell suspension obtained was used for dye loading.
Dye loading and measurement of [Ca2+]i
The dissociated supraoptic cells were loaded by incubation with the Ca2+-sensitive dye fura-2 AM (2.5 μm, dissolved in DMSO) plus 0.02 % Pluronic F-127 (dissolved in water; Molecular Probes Inc.) in Locke buffer. Dye loading was carried out for 40 min at 37°C in a humidified atmosphere. [Ca2+]i in single cells was measured as described previously (Dayanithi et al. 1996). Briefly, fluorescence measurements of [Ca2+]i were performed with the Zeiss Microscope Photometer System (FFP, Zeiss, Oberkochen, Germany), based on an inverted microscope (Axiovert 100, Zeiss) equipped for epifluorescence (objective, Plan-Neofluar × 100/1.30 oil immersion). With fluorescence values corrected for background and dark current, the [Ca2+]i values were calculated from the ratio between 340 and 380 nm recordings, in accordance with the equation given by Grynkiewicz et al. (1985). Fura-2 calibration was performed following the same procedure as described previously (Lambert et al. 1994).
Drugs and solutions
The Locke buffer described above was used as the control medium. Stock solutions of vasopressin, oxytocin (Boehringer Mannheim) and PACAP-38 (Isochem, Strasbourg, France) were prepared in distilled water and diluted to working concentrations in the Locke buffer before use. All pharmacological compounds used to interfere with the different stages of intracellular transduction pathways were obtained from Biomol Research Laboratories Inc. (Plymouth Meeting, PA, USA) except 8-Br-cAMP, which was purchased from Sigma. Concentrated stocks of SQ-22536 (AC inhibitor), 8-Br-cAMP (cAMP analogue), Sp-cAMPS (SP-cyclic 3′,5′-hydrogen phosphorothioate adenosine triethylammonium salt; cAMP analogue, protein kinase A (PKA) activator) and Rp-cAMPS (RP-3′,5′-hydrogen phosphorothioate adenosine triethylammonium salt; cAMP analogue, PKA inhibitor) were prepared in distilled water, stored at −20°C and dissolved in Locke buffer at appropriate concentrations before use. Stock solutions of forskolin (AC activator), 3-isobutyl-methyl-xanthine (IBMX, phosphodiesterase inhibitor), H-89 (PKA inhibitor) and calphostin C (PKC inhibitor) were prepared in DMSO and diluted to working concentration. As has been suggested by the drug company, (Biomol Research Laboratories), U-73122 (PLC inhibitor) was dissolved in chloroform at stock concentration, aliquots were left to evaporate at room temperature and stored at −20°C. Immediately before use, dried aliquots were first diluted in DMSO and finally dissolved in Locke buffer to the appropriate concentration.
Drug application
In contrast to the methods used to apply the drugs in our previous studies (Lambert et al. 1994; Dayanithi et al. 1996; Sabatier et al. 1997), the control and test solutions (vasopressin, PACAP-38, 8-Br-cAMP, IBMX, Sp-cAMPS) were applied using a multiple capillary perfusion system (200 μm inner diameter capillary tubing, flow rate 100 μl min−1) placed to the proximity of each cell tested (< 0.5 mm). Each capillary was fed by a reservoir 50 cm above the bath. Complete solution changes were made by switching the opening from one capillary to the next. After each application, the cells were washed with the Locke buffer. Under these conditions, the responses to vasopressin showed no desensitization after repeated applications (L. Gouzènes, N. Sabatier, Ph. Richard, F. Moos & G. Dayanithi, unpublished results) in contrast to long-duration applications (Dayanithi et al. 1996). It should be noted that incubations with inhibitory substances were carried out in a 500 μl bath containing the inhibitors diluted in Locke buffer.
Data analysis and statistical methods
The results were analysed by Student's paired t test. Unless otherwise stated, all inhibitors used in this study showed a ‘significant’ effect at P < 0.001. The results are expressed as means ±s.e.m. The value of sample size (n) quoted in the paper is based on the number of cells tested with the same protocol (control, test drug, recovery) for each group (i.e. for example, n cells tested with vasopressin, vasopressin + U-73122, recovery). The figures (traces) represent on-line measurements showing the [Ca2+]i levels both before and after application of the test substances whereas the bar diagrams or the data given as means with s.e.m. represent the ‘mean evoked [Ca2+]i increase’. The mean evoked [Ca2+]i is calculated by subtracting the [Ca2+]i levels under basal conditions, i.e. prior to the stimulus, from the peak amplitude observed after the application of the test substances.
RESULTS
Freshly isolated supraoptic magnocellular neurones of adult male rats were chosen following the morphological criteria as described in our previous papers (Lambert et al. 1994; Dayanithi et al. 1996). Consistent with our previous findings, in the present investigation, the neurones showed an increase in [Ca2+]i in response to either vasopressin or to oxytocin or to both. Moreover, in a previous study, the immunocytochemistry performed after calcium measurements has revealed that the neurones responding to vasopressin contained vasopressin and the neurones responding to oxytocin contained oxytocin (Dayanithi et al. 1996). Although the intracellular transduction signals activated by oxytocin on oxytocin neurones as well as on the population of neurones that responded to both peptides are under investigation, the present paper focuses only on the neurones that responded selectively to vasopressin.
Since the effects of vasopressin on supraoptic magnocellular vasopressin-sensitive neurones partially involves Ca2+ mobilization from thapsigargin-sensitive intracellular Ca2+ stores, suggesting activation of inositol 1,4,5-trisphosphate (IP3) receptors (Dayanithi et al. 1996), we first investigated the involvement of PLC-linked intracellular mechanisms. We then analysed whether the AC-coupled transduction pathway was also activated. To do this, specific pharmacological agents were used to directly modulate different stages of these intracellular pathways and the consequences on vasopressin-elicited [Ca2+]i responses were observed. Unless otherwise stated in the text, vasopressin was applied at 100 nm. It should be noted that brief repeated applications of vasopressin induced reproducible [Ca2+]i increases, the response to the second application being 98.5 % of the response to the first application (first application, AVP = 632 ± 60 nm; second application, AVP = 623 ± 58 nm; n = 6). In general, the response to vasopressin was single and transient but some neurones, after vasopressin application, showed a second or biphasic response (example: Figs 1, 2, 4 and 6) suggesting a release of calcium from internal stores (Dayanithi et al. 1996).
