Abstract
Whole-cell patch clamp recordings were made from substantia nigra pars reticulata (SNr) neurones in rat midbrain slices. Monosynaptic IPSCs were evoked by electrical stimulation of the cerebral peduncle in the presence of the glutamate receptor antagonists CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) and AP5 (2-amino-5-phosphonopentanoic acid).
IPSCs were predominantly outward at −70 mV (in 124/135 cells), with a reversal potential of −83 mV, a time to peak of 2.6 ms and a decay time constant of 6.5 ms. Faster inward IPSCs were also observed in thirty-five cells, with a time to peak of 1.0 ms, a decay time constant of 2.3 ms, and a reversal potential of −61 mV. Both IPSCs were sensitive to the GABAA receptor antagonists picrotoxin or bicuculline.
In cells recorded with Cs+-filled pipettes, the outward IPSC reversal potential was shifted to −76 mV, closer to the estimated Cl− equilibrium potential of −56 mV, while that of the inward IPSC was unchanged at −64 mV.
The outward IPSC was reversibly depressed by up to 100 % by dopamine in a concentration-dependent manner with an IC50 of 10.5 μm, while the inward IPSC was relatively insensitive.
Dopamine was without effect on cell holding current, or on outward IPSC reversal potential, but it increased paired-pulse IPSC facilitation, consistent with a presynaptic site of action.
The D1-like dopamine receptor agonist SKF 38393 (10 μm) depressed the outward IPSC by 43 %, while the D2-like dopamine receptor agonist quinpirole (10 μm) was without effect.
It is concluded that GABA-ergic synaptic input onto distal rather than proximal regions of SNr neurones is susceptible to presynaptic inhibition via a D1-like receptor. These inputs are probably from striato-nigral fibres, and their inhibition by dopamine is likely to influence the patterning of basal ganglia output.
The substantia nigra (SN) in the ventral midbrain occupies a pivotal position in the circuitry of the basal ganglia, which appears collectively to integrate sensorimotor information of cortical and thalamic origin within the caudate-putamen (striatum in rodents), before its re-presentation to motor cortical regions via the ventrolateral and ventromedial nuclei of the thalamus (Alexander & Crutcher, 1990; Wichmann & Delong, 1996). The GABA-containing projection neurones of the substantia nigra pars reticulata (SNr), along with those of the internal segment of the globus pallidus (GPi; or its rodent homologue, the entopeduncular nucleus), comprise the principal output relays of the basal ganglia. Prevailing models of basal ganglia organization argue that inhibition of ongoing activity in GPi/SNr is necessarily permissive for voluntary movement. This is brought about by both increased activity in the ‘direct’ striato-nigral/pallidal GABA-ergic pathway, and a net reduction in the excitatory drive derived from the so-called ‘indirect’ pathway, which relays striatal output to GPi/SNr via the external globus pallidus and the subthalamic nucleus. Basal ganglia output is disrupted in the akinetic disorder of Parkinson's disease (PD), which results from degeneration of the dopamine-containing neurones of the substantia nigra pars compacta (SNc) that project predominantly to the striatum. In animal models of PD, firing rates of GPi/SNr neurones are increased, and it is commonly thought that this results from alterations in the inputs to these regions following dopamine depletion in the striatum (Gerfen, 1992).
In addition to releasing dopamine from axon terminals in the striatum, midbrain dopamine neurones can also release dopamine locally from their cell bodies and dendrites (Geffen et al. 1976; Gauchy et al. 1987; Jaffe et al. 1998). In addition to the inhibitory D2-like dopamine ‘autoreceptors’ on dopamine neurones themselves (Lacey et al. 1987), it has long been suspected that D1-like receptors on the terminals of striato-nigral neurones (Quik et al. 1979; Yung et al. 1995), which innervate both dopaminergic and GABA-ergic neurones of the SN (Smith & Bolam, 1990), could be functionally important targets of this locally released dopamine. This possibility of an extrastriatal role for dopamine finds behavioural support in the involvement of intranigral D1-type dopamine receptor activation in promoting movement in Parkinsonian rats (Robertson & Robertson, 1989), the induction of behavioural sensitization to repeated administration of amphetamine (Stewart & Vezina, 1989), and increasing susceptibility to epileptiform seizures (reviewed by Starr, 1996). Moreover, inactivation of D1 (or D2) receptors in the SN (but not striatum) promotes an akinetic muscular rigidity in rats (Double & Crocker, 1995).