Figure 1. Effects of the phospholipase C inhibitor U-73122 on the [Ca2+]i rise induced by arginine vasopressin (AVP) in supraoptic neurones.
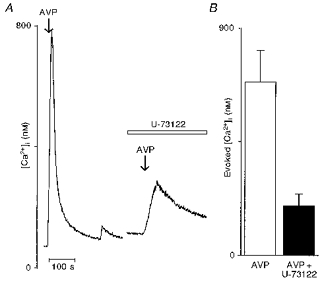
A, left trace shows a typical transient [Ca2+]i response after a brief application of vasopressin (100 nm) in a representative vasopressin-sensitive neurone. The same neurone was exposed to U-73122 (5 μm) for 15 min and then challenged with vasopressin. Note that the vasopressin-induced [Ca2+]i rise was strongly depressed by U-73122 (right trace). B, bar diagram representing the mean evoked peak amplitude of the [Ca2+]i response triggered by vasopressin before (□) and after (▪) exposure to U-73122. Arrows indicate brief applications (10 s) of drugs and the open bar represents the actual duration of application.
Figure 2. Effects of the protein kinase C inhibitor, calphostin C, on the [Ca2+]i increase elicited by arginine vasopressin.
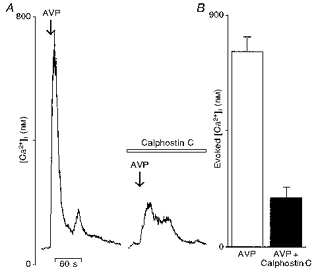
A, vasopressin (AVP) applied briefly (arrow) at 100 nm triggered a rise in [Ca2+]i (left trace). Neurones were then subjected to calphostin C (100 nm) for 15 min and challenged again with vasopressin. Note the strong inhibitory effect of calphostin C on the vasopressin-induced [Ca2+]i response (right trace). B, the mean evoked [Ca2+]i increase elicited by vasopressin before (□) and after application of protein kinase C inhibitor (▪), represented by a bar graph.
Figure 4. Effects of the adenylate cyclase inhibitor SQ-22536 on the vasopressin-induced [Ca2+]i rise in supraoptic neurones.
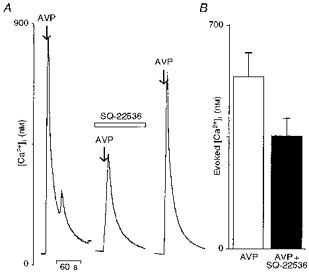
A, left trace shows a [Ca2+]i response induced by a brief application (arrow) of vasopressin (AVP; 100 nm). The cells were then pre-incubated for 15 min with the AC inhibitor SQ-22536 (100 μm), and then challenged with vasopressin and the vasopressin-induced [Ca2+]i rise was reduced (n = 11) by SQ-22536 (right trace). Note that the SQ-22536 block was reversible. B, the mean evoked [Ca2+]i increase elicited by vasopressin in the absence (□) and in the presence (▪) of the inhibitor SQ-22536, represented by a bar graph.
Figure 6. Effects of the protein kinase A inhibitors Rp-cAMPS and H-89 on the vasopressin-induced [Ca2+]i rise.
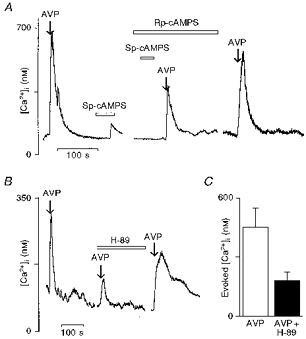
A, the [Ca2+]i responses to vasopressin (100 nm) and Sp-cAMPS (100 μm) (left trace). The neurones were pre-incubated for 15 min with Rp-cAMPS (500 μm) which abolished the [Ca2+]i rise elicited by Sp-cAMPS and strongly reduced the response induced by vasopressin (right trace). B, neurones were subjected to a second protein kinase A inhibitor, H-89, applied at 10 μm for 15 min. Note that H-89 also strongly depressed the vasopressin-induced response. It should be noted that the effects of these inhibitors were reversible. C, bar diagram shows the mean vasopressin-induced [Ca2+]i rise before (□) and after H-89 incubation (▪).
Effects of PLC and PKC inhibitors
In order to determine whether the vasopressin action was mediated by a PLC-coupled receptor, we first tested the effects of U-73122, an inhibitor of PLC (Smith et al. 1990) on the vasopressin-induced [Ca2+]i rise. Figure 1A shows that 15 min pre-incubation with U-73122 (5 μm) inhibited the [Ca2+]i rise elicited by vasopressin by 75 %. For the nine cells tested with this protocol, the mean evoked [Ca2+]i response induced by vasopressin under control conditions was 687 ± 125 nm. After incubation with U-73122, the [Ca2+]i rise was reduced to 197 ± 47 nm (Fig. 1B; n = 9).
PLC activation leads to production of both IP3, an intracellular Ca2+ mobilizer, and diacyl glycerol, which is a protein kinase C activator. Thus, in a second series of experiments, vasopressin-sensitive neurones were treated with calphostin C, an inhibitor of PKC (Kobayashi et al. 1989). Brief application of vasopressin triggered a [Ca2+]i rise that was reduced by 75 % during calphostin C (100 nm) administration (Fig. 2A). Reduction of the mean evoked [Ca2+]i response generated by vasopressin before and after calphostin C treatment is quantified in Fig. 2B (vasopressin, 760 ± 54 nm; calphostin C, 192 ± 39 nm; n = 17). Together, these results suggest that both PLC and PKC activation are involved in the vasopressin-induced [Ca2+]i rise in vasopressin-sensitive neurones.