The precise way in which intranigral dopamine might modulate the GABA released from striato-nigral fibres is not clear. Studies of either basal or potassium-stimulated GABA release using in vivo microdialysis or the push-pull cannula technique, or in brain slices, have provided inconsistent findings. Thus nigral GABA release in response to directly or indirectly acting D1 receptor agonists shows variously no change (Arbilla et al. 1981; Starr, 1987), an increase (Reubi et al. 1977; Timmerman & Westerink, 1995), or both an increase and decrease (van der Heyden et al. 1980), sometimes dependent on the precise conditions used. Electrophysiological studies are likewise not in agreement. Intranigral application in vivo of directly or indirectly acting D1 receptor agonists reportedly causes excitation of SNr neurones (Ruffieux & Schultz, 1980), an effect apparently dependent on an intact striato-nigral pathway (Martin & Waszczak, 1994), or inhibition (Gauchy et al, 1987; Timmerman & Abercrombie, 1996). On the other hand, studies in brain slices have provided evidence for activation of presynaptic D1-like receptors increasing the amplitude of evoked GABA-mediated IPSCs in both dopaminergic (Cameron & Williams, 1993) and GABA-ergic SNr (Radnikow & Misgeld, 1998) neurones. Here we have used whole-cell patch clamp recording in rat midbrain slices to examine the effect of dopamine on GABA-ergic IPSCs evoked by extranigral electrical stimulation of the descending fibres innervating SNr neurones. We have identified a component of the IPSC that is strongly and selectively depressed by D1-like dopamine receptor activation, and we argue that its properties are consistent with those expected for striatal afferents. Some of this work has been presented previously in abstract form (Miyazaki & Lacey, 1997).
METHODS
Brain slices were prepared from freshly excised brains of 24–30 day old (weight, 80–130 g) male Wistar rats killed by cervical dislocation under halothane (4 % isoflurane) anaesthesia. Parasagittal slices (300 μm thick), cut at an angle of approximately 20 deg to the mid-line in order to optimize the preservation of descending input to the ventral midbrain within the internal capsule, were prepared using a tissue slicer (DTK-1000, Dosaka Co., Kyoto, Japan) from a block of ventral midbrain. Cutting was performed in a solution containing (mm): NaCl, 126; KCl, 3; NaH2PO4, 1.2; MgCl2, 5; CaCl2, 1; and glucose, 20, buffered to pH 7.4 with Na2HCO3 (26 mm) and saturated with 95 % O2-5 % CO2, with added 4 mm lactate, cooled to 4°C. Slices were then stored in lactate-containing artificial cerebrospinal fluid (ACSF) containing (mm): NaCl, 126; KCl, 2.5; NaH2PO4, 1.2; MgCl2, 1.3; CaCl2, 2.4; and glucose, 10, buffered to pH 7.4 with Na2HCO3 (26 mm) and saturated with 95 % O2-5 % CO2 at 31°C, for at least 60 min, prior to transfer to the lactate-containing ACSF at room temperature (18–25°C). For recording, slices were placed onto the glass coverslip that formed the base of the recording chamber (volume, 1 ml), held in position with a titanium electron microscopy grid and 0.5 mm diameter pieces of platinum wire, and continuously superperfused at 2–3 ml min−1 with lactate-free ACSF at 34 ± 1°C. Individual neurones of the substantia nigra pars reticulata (SNr) were visualized in situ using a differential interference contrast (Nomarski) optical system (non-inverted Zeiss Axioscop FS microscope, Oberkochen, Germany) with a × 40 water-immersion objective, combined with an infrared filter and video enhancement performed with a Hitachi monochrome CCD camera (model KP-M1E/K) in conjunction with a video contrast enhancer (CE-4, Brian Reece Scientific, Newbury, UK).
Recordings were made with borosilicate glass pipettes (3–5 MΩ) with heat-polished tips containing (mm): potassium gluconate, 125; NaCl, 10; CaCl2, 1.0; MgCl2, 2.0; BAPTA, 10; Hepes, 10; GTP, 0.3; and MgATP, 2.0, adjusted to pH 7.3 with KOH. Caesium gluconate was substituted for potassium gluconate in some experiments, with pH adjustment performed with CsOH. Recording pipettes were advanced with their contents under positive pressure towards individual cells in the slice and, on contact, tight seals were made by applying negative pressure. The membrane patch was then ruptured by suction, and membrane current and potential monitored using an Axopatch 200B patch clamp amplifier (Axon Instruments). Whole-cell access resistances measured in voltage clamp were in the range 5–15 MΩ prior to electronic compensation (80 % was routinely used). After initial determination, the access resistance and cell input resistance were continuously monitored in voltage clamp by measuring the sizes of the capacitance transient and steady-state current in response to a 5 mV hyperpolarizing step. Experiments were abandoned if the capacitance transients changed by more than 10 %. Holding potential was −70 mV, unless otherwise indicated. All values of membrane potentials given were corrected for a liquid junction potential value of −10 mV, close to the measured value of −8 ± 0.4 mV (n = 10).
Synaptic events were evoked by focal stimulation of the slice using a twisted Nichrome wire bipolar stimulating electrode positioned by visual inspection in the cerebral peduncle, outside the grey matter of the substantia nigra, approximately 300–1000 μm rostro-ventral to the recording site. In order to block fast glutamatergic excitatory postsynaptic currents, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μm) and dl-2-amino-5-phosphonopentanoic acid (dl-AP5; 100 μm) were routinely added to the perfusate. Clusters of three to five consecutive single shock stimulations (0.1 ms, 0.04–3 mA) were delivered at 0.067 Hz at 2 min intervals using a constant current stimulation unit (AMPI Isoflex, Jerusalem, Israel). Voltage steps and stimulus protocols were generated using pCLAMP version 6.0 software (Axon Instruments). The resulting membrane currents, and also those resulting from synaptic activation, were filtered at 5 kHz and then stored to computer for subsequent analysis and display on a chart recorder (Gould Easygraph, Hainault, UK). Numerical data derived from experimental manipulations on synaptic currents were quantified from the mean of the three to five consecutive single events in each cluster. Fitting of synaptic current waveforms to exponential functions was performed with Clampfit (pCLAMP 6.0). All numerical data are expressed as means ± standard error of the mean (s.e.m.); n refers to the number of neurones tested. Statistical significance was assessed by Student's t test and P < 0.05 was accepted as significant, unless otherwise stated.
Drugs were applied to the superfusate by exchanging the ACSF for one differing only by the addition of a known concentration of drug, with the exchange beginning after a dead time of around 20 s. Drugs used were: CNQX, dl-AP5, bicuculline methiodide and picrotoxin (all from Tocris Cookson, Bristol, UK); dopamine HCl, strychnine HCl and tetrodotoxin (all from Sigma); (±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol HCl (SKF 38393) and (−)-quinpirole HCl (both from Research Biochemicals International, distributed by Sigma).
RESULTS
Characteristics of SNr neurones
A total of 167 non-dopaminergic SNr neurones (recorded using potassium gluconate-containing pipettes) were used for this study. They were readily identified as such on the basis of their spontaneous action potential firing rate and pattern (Fig. 1Aa), their relatively short duration action potential after-hyperpolarization (Fig. 1Ab), and the absence of the hyperpolarization-activated time-dependent slow inward current (Ih; Fig. 1Ba), as previously described (see Stanford & Lacey, 1996, and references therein). SNr cells with such characteristics have been shown to be GABA containing (Richards et al. 1997). In quiescent cells, and in those recorded with caesium gluconate-filled pipettes (which markedly altered spike firing pattern and action potential waveform), the absence of Ih was used as the principal indicator of non-dopaminergic SNr neurones. Five minutes after gaining whole-cell access, 94 out of 167 neurones (58 %) exhibited spontaneous spike firing at rest at a mean rate of 18 ± 1.2 Hz (range, 3–52 Hz; Fig. 1Aa), with mean action potential duration (taken as the time between the point of initiation of the maximum rate of depolarization and the corresponding equipotential point during spike repolarization) of 0.82 ± 0.02 ms (range, 0.4–1.68 ms; n = 94; Fig. 1Ab). A smaller number (n = 39) of SNr neurones showed spontaneous burst firing activity at rest after 5 min or more of whole-cell recording. The remainder of the non-dopaminergic neurones (n = 34) showed no spontaneous firing, and had resting membrane potentials of −73 ± 1.2 mV. About 15 min after gaining whole-cell access, transitions were occasionally observed from a resting pattern of tonic firing to bursting activity (13 cells), from tonic activity to quiescence (n = 5), or from tonic activity to quiescence after a phase of bursting activity (n = 3). Such transitions suggest that rather than representing subtypes of SNr neurones, these different firing patterns probably result from changes of the cytoplasmic constituents as a result of the intracellular dialysis.
Figure 1. Electrophysiological characteristics of non-dopaminergic substantia nigra pars reticulata neurones.
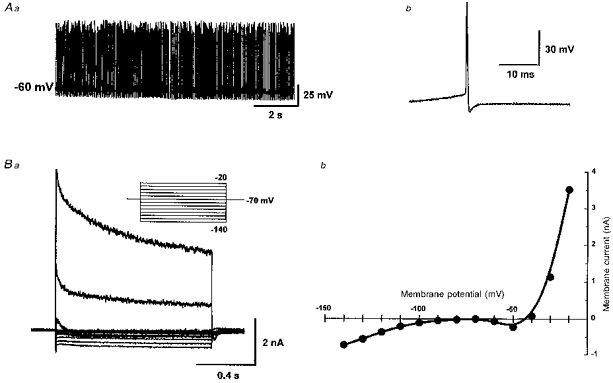
Aa, spontaneous, tonic action potential firing at rest at a rate of 21 Hz (full amplitude of spikes not faithfully represented by chart recording). Ab, a single spontaneous action potential, duration 0.62 ms. Ba, superimposed records of membrane current obtained under voltage clamp during a series of voltage steps (1 s duration) from a holding potential of −70 mV represented in the inset. Bb, steady-state I-V curve plotted from currents in Ba. All records from the same cell.
Under voltage clamp, steady-state input resistance measured with a 1 s hyperpolarizing step from −70 to −80 mV (Fig. 1Ba) was 0.39 ± 0.03 GΩ (range, 0.11–2 GΩ; n = 167), comparable to those reported previously (Stanford & Lacey, 1996). Whole-cell capacitance was 24.4 ± 0.45 pF (range, 13–43 pF; n = 167). Steady-state current-voltage (I-V) relationships derived from a family of such voltage steps indicated a distinct region of negative slope conductance at around −50 mV in 128 out of 167 neurones (Fig. 1Bb).