Modulation of cAMP signal transduction pathway
We next investigated the possible involvement of the cAMP messenger pathway in these responses. The effects of 8-Br-cAMP, a cAMP analogue, on vasopressin-sensitive neurones were first investigated. Figure 3A shows that in vasopressin-sensitive neurones, application of 1 mm 8-Br-cAMP during 70 s induced a small increase in [Ca2+]i (vasopressin, 607 ± 97 nm; 8-Br-cAMP, 170 ± 33 nm; n = 16). We then tested IBMX, which inhibits the enzyme phosphodiesterase and thus prevents cAMP degradation. For this particular test, we used vasopressin at 1 nm instead of 100 nm to get a small [Ca2+]i increase in order to detect whether IBMX potentiates the response to vasopressin. Applied separately during 50 s, vasopressin at the lower concentration (1 nm) and IBMX at 100 μm induced a moderate increase of [Ca2+]i but when IBMX was given in combination with vasopressin we observed a potentiation of the vasopressin-elicited [Ca2+]i rise (Fig. 3B; vasopressin (1 nm), 55 ± 8 nm; IBMX, 130 ± 40 nm; vasopressin (1 nm) + IBMX, 570 ± 75 nm; n = 14).
Figure 3. Effects of compounds increasing intracellular cAMP levels in vasopressin-sensitive supraoptic neurones on vasopressin-induced [Ca2+]i increase.
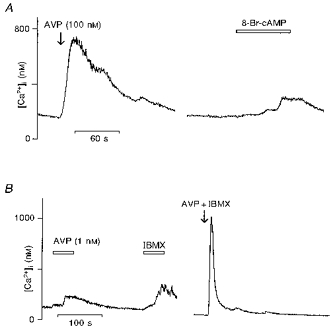
A, 8-Br-cAMP, an analogue of cAMP, was tested at 1 mm on vasopressin (AVP)-responsive neurones. A moderate increase of [Ca2+]i could be observed in response to 8-Br-cAMP about 1 min after the beginning of the application. B, vasopressin was tested at a lower concentration (1 nm) followed by the phosphodiesterase inhibitor IBMX at 100 μm. Left trace shows that both vasopressin and IBMX triggered a small [Ca2+]i increase which appeared after about 30 s of application. After washout, the same neurone was subjected to administration of a mixture of 1 nm vasopressin and 100 μm IBMX (right trace). Note that the vasopressin-induced [Ca2+]i response at 1 nm was greatly increased in the presence of IBMX.
These data suggest that an increase in intracellular cAMP level might be involved in the vasopressin-elicited [Ca2+]i increase. In order to further investigate this possibility, we examined the effect of SQ-22536, an adenylate cyclase inhibitor (Madison & Nicoll, 1986). After pre-incubation with SQ-22536 (100 μm), 7 out of 18 vasopressin-sensitive neurones showed no inhibition of the vasopressin-induced [Ca2+]i response, while the response to vasopressin was reduced by 35 % in the other 11 neurones (Fig. 4A). For this set of 11 neurones, the mean evoked [Ca2+]i increase before and after SQ-22536 is illustrated in Fig. 4B (vasopressin, 538 ± 73 nm; SQ-22536, 354 ± 55 nm). The statistical test was not applied here because the SQ-22536 was ineffective in some vasopressin-sensitive neurones. The SQ-22536 inhibitory effect was reversed after washing. These results reveal that modulation of cAMP intracellular concentration might interfere with the level of [Ca2+]i rise triggered by vasopressin in about 60 % of the vasopressin-sensitive neurones.
To determine whether the cAMP-activated protein kinase PKA plays a role in the actions of vasopressin, we tested the effects of Sp-cAMPS (the SP-diastereomer of cAMPS), an activator of PKA and of two inhibitors of PKA, Rp-cAMPS (the RP-diastereomer of cAMPS) and H-89 (Chijiwa et al. 1990). In vasopressin-sensitive neurones, application of Sp-cAMPS (100 μm) over 50 s induced a rise in [Ca2+]i (Fig. 5A; vasopressin, 781 ± 133 nm; Sp-cAMPS, 190 ± 47 nm; n = 9). Figure 5B shows [Ca2+]i responses elicited by a lower concentration of vasopressin (1 nm) alone, by Sp-cAMPS (100 μm) alone and by a mixture of both compounds. The effects of vasopressin and Sp-cAMPS were additive (vasopressin (1 nm), 76 ± 6 nm; Sp-cAMPS, 101 ± 20 nm; vasopressin (1 nm) + Sp-cAMPS, 141 ± 20 nm; n = 11). After administration of Rp-cAMPS (500 μm) for 15 min to vasopressin-sensitive neurones, the [Ca2+]i increase induced by Sp-cAMPS was totally abolished while the vasopressin-elicited [Ca2+]i increase was inhibited by 71 % (Fig. 6A; vasopressin, 547 ± 70 nm; Rp-cAMPS, 156 ± 24 nm; n = 12). Similarly to Rp-cAMPS, incubation with H-89 (10 μm) resulted in a reduction of the response to vasopressin by 70 % (Fig. 6B). The mean vasopressin-evoked [Ca2+]i response in control conditions and during H-89 treatment is shown in Fig. 6C (vasopressin, 453 ± 96 nm; H-89, 181 ± 42 nm; n = 13). The inhibitory effects of both Rp-cAMPS and H-89 were reversed after several washings.
Figure 5. Effects of the protein kinase A activator Sp-cAMPS on the vasopressin-induced [Ca2+]i rise in vasopressin-sensitive neurones.
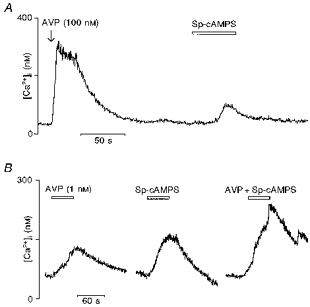
A, neurones responding to vasopressin (AVP; 100 nm) (left trace) were subjected after a wash to the application of Sp-cAMPS at 100 μm (right trace). Sp-cAMPS alone triggered a small [Ca2+]i increase. B, the [Ca2+]i rise induced by vasopressin at 1 nm was similar to the Sp-cAMPS-elicited rise (left trace) and no potentiation of response to vasopressin in the presence of Sp-cAMPS was observed (right trace).