Monosynaptic outward IPSCs evoked in SNr neurones
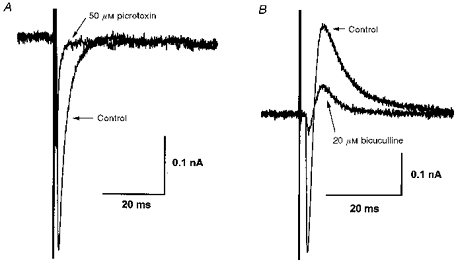
IPSCs evoked by electrical stimulation of the cerebral peduncle were examined at a holding potential of −70 mV. By far the most common event - seen in 102 out of 137 cells - was an outward IPSC (oIPSC) with an amplitude that increased with stimulus strength, unaccompanied by a change in the latency or time to peak (n = 3; Fig. 2A). No failure was observed even at 100 Hz stimulation (n = 3; Fig. 2B). The oIPSC was abolished by tetrodotoxin (1 μm, n = 3), or by superfusion with a nominally Ca2+-free ACSF, in which the added CaCl2 had been substituted with MgCl2 (n = 3). The oIPSC was abolished or greatly reduced by the GABAA receptor antagonists picrotoxin (50 μm, n = 5) or bicuculline (20 μm; n = 5; Fig. 2C). In three of these cells, a portion of the oIPSC was resistant to bicuculline and picrotoxin, and in these cases a complete inhibition was achieved with the additional application of strychnine (10 μm), the antagonist of taurine and glycine receptors (n = 3; Fig. 2D). These results indicated that the oIPSC was a monosynaptic event, mediated largely (but not solely) by GABA released in a Ca2+-dependent manner, acting on GABAA receptors. The reversal potential of this oIPSC was −83 ± 2.0 mV (n = 21; see Figs 5A, 7B and 10). This value is appreciably different from the estimated equilibrium potential for Cl− (ECl; −56.1 mV), which is usually the charge carrier for GABAA currents.
Figure 2. Outward IPSCs are monosynaptic, and predominantly GABAA receptor mediated.
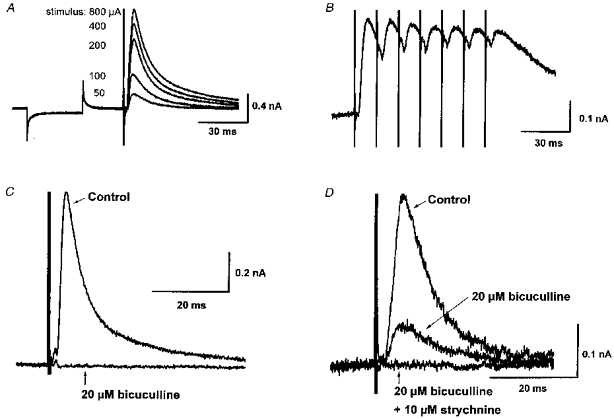
A, superimposed records of membrane current showing response to a −5 mV step, followed by outward synaptic currents evoked by single-shock electrical stimulation (for 0.1 ms) of the cerebral peduncle at the currents indicated. oIPSC amplitude is graded proportional to stimulus strength. B, oIPSCs evoked by 7 stimuli (0.1 ms, 50 μA) at 100 Hz show no fatigue or failure. C, oIPSC can be completely abolished by the GABAA receptor antagonist bicuculline (20 μM). D, in some cells, a bicuculline-resistant, strychnine-sensitive portion of the oIPSC was observed. Means of 5 successive events at a holding potential of −70 mV in all records. dl-AP5 (100 μM) and CNQX (20 μM) were present in all cases.
Figure 5. The oIPSC reverses at a less negative potential in cells recorded with caesium gluconate-containing pipettes, suggesting that it may have a distal location.
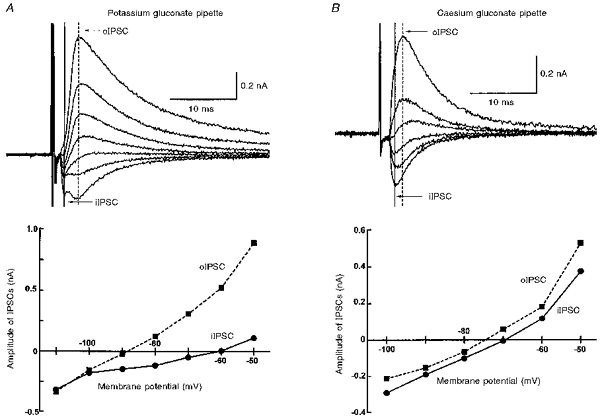
A, upper panel, superimposed records of membrane current from a single cell recorded with a potassium gluconate-containing pipette held at potentials in the range −50 to −110 mV showing a biphasic IPSC. Peak amplitudes of iIPSCs (taken from the vertical continuous line) and oIPSCs (vertical dashed line) are plotted against membrane potential in the lower panel. Reversal potential of the iIPSC is −60 mV, and the oIPSC reverses at −88 mV. B, records from another cell recorded with a caesium gluconate-containing pipette, showing IPSCs recorded in the range −50 to −100 mV. The iIPSC reverses at −69 mV, and the oIPSC at −74 mV.
Figure 7. oIPSC reversal potential is unaffected when depressed by dopamine, in accordance with a presynaptic site of dopamine action.
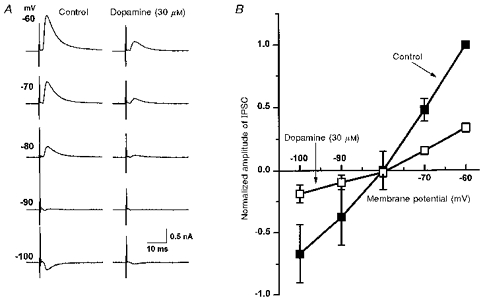
A, IPSCs from a single cell recorded at membrane potentials in the range −60 to −100 mV under control conditions (left), and in dopamine (30 μM; right), which depresses the IPSCs in a voltage-independent manner. B, plot of pooled data from 3 experiments such as that in A, with IPSC amplitude in each experiment normalized to that seen under control conditions at −60 mV. The IPSC amplitude in dopamine (□) was reduced in a voltage-independent manner relative to control (▪), but with its reversal potential (of −78 mV) essentially unchanged from that in control (−80 mV).
Figure 10. While the oIPSC can be completely depressed by dopamine, the iIPSC is relatively unaffected.
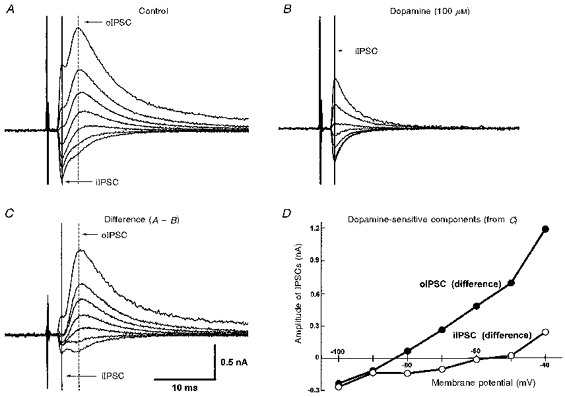
A, superimposed records of a biphasic IPSC at potentials in the range −40 to −100 mV. The peak of the oIPSC is indicated by the vertical dashed line, and that of the iIPSC by the vertical continuous line. B, in the presence of dopamine (100 μM), the oIPSC is undetectable, while the iIPSC persists. C, digital subtraction of currents in B from those in A, showing that the dopamine-sensitive component of the IPSC is principally the late oIPSC. D, dopamine-sensitive portions of both the oIPSC (•) and the iIPSC (○), derived from C, plotted against membrane potential. The effect of dopamine is predominantly upon the oIPSC, whereas the iIPSC is only minimally affected.
Inward IPSCs evoked in SNr neurones - a more proximal input
In some neurones, a fast inward IPSC (iIPSC) was observed at the holding potential of −70 mV, either in isolation (n = 13; Fig. 3A), or preceding an oIPSC as a component of a biphasic synaptic event (n = 22; Fig. 3B). The iIPSC was also sensitive to bicuculline (20 μm; n = 3) and picrotoxin (50 μm; n = 3), but, like the oIPSC, was also incompletely blocked by the GABAA antagonists in four out of seven cells (Fig. 3).
Figure 3. Inward IPSCs are predominantly GABAA receptor mediated.
A and B, iIPSCs in 2 different cells are depressed in amplitude by > 85 % by GABAA receptor antagonists picrotoxin (50 μM) or bicuculline (20 μM). In B, the outward component of the IPSC is also depressed by bicuculline. Means of 5 successive events at a holding potential of −70 mV.
The IPSC time to peak and the decay time constant were measured in several different cells in which only a monophasic IPSC (either pure inward or pure outward) was observed (at −70 mV). The time-to-peak values were 1.0 ± 0.12 ms (n = 8) and 2.6 ± 0.16 ms (n = 13) for the iIPSC and oIPSC, respectively (Fig. 4), which differ significantly from each other (P < 0.01, unpaired t test). The decay time courses of both types of IPSC were well fitted to a single exponential function with time constants of 2.3 ± 0.38 ms (n = 8) and 6.5 ± 0.32 ms (n = 13) for the iIPSC and oIPSC, respectively (Fig. 4), also significantly different from each other (P < 0.05, unpaired t test). These differences suggest that the iIPSC may be the consequence of activating a more proximally located set of presynaptic terminals than those giving rise to the oIPSC.
Figure 4. The iIPSC shows a faster time to peak and decay time constant than the oIPSC, suggesting that it may have a more proximal origin.