The use of all of these compounds which interfere with the major steps of the cAMP signalling cascade allowed us to reveal that each of these steps might play a role in [Ca2+]i responses induced by vasopressin in vasopressin-sensitive neurones.
AC- and PLC-coupled transduction pathways are both involved in vasopressin action
The findings so far described indicate that the activation of both AC- and PLC-coupled intracellular mechanisms are required for the vasopressin-induced [Ca2+]i rise in vasopressin-sensitive neurones. Furthermore, we checked this hypothesis by using combinations of inhibitors of both transduction pathways. Figure 7A shows a vasopressin-sensitive neurone treated successively with SQ-22536 (100 μm) and U-73122 (5 μm) in which the mean vasopressin-evoked [Ca2+]i response is reduced by 45 % by the adenylate cyclase inhibitor and nearly totally abolished (93 % reduction) by further treatment with the PLC inhibitor (Fig. 7B; vasopressin, 413 ± 60 nm; SQ-22536, 228 ± 61 nm; SQ-22536 + U-73122, 30 ± 9 nm; n = 10). It should be noted that the effects of a combination of SQ-22536 and U-73122 were tested only on the cells which were affected by SQ-22536 and considered for statistical analysis. In another batch of experiments, the vasopressin-evoked [Ca2+]i response was monitored in the presence of U-73122 alone or in combination with H-89 (Fig. 8A). The PLC inhibitor first reduced the mean vasopressin-evoked [Ca2+]i rise by 68 % and this rise was further inhibited by 92 % with the PKA inhibitor (Fig. 8B; vasopressin, 486 ± 79 nm; U-73122, 156 ± 27 nm; U-73122 + H-89, 40 ± 6 nm; n = 10).
Figure 7. Effect of combined application of the AC inhibitor SQ-22536 and PLC inhibitor U-73122 on the vasopressin-induced [Ca2+]i rise.
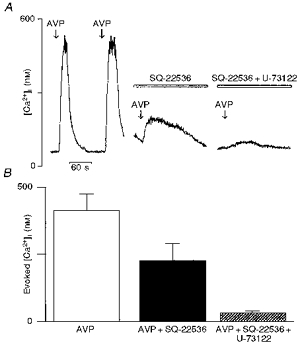
A, brief repeated applications (arrows) of vasopressin (AVP; 100 nm) elicited reproducible [Ca2+]i responses (left trace). This representative vasopressin-sensitive neurone was first pre-incubated with 100 μm SQ-22536 for 15 min (middle trace) and then challenged with vasopressin. After washout, the cells were subjected to a mixture of both SQ-22536 (100 μm) and U-73122 (5 μm) for 15 min (right trace). The reduction of the [Ca2+]i response by adenylate cyclase inhibitor SQ-22536 was further decreased in the presence of the phospholipase C inhibitor U-73122. For statistical analysis, the comparisons were made between each group (SQ-22536 and SQ-22536 + U-73122) compared with the control vasopressin. B, the nearly complete blockade of vasopressin response by combination of inhibitors of cAMP and IP3 second messenger pathways is illustrated by graphical representation of the evoked mean vasopressin [Ca2+]i responses.
Figure 8. Effects of a combination of the phospholipase C inhibitor U-73122 and the protein kinase A inhibitor H-89 on vasopressin neurones.
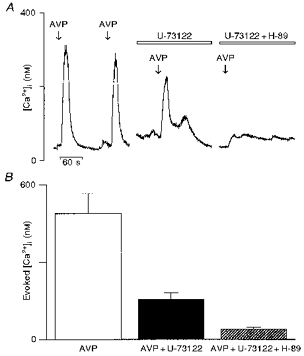
The same experimental design was used as described in Fig. 7. A, selected neurones responding to 100 nm vasopressin (left trace) were first pre-incubated with U-73122 (5 μm) for 15 min before being challenged for a second time with vasopressin. Following a wash, the cells were pre-incubated with a buffer containing both U-73122 (5 μm) and H-89 (10 μm) for 15 min and then challenged with vasopressin. The reduction of the [Ca2+]i response by the phospholipase C inhibitor U-73122 was further decreased in the presence of the protein kinase A inhibitor H-89. For statistical analysis, comparisons were made between each group (U-73122 and (H-89 + U-73122) and the control vasopressin response. B, the bar diagram shows the mean amplitude of the [Ca2+]i responses evoked by vasopressin and in the absence and presence of inhibitors.
Comparison of the mechanisms of vasopressin- and PACAP-induced [Ca2+]i rise
We performed experiments in vasopressin-sensitive neurones to demonstrate the parallel regulation of the [Ca2+]i increase induced either by activation of vasopressin receptors or by activation of neuropeptide receptors known to be linked to both PLC and AC, that is, PACAP type I receptors (Spengler et al. 1993).
Figure 9 shows that vasopressin-sensitive neurones are also sensitive to PACAP which induced a rise in [Ca2+]i at 10 nm. The [Ca2+]i response induced by PACAP is reproducible without any desensitization (results not shown). The same neurone was first incubated with U-73122, which reduced both vasopressin- and PACAP-induced [Ca2+]i responses (vasopressin, 792 ± 130 nm; PACAP, 662 ± 127 nm; vasopressin + U-73122, 253 ± 55 nm; PACAP + U-73122, 275 ± 61 nm). In this neurone, further treatment with Rp-cAMPS abolished both responses (vasopressin + U-3122 + Rp-cAMPS, 22 ± 3 nm; PACAP + U-73122 + Rp-cAMPS, 31 ± 4 nm, n = 7). Furthermore, in the presence of the V1a-type vasopressin receptor antagonist SR 49059 (10 nm), shown to inhibit the response to vasopressin in supraoptic neurones (Dayanithi et al. 1996), the PACAP-induced [Ca2+]i rise was not affected (vasopressin, 415 ± 65 nm; PACAP, 452 ± 63 nm; PACAP + SR 49059, 504 ± 69 nm; n = 8).
Figure 9. Inhibition of the PACAP-induced [Ca2+]i response by both phospholipase C and protein kinase A inhibitors.
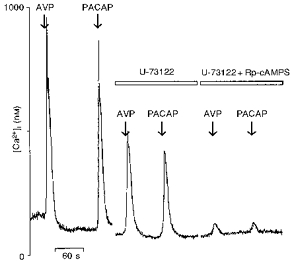
Left traces show the transient [Ca2+]i response induced by 10 nm PACAP in a vasopressin-sensitive neurone. Neurones were then pre-incubated for 15 min in a medium containing U-73122 (5 μm) and challenged again with vasopressin (AVP) and PACAP (middle traces). After washout, the cells were incubated in a mixture of U-73122 and Rp-cAMPS for about 15 min and the responses to vasopressin and PACAP were monitored (right traces). Note that the responses to both vasopressin and PACAP were significantly reduced by U-73122 and the remaining response was almost completely abolished by Rp-cAMPS.
DISCUSSION
In the present study, we characterized the intracellular mechanisms involved in vasopressin-induction of [Ca2+]i increases in vasopressinergic magnocellular supraoptic neurones. The use of compounds that inhibited the PLC pathway (U-73122 and calphostin C) and that inhibited (SQ-22536 and PKA inhibitors) or activated (cAMP analogues and IBMX) the AC pathway allowed us to interfere with several stages of these mechanisms. Our findings suggest that both PLC- and AC-linked signal transduction pathways play an important role in the vasopressin action on intracellular Ca2+. Results obtained by affecting these two pathways during activation of vasopressin receptors were similar to those observed during activation of PACAP receptors, which are known to couple to both pathways.
Vasopressin activates phospholipase C pathway
PLC-mediated hydrolysis of phosphatidylinositol phosphates leads to the production of the second messenger IP3. This messenger binds to IP3 receptors expressed on the membranes of intracellular Ca2+ stores to release Ca2+ from these stores. In this study, we demonstrated the involvement of PLC in the [Ca2+]i increase elicited by vasopressin in isolated vasopressin-sensitive neurones, suggesting the activation of a V1-type vasopressin receptor. This is in agreement with our previous findings that an antagonist of V1a-receptors, SR 49059, specifically inhibited the vasopressin-induced [Ca2+]i rise (Dayanithi et al. 1996). In addition, studies on IP3 accumulation measurements have shown that in many brain areas, vasopressin stimulation of the production of phosphatidyl-inositols is inhibited by a V1-type antagonist (Stephens & Logan, 1986; Moratalla et al. 1988; Shewey & Dorsa, 1988; Hatton et al. 1992). In the supraoptic nucleus, the vasopressin-induced [Ca2+]i increase is totally dependent upon extracellular Ca2+ but nevertheless is also significantly depressed by emptying the Ca2+ from intracellular stores by thapsigargin (Dayanithi et al. 1996). Thus, the IP3 production triggered by vasopressin is probably not sufficient to lead to a release of stored Ca2+ (Berridge, 1993; Diaz-Brinton et al. 1994).
Diacylglycerol (DAG) is the other second messenger produced by PLC-mediated phosphatidylinositol hydrolysis and is known to activate PKC. We found that PKC played an important role in [Ca2+]i increase induced by vasopressin. Activation of PKC by phorbol esters has been shown to modulate Ca2+ currents in various neuronal models and notably, stimulation of PKC by 12,13-phorbol dibutyrate enhances N- and L-type Ca2+ channel currents in frog sympathetic neurones (Yang & Tsien, 1993). Similarly, the constitutively active form of PKC increases high threshold voltage-activated Ca2+ currents in rat dorsal root ganglion neurones (Hall et al. 1995). In supraoptic neurones, Ca2+ currents expressed on the plasma membrane (Foehring & Armstrong, 1996) and involved in the vasopressin-elicited [Ca2+]i rise (Sabatier et al. 1997) might be modulated by PKC.
Vasopressin activates adenylate cyclase pathway
The cAMP signal transduction pathway also clearly appeared to be involved in the [Ca2+]i rise triggered by vasopressin. In vasopressin-sensitive neurones, we first observed that increasing cAMP intracellular levels by using phosphodiesterase-resistant cAMP analogues (8-Br-cAMP and Sp-cAMPS) or by preventing cAMP degradation with the phosphodiesterase inhibitor IBMX, produced a [Ca2+]i response. Potentiation or inhibition of vasopressin-induced [Ca2+]i increase by IBMX and SQ-22536, respectively, provided evidence of the contribution of AC activation to this increase. Direct induction of cAMP production by vasopressin is uncommon in the central nervous system. In membranes prepared from the hippocampus and septum, specific vasopressin binding sites have been revealed but AC assays failed to detect any cAMP accumulation in response to vasopressin treatment (Barberis, 1983; Dorsa et al. 1983; Audigier & Barberis, 1985). However, it has been shown that the vasopressin can act through the cAMP system in guinea-pig supraoptic neurones in vitro (Abe et al. 1983). Indeed, vasopressin and dibutyryl-cAMP were shown to induce similar electrophysiological effects on the supraoptic neurone membrane which could be potentiated by papaverine, a phosphodiesterase inhibitor. In the same study, the cAMP levels in the supraoptic tissues incubated with vasopressin were significantly higher than in control tissues.
The results obtained with specific PKA inhibitors further support the findings that the cAMP-linked intracellular cascade plays an important role in the action of vasopressin. An increasing body of evidence suggests that the regulation of Ca2+ influx through voltage-dependent Ca2+ channels by PKA could influence Ca2+-dependent neurotransmitter release as well as Ca2+-activated enzymes and gene expression within hippocampal neurones (Hell et al. 1995). There are other possible targets for the PKA and PKC modulation: for example, cation channel opening is reported to occur upon vasopressin and PACAP receptor activation in various types of cells. In our hands, the most probable functional role for PKA during the action of vasopressin is to modulate L- and N-type Ca2+ channels or it could be multiple types of ion channels and this remains to be clearly established by measuring membrane currents or phosphorylation of channel proteins.