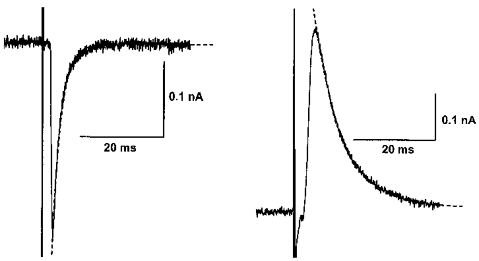
IPSCs from 2 different cells where electrical stimulation evoked either a pure iIPSC (left) or a pure oIPSC (right) at the holding potential of −70 mV. Time to peak of the iIPSC is 0.8 ms, while that of the oIPSC is 2.7 ms. Decay time constants, estimated by fitting to a single exponential curve (dashed lines), were 2.0 ms (iIPSC) and 6.9 ms (oIPSC), respectively.
Reversal potentials of inward and outward IPSCs are differentially sensitive to changes in electrotonic length
The reversal potentials for the iIPSC and oIPSC were measured in eight cells displaying a biphasic IPSC, to permit a direct comparison. The iIPSC reversed polarity at −61 ± 3.5 mV (n = 8; data include 2 extrapolated reversal potential estimates), while in the same eight neurones, the oIPSC reversed at −84 ± 4.0 mV (Fig. 5A). The reversal potential of the iIPSC was at a significantly (P < 0.01; paired t test) more depolarized level than that of the oIPSC, and considerably closer to the estimated value of ECl (−56.1 mV).
In a separate series of experiments conducted on cells recorded with caesium gluconate-containing pipettes, the oIPSCs reversed polarity at −76 ± 1.7 mV (n = 10, including 6 with biphasic IPSC; Fig. 5B), which was significantly more depolarized than that recorded using potassium gluconate-containing pipettes (P < 0.05; unpaired t test), although still different from the estimated ECl. Under these conditions the iIPSC reversed its polarity at −64 ± 3.9 mV (n = 6, all with biphasic IPSC), which was not significantly different from the value obtained when potassium gluconate-containing pipettes were used (Fig. 5B). In the four cells recorded with caesium gluconate-containing pipettes that exhibited pure oIPSCs, the mean oIPSC time to peak and decay time constant were 2.1 ± 0.3 ms and 5.4 ± 0.81 ms, respectively, not significantly different (P > 0.05) from those measured using potassium gluconate-containing pipettes.
Cells dialysed with Cs+ had input resistances of 0.87 ± 0.22 GΩ (n = 10), significantly higher than under control conditions (P < 0.001, unpaired t test). The increased resistance (due to potassium channel blockade) would be expected to reduce the electronic length of the recorded cell, and improve the voltage control (‘space clamp’) of distal portions of the dendritic tree. The shift under these conditions of the oIPSC reversal potential towards that of the iIPSC, and ECl, is consistent with it being due to a conductance increase predominantly to Cl−, like the iIPSC, but occurring at a more distal location on the neuraxis where voltage control is poorer.
Dopamine depresses the outward IPSC
Dopamine (1–100 μm), applied in the superfusion medium, decreased the amplitude of the oIPSC, without effect on the holding current or membrane conductance at −70 mV. This effect of dopamine was maximal within 10 min of commencing application, reversed within 15 min of washout (Fig. 6A), and was reproduced on repeated application. The inhibition of the oIPSC by dopamine was concentration dependent, with an IC50 value of around 10.5 μm and a maximal effect - obtained with around 100 μm dopamine - of 100 % oIPSC depression (Fig. 6B). Dopamine caused a clear depression of the oIPSC in all neurones tested (n = 106) when applied in concentrations of 10–100 μm. Dopamine (100 μm) also depressed the oIPSC in all five cells tested that were recorded with caesium gluconate-containing pipettes.
Figure 6. Dopamine depresses the oIPSC in a concentration-dependent manner, and by up to 100 %.
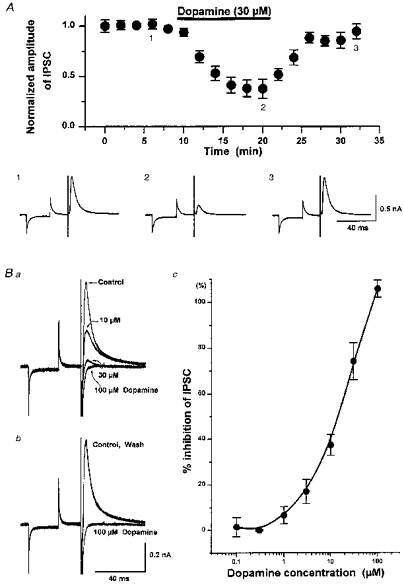
A, data pooled from 6 cells showing (upper panel) the time course of depression of the oIPSC by dopamine (30 μM; filled bar), and recovery on washout. Lower panel, sample records (mean of 5 successive events) from 1 of these cells, taken at the time points indicated (1, 2 and 3). Ba and b, superimposed records of oIPSCs from a single cell showing concentration-dependent depression by dopamine (10, 30 and 100 μM; Ba) and recovery from complete oIPSC depression by dopamine (100 μM; Bb) on washout. Bc, pooled concentration-effect data showing the extent of oIPSC depression by dopamine (0.1–100 μM). The maximal effect (100 % depression) is obtained with 100 μM dopamine, and the half-maximal effect (IC50) by 10.5 μM dopamine. Each point represents the mean from 5 or 6 different cells. IPSCs recorded at −70 mV in all cases.