Receptor-mediated actions of vasopressin
Here, we have clearly demonstrated that the vasopressin-induced [Ca2+]i increase in vasopressin-sensitive neurones involves multiple intracellular mechanisms. These findings raise the question of the precise nature of vasopressin receptors expressed on vasopressin neurones of the supraoptic nucleus. To date, different types of vasopressin receptors have been identified in peripheral tissues, such as V1a, V1b and V2 (for review, see Barberis & Tribollet, 1996). In peripheral targets, V1a-type (liver, vascular smooth muscle and most of peripheral vasopressin receptor) and V1b-type receptors (adenohypophysis) stimulate PLC, while V2-type (kidney) activate AC to increase intracellular cAMP levels. Recently, a vasopressin-activated Ca2+-mobilizing receptor (VACM-1) has been cloned from rabbit kidney medulla but this receptor seems to be totally different from G protein-coupled receptors (Burnatowska-Hledin et al. 1996). In vasopressin-sensitive neurones, according to the double intracellular signal transduction profile involved in the vasopressin-elicited [Ca2+]i response, we may first hypothesize that both V1- and V2-type receptors are expressed at the plasma membrane (see Fig. 10A). We have shown that V1a-receptors are probably present on vasopressin-sensitive neurones (Dayanithi et al. 1996). The lack of specific and selective pharmacological agents does not allow a precise characterization of V1b-receptors with our technique but reverse transcriptase (RT)-nested polymerase chain reaction (PCR) and in situ hybridization have revealed that V1b-receptor mRNA is expressed in low quantities in vasopressin neurones (Hurbin et al. 1998). The [Ca2+]i measurement experiments using currently available V2-receptor agonists and antagonists provide direct pharmacological evidence that the V2-type receptor is involved in the [Ca2+]i response induced by vasopressin (Gouzènes et al. 1998). However, so far, autoradiographic and in situ hybridization studies have failed to clearly detect any V2-receptor protein or mRNA in the central nervous system (for review, see Barberis & Tribollet, 1996). In addition, in the supraoptic nucleus, the presence of V2-receptor mRNA was not detectable using RT-nested PCR (Hurbin et al. 1998).
Figure 10. Schematic representations of possible mechanisms of action of vasopressin on vasopressin neurones of the supraoptic nucleus.
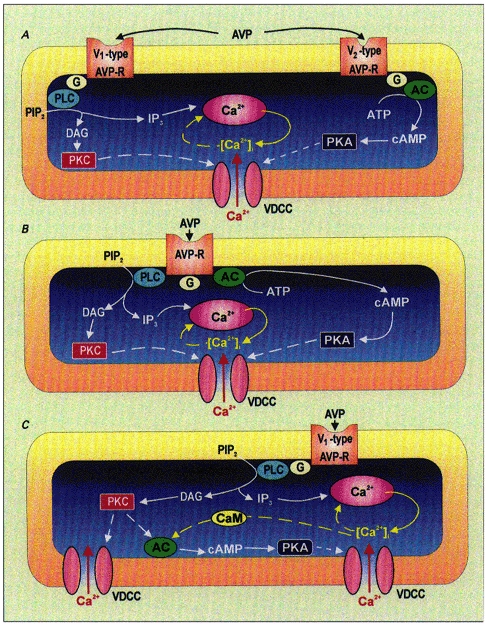
A, vasopressin (AVP) binds to both V1- and V2-type vasopressin receptors, activating both PLC- and AC-coupled intracellular mechanisms. PLC hydrolyses phosphatidylinositol 4,5-diphosphate (PIP2) leading to production of the second messengers IP3, DAG and cAMP. IP3 releases Ca2+ from intracellular stores while DAG and cAMP activate PKC and PKA, respectively. Both protein kinases might modulate voltage-dependent Ca2+ channels (VDCC). The rise in [Ca2+]i may in turn modulate IP3 receptors following a ‘calcium-induced calcium release’ phenomenon. B, another possibility would be that vasopressin binds to a single vasopressin receptor coupled to both PLC and AC, leading then to the same intracellular events as described in A above. C, vasopressin binds to the V1-type vasopressin receptors linked to PLC, leading to activation of PKC and release of Ca2+ from intracellular stores. The resulting PKC as well as calmodulin (CaM) activated by intracellular Ca2+ might stimulate AC that, in turn, produces cAMP which activates PKA. Dashed arrows represent hypothetical mechanisms.
A second possibility may be the presence of a unique vasopressin receptor on vasopressin neurones that could be coupled to both PLC and AC (see Fig. 10B). In various studies, V1-receptors have been shown to stimulate multiple signalling pathways when expressed in tissues or cell lines (Thibonnier et al. 1991; Briley et al. 1994). Notably, when expressed in Chinese hamster ovary (CHO) cells, V1b-type pituitary vasopressin receptors have been found to activate several signalling pathways including PLC, PLA2 and AC, via different G proteins and depending on receptor density (Thibonnier et al. 1997). In the present study, we have demonstrated that the [Ca2+]i response induced by vasopressin was regulated by the same mechanisms as those observed for the PACAP-induced [Ca2+]i response. At concentrations varying from 10−12 to 10−7 M, PACAP increases [Ca2+]i in freshly dissociated supraoptic neurones by enhancing Ca2+ entry via voltage-dependent Ca2+ channels and consequently these Ca2+ events resulted in stimulation of somatodendritic vasopressin release (Shibuya et al. 1998). In our hands, PACAP (10−8 M) induced an increase in [Ca2+]i in vasopressin-sensitive neurones. At this concentration, PACAP binds to high affinity PACAP type I receptors that are both PLC- and AC-positively coupled receptors (Spengler et al. 1993). In addition, gene expression of type I receptors has been detected throughout the paraventricular and supraoptic nucleus of the rat (Nomura et al. 1996). Furthermore, the V1a-type vasopressin receptor antagonist SR 49059 did not alter the PACAP-induced [Ca2+]i rise in vasopressin-sensitive neurones. This confirms that the response to PACAP is due solely to a direct action of PACAP rather than an effect of vasopressin released somatodendritically as a result of PACAP stimulation (Shibuya et al. 1998). Taken together, our results strongly suggest that the intracellular mechanisms involved in the vasopressin actions on supraoptic nucleus neurones are somewhat similar to those observed for the actions of PACAP.
A third possibility is that vasopressin receptors on vasopressin neurones are V1-type receptors uniquely coupled to PLC whose intracellular cascade could, in turn, stimulate the cAMP second messenger system (see Fig. 10C). Indeed, various studies have revealed an effective cross-talk between both pathways. Types II and VII isoforms of AC are activated by PKC and by βγ subunits of G proteins while types I, III and VIII isoforms are stimulated by Ca2+ via calmodulin (for review, see Cooper et al. 1995). A direct activation of AC by βγ subunits of G proteins that are released upon V1-receptor activation is not excluded. Interestingly, in plasma membranes prepared from the hypothalamus, Ca2+-calmodulin stimulated AC activity (Mons & Cooper, 1994).
In summary, our results show that the [Ca2+]i rise induced by vasopressin autocontrol of vasopressin neurones results from multiple intracellular transduction signals including at least PLC- and AC-linked pathways. It is important to understand the second messengers that participate in such peptide modulation of neuronal excitability and neuropeptide release, particularly in vasopressin neurones, as this represents an autocontrol processes. The precise mechanisms by which these second messengers, activated by vasopressin binding on vasopressin receptors, open specific Ca2+ channel types, are under investigation.
References
- Abe H, Inoue M, Matsuo T, Ogata N. The effects of vasopressin on electrical activity in the guinea-pig supraoptic nucleus in vitro. The Journal of Physiology. 1983;337:665–685. doi: 10.1113/jphysiol.1983.sp014648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audigier S, Barberis C. Pharmacological characterization of two specific binding sites for neurohypophyseal hormones in hippocampal synaptic plasma membranes of the rat. EMBO Journal. 1985;4:1407–1412. doi: 10.1002/j.1460-2075.1985.tb03794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis C. [3H]Vasopressin binding to rat hippocampal synaptic plasma membrane. FEBS Letters. 1983;162:400–405. doi: 10.1016/0014-5793(83)80795-9. [DOI] [PubMed] [Google Scholar]
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Critical Reviews in Neurobiology. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol triphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Briley EM, Lolait SJ, Axelrod J, Felder CC. The cloned vasopressin V1a receptor stimulates phospholipase A2, phospholipase C, and phospholipase D through activation of receptor-operated calcium channels. Neuropeptides. 1994;27:63–74. doi: 10.1016/0143-4179(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Brinton RE, McEwen BS. Vasopressin neuromodulation in the hippocampus. Journal of Neuroscience. 1989;9:752–759. doi: 10.1523/JNEUROSCI.09-03-00752.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnatowska-Hledin MA, Spielman WS, Smith WL, Shi P, Meyer JM, Dewitt DL. Expression cloning of an AVP-activated, calcium mobilizing receptor from rabbit kidney medulla. American Journal of Physiology. 1996;268:F1198–1210. doi: 10.1152/ajprenal.1995.268.6.F1198. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. Journal of Biological Chemistry. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Cooper DMF, Mons N, Karpen JW. Adenylyl cyclase and the interaction between calcium and cAMP signalling. Nature. 1995;374:421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- Dayanithi G, Widmer H, Richard Ph. Vasopressin-induced intracellular Ca2+ increase in isolated rat supraoptic cells. The Journal of Physiology. 1996;490:713–727. doi: 10.1113/jphysiol.1996.sp021180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Brinton R, Brownson EA. Vasopressin-induction of cyclic AMP in cultured hippocampal neurons. Developmental Brain Research. 1993;71:101–105. doi: 10.1016/0165-3806(93)90110-v. [DOI] [PubMed] [Google Scholar]
- Diaz-Brinton R, Gonzalez TM, Cheung WS. Vasopressin-induced calcium signaling in cultured hippocampal neurons. Brain Research. 1994;661:274–282. doi: 10.1016/0006-8993(94)91194-0. 10.1016/0006-8993(94)91204-1. [DOI] [PubMed] [Google Scholar]
- Dorsa DM, Majumdar LA, Petracca FM, Baskin D, Cornett LE. Characterization and localization of [3H]-arginine8-vasopressin binding to rat kidney and brain tissue. Peptides. 1983;4:699–706. doi: 10.1016/0196-9781(83)90021-9. 10.1016/0196-9781(83)90021-9. [DOI] [PubMed] [Google Scholar]
- Dyball REJ. The importance of bursting in determining secretory response: how does a phasic firing pattern influence peptide release from neurohypophyseal vasopressin terminals. In: Leng G, editor. Pulsatility in Neuroendocrine Systems. Boca Raton, FL, USA: CRC Press, Inc.; 1988. pp. 181–186. [Google Scholar]
- Foehring RC, Armstrong WE. Pharmacological dissection of high-voltage-activated Ca2+ current types in acutely dissociated rat supraoptic magnocellular neurons. Journal of Neurophysiology. 1996;76:977–983. doi: 10.1152/jn.1996.76.2.977. [DOI] [PubMed] [Google Scholar]
- Gouzènes L, Desarménien M, Hussy N, Richard Ph, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular neurons. Journal of Neuroscience. 1998a;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzènes L, Sabatier N, Richard Ph, Moos F, Dayanithi G. V1a- and V2-vasopressin receptor agonist-induced [Ca2+]i responses in vasopressin-sensitive neurones of the rat supraoptic nucleus. The Journal of Physiology. 1998b;509.P:86–87P. doi: 10.1111/j.1469-7793.1999.0771s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hall KE, Browning MD, Dudek EM, Macdonald RL. Enhancement of high threshold calcium currents in rat primary afferent neurons by constitutively active protein kinase C. Journal of Neuroscience. 1995;15:6069–6076. doi: 10.1523/JNEUROSCI.15-09-06069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI, Bicknell RJ, Hoyland J, Bunting R, Mason WT. Arginine vasopressin mobilises intracellular calcium via V1-receptor activation in astrocytes (pituicytes) cultured from adult rat neural lobes. Brain Research. 1992;588:75–83. doi: 10.1016/0006-8993(92)91346-g. 10.1016/0006-8993(92)91346-G. [DOI] [PubMed] [Google Scholar]