Depression of the outward IPSC by dopamine is due to a presynaptic action
The inhibition by dopamine of the oIPSC was independent of membrane potential in the range −60 to −100 mV. Thus while the oIPSC conductance was reduced by dopamine (30 μm), its reversal potential was unchanged (−81 ± 4.0 mV in dopamine, compared with −82 ± 4.8 mV in control, in the same 3 neurones; Fig. 7). Application of two consecutive stimuli at an interval of 30 ms caused a facilitation of the second oIPSC amplitude by 151 ± 10 % (n = 6; Fig. 8). In the presence of dopamine (30 μm), this paired-pulse ratio was increased to 466 ± 178 % (in the same 6 cells; Fig. 8). These two findings, together with the absence of any effect of dopamine on membrane holding current, indicate that dopamine was acting presynaptically to inhibit the evoked release of transmitter from the nerve terminals mediating the oIPSC.
Figure 8. Paired-pulse IPSC facilitation is enhanced by dopamine, consistent with a presynaptic site of action.
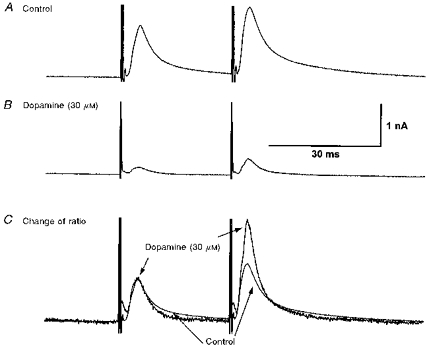
A, a pair of oIPSCs, evoked at an interval of 30 ms with the same stimulus at −70 mV. The amplitude of the second is facilitated relative to the first. B, in the presence of dopamine (30 μM), both oIPSCs are depressed, but the second less so than the first. C, the records in B (dopamine, 30 μM) are scaled up in amplitude so that the first IPSC matches the first IPSC in A (Control; superimposed records). The facilitation of the second IPSC is enhanced by dopamine, relative to control.
Dopamine acts through a D1-like receptor
The D1-type dopamine receptor agonist SKF 38393 (10 μm) reversibly depressed the oIPSC amplitude by 43.3 ± 5.0 % (n = 6), compared with a depression of 55.3 ± 8.1 % by dopamine (30 μm) in the same six neurones (Fig. 9). However, in a different set of experiments, the selective D2-type dopamine receptor agonist quinpirole (10 μm) reduced oIPSC amplitude by only 6.8 ± 1.4 %, as opposed to 57.8 ± 3.0 % by dopamine (30 μm; n = 5).
Figure 9. oIPSC depression by dopamine is mimicked by the D1-like receptor agonist SKF 38393.
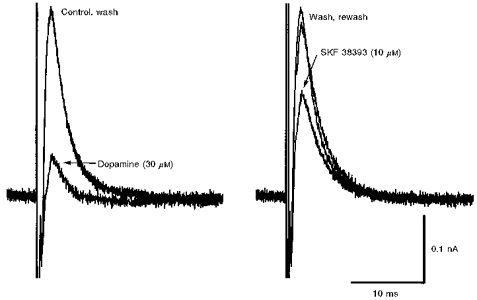
Superimposed records of oIPSC from a single cell showing depression by dopamine (30 μM) relative to control, and recovery on wash (left panel), and then subsequent depression by SKF 38393 (10 μM) and recovery on rewash (right panel).
The inward IPSC is relatively insensitive to dopamine
In cells that displayed both oIPSC and iIPSC, a direct comparison of the effect of dopamine on both components of the IPSC was made. In nine cells tested with dopamine (30 μm), the oIPSC amplitude was reduced by 60.7 ± 6.5 %, while the iIPSC was depressed by only 1.7 ± 12.3 %. Even in 100 μm dopamine, which completely abolished the oIPSCs, the iIPSC was depressed by only 15.0 ± 12.5 % (n = 3) of control amplitude (Fig. 10).
DISCUSSION
Presynaptic inhibition of oIPSC by dopamine
The predominant component of the IPSC was the oIPSC (as previously described by Stanford & Lacey, 1996; Shen & Johnson, 1997), and this was robustly and consistently depressed by dopamine. The enhancement of paired-pulse facilitation, together with the lack of any postsynaptic action of dopamine on holding current or membrane conductance, or on IPSC reversal potential, all point to a presynaptic site for this action, and the conclusion that dopamine was depressing the release of GABA evoked by electrical stimulation. The ability of the agonist of D1-like dopamine receptors SKF 38393, but not the D2-like agonist quinpirole (Seeman & Van Tol, 1994), to mimic this effect, points towards the involvement of D1-like receptors. Indeed, apart from the D2-like receptors, which are exclusively located on dopaminergic neurones, the only dopamine receptors in the SNr are of the D1 type, and these are generally considered to be located exclusively on the terminals of striato-nigral fibres (Quik et al. 1979; Yung et al. 1995). This is not a unique mechanism, as there is electrophysiological evidence for presynaptic inhibition by D1-like receptors of GABA release in basal forebrain (Momiyama & Sim, 1996), entorhinal cortex (Pralong & Jones, 1993) and nucleus accumbens (Nicola & Malenka, 1997).