- Hell JW, Yokoyama CT, Breeze LJ, Chavkin C, Catterall WA. Phosphorylation of presynaptic and postsynaptic calcium channels by cAMP-dependent protein kinase in hippocampal neurons. EMBO Journal. 1995;14:3036–3044. doi: 10.1002/j.1460-2075.1995.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurbin A, Boissin-Agasse L, Orcel H, Rabié A, Joux N, Desarménien MG, Richard Ph, Moos F. The magnocellular vasopressin neurones of the rat hypothalamus express V1a and V1b but not V2 vasopressin receptor mRNAs. The Journal of Physiology. 1998;509.P:87P. doi: 10.1210/endo.139.11.6320. [DOI] [PubMed] [Google Scholar]
- Inenaga K, Yamashita H. Excitation of neurones in the rat paraventricular nucleus in vitro by vasopressin and oxytocin. The Journal of Physiology. 1986;370:165–180. doi: 10.1113/jphysiol.1986.sp015928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochemical and Biophysical Research Communications. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Dayanithi G, Moos FC, Richard Ph. A rise in intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. The Journal of Physiology. 1994;478:275–288. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R. Central release of vasopressin: stimuli, dynamics, consequences. Progress in Brain Research. 1992;91:29–39. doi: 10.1016/s0079-6123(08)62313-4. [DOI] [PubMed] [Google Scholar]
- Lebrun C, Gruber M, Meister M, Unger T. Central vasopressin pretreatment sensitizes phosphoinositol hydrolysis in the rat system. Brain Research. 1990;531:167–172. doi: 10.1016/0006-8993(90)90770-c. 10.1016/0006-8993(90)90770-C. [DOI] [PubMed] [Google Scholar]
- Leng G, Mason WT. Influence of vasopressin upon firing patterns of supraoptic neurones: a comparison of normal and Brattleboro rats. Annals of the New York Academy of Sciences. 1982;394:153–158. doi: 10.1111/j.1749-6632.1982.tb37422.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. European Journal of Neuroscience. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Cyclic adenosine 3′,5′-monophosphate mediates receptor actions of noradrenaline in rat hippocampal pyramidal cells. The Journal of Physiology. 1986;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mons N, Cooper DMF. Adenylyl cyclase mRNA expression does not reflect the predominant Ca2+/calmodulin-stimulated activity in the hypothalamus. Journal of Neuroendocrinology. 1994;6:665–671. doi: 10.1111/j.1365-2826.1994.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Vallejo M, Lightman S. Vasopressin stimulates inositol phospholipid metabolism in rat medulla oblongata in vivo. Brain Research. 1988;450:398–402. doi: 10.1016/0006-8993(88)91583-1. 10.1016/0006-8993(88)91583-1. [DOI] [PubMed] [Google Scholar]
- Nomura M, Ueta Y, Serino R, Kabashima N, Shibuya I, Yamashita H. PACAP type I receptor gene expression in the paraventricular and supraoptic nuclei of rats. NeuroReport. 1996;8:67–70. doi: 10.1097/00001756-199612200-00014. [DOI] [PubMed] [Google Scholar]
- Poulin P, Pittman QJ. Arginine vasopressin-induced sensitization in brain: facilitated inositol phosphate production without changes in receptor number. Journal of Neuroendocrinology. 1993;5:23–31. doi: 10.1111/j.1365-2826.1993.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Richard Ph, Moos F, Freund-Mercier MJ. Central effects of oxytocin. Physiological Reviews. 1991;71:331–370. doi: 10.1152/physrev.1991.71.2.331. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Richard Ph, Dayanithi G. L-, N- and T- but neither P- nor Q-type Ca2+ channels control vasopressin-induced Ca2+ influx in magnocellular vasopressin neurones isolated from the rat supraoptic nucleus. The Journal of Physiology. 1997;503:253–268. doi: 10.1111/j.1469-7793.1997.253bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Richard Ph, Dayanithi G. Activation of multiple intracellular transduction signals by vasopressin (AVP) in AVP-sensitive neurones of the rat supraoptic nucleus. The Journal of Physiology. 1998;509.P:86P. doi: 10.1111/j.1469-7793.1998.699ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewey LM, Dorsa DM. V1-type vasopressin receptors in rat brain septum: binding characteristics and effects on inositol phospholipid metabolism. Journal of Neuroscience. 1988;5:1671–1677. doi: 10.1523/JNEUROSCI.08-05-01671.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya I, Noguchi J, Tanaka K, Harayama N, Inoue Y, Kabashima N, Ueta Y, Hattori Y, Yamashita H. PACAP increases the cytosolic Ca2+ concentration and stimulates somatodendritic vasopressin release in rat supraoptic neurons. Journal of Neuroendocrinology. 1998;10:31–42. doi: 10.1046/j.1365-2826.1998.00168.x. 10.1046/j.1365-2826.1998.00168.x. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. Journal of Pharmacology and Experimental Therapeutics. 1990;253:688–697. [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Stephens LR, Logan SD. Arginine-vasopressin stimulates inositol phospholipid metabolism in rat hippocampus. Journal of Neurochemistry. 1986;46:649–651. doi: 10.1111/j.1471-4159.1986.tb13016.x. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Bayer AL, Simonson MS, Kester M. Multiple signaling pathways of V1-vascular vasopressin receptor of A7r5 cells. Endocrinology. 1991;129:2845–2856. doi: 10.1210/endo-129-6-2845. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Preston JA, Dulin N, Wilkins PL, Berti-Mattera LN, Mattera R. The human V3 pituitary vasopressin receptor: ligand binding profile and density-dependent signalling pathways. Endocrinology. 1997;138:4109–4122. doi: 10.1210/endo.138.10.5432. 10.1210/en.138.10.4109. [DOI] [PubMed] [Google Scholar]
- Yang J, Tsien RW. Enhancement of N- and L-type calcium channel currents by protein kinase C in frog sympathetic neurons. Neuron. 1993;10:127–136. doi: 10.1016/0896-6273(93)90305-b. 10.1016/0896-6273(93)90305-B. [DOI] [PubMed] [Google Scholar]