The dual-component IPSC
The proposition that the oIPSC arose from synaptic contacts at more distal points on the dendritic tree than the iIPSC is supported principally by the shift in its reversal potential towards the Cl− equilibrium potential in recordings made with Cs+-filled pipettes, relative to that of the iIPSC. Under these conditions voltage clamp of the dendrites would be improved on account of the (2.2-fold) increase in membrane resistance. However, complete elimination of the ‘space-clamp’ problem would still be unlikely with somatic Cs+ loading, as these cells can have dendritic fields extending over 1 mm in the rostrocaudal direction (Grofova et al. 1982). The different reversal potentials of the two IPSCs (which still persisted with Cs+-filled pipettes, albeit to a lesser degree) might additionally be accounted for by (1) regional differences in intracellular Cl− concentration, possibly as a result of incomplete equilibration with the pipette Cl− concentration in distal dendrites, and/or (2) the two IPSCs resulting from operation of two fundamentally different types of GABAA conductances, with one having a significantly different permeability to ions in addition to Cl−, such as HCO3− (Staley et al. 1995). The present study does not permit definitive elimination of any of these possibilities. The slower time to peak and decay time of the oIPSC relative to the iIPSC in the present study also suggests relatively greater electrotonic decay for the oIPSC, but as these variables were not significantly changed by intracellular Cs+, this alone does not provide support for the oIPSC being more distal in origin. Indeed, the kinetic differences between the two IPSC types might reflect different GABAA receptor subtypes, as well as different locations. Similar conclusions have been reached in a study of cortical pyramidal neurones, where two IPSC components with clearly different loci and kinetics have been described (Kapur et al. 1997), although in these studies the decay time constants (at 29°C) of the ‘fast’ and ‘slow’ IPSCs were around 10 and 40 ms, respectively - appreciably slower than those observed here. The oIPSC and iIPSC in the present study sometimes exhibited a bicuculline-insensitive component which (in the case of the oIPSC) was demonstrably strychnine sensitive. This opens the possibility that glycine, or taurine, as well as GABA, might be released by the stimulation protocol employed. While this was not further characterized, the co-activation of glycine/taurine receptors with GABAA receptors is unlikely to have greatly influenced the reversal potential data, as Cl− would also be expected to be the principal charge carrier. The precise anatomical origin of this strychnine-sensitive IPSC is unclear, although taurine has been proposed to be released from striato-nigral terminals (Della Corte et al. 1990).
The prospect of there being two different principal loci for GABA-releasing synaptic contacts on SNr neurones has been raised independently by results from double labelling anterograde tracer studies. SNr neurones receive GABA-containing synaptic contacts from neurones of the globus pallidus (external segment; GPe) on their cell bodies and proximal dendrites, while GABA-containing striatal afferents terminate almost exclusively on distal dendritic locations (Smith & Bolam, 1991). The sensitivity of solely the oIPSC to dopamine strengthens the proposition that it does indeed represent a predominantly distal input, and that it is of striatal origin. The small depression of the iIPSC at the highest concentrations of dopamine tested may reflect a minor contribution of more proximally distributed striato-nigral input to the iIPSC.
Dopamine promotes increased GABA release in substantia nigra
It is difficult to see how the present results might be interpreted as a consequence of dopamine elevating GABA release in SN, in line with the conclusions of several other studies (see Introduction). In many of these studies, the precise source of the GABA being measured is unclear. Thus intranigral GABA may originate not only from striato-nigral terminals, but also from at least two other distinct sources - terminals arising from the GPe (Smith & Bolam, 1991) and from the axon collaterals of the spontaneously active SNr neurones themselves (Deniau et al. 1982; Rick & Lacey, 1994; Stanford & Lacey, 1996). These are unlikely to be completely independent. In particular, as SNr neurones have extensively ramifying axon collaterals with intranigral terminations (Grofova et al. 1982), increased GABA release may result from (an indirect) disinhibition of SNr neurones, rather than increased release from extrinsic afferents to the SN. Indeed, as these collaterals may promote lateral inhibition of SNr neurones (Deniau et al. 1982; Rick & Lacey, 1994), this might account for the inhibition of SNr neurones seen in vivo in response to dopamine released by local amphetamine administration (Gauchy et al. 1987; Timmerman & Abercrombie, 1996). Thus the extensive recurrent inhibitory network in SNr may be highly influential, and liberate significantly more GABA when activated (by disinhibition, for example) than the extrinsic GABA-ergic striato-nigral afferent system which, as we report here, is itself inhibited by dopamine. A similar argument has been proposed to reconcile the inhibitory action on SNr neurones of 5-HT in vivo with the (direct) excitation observed in vitro (Stanford & Lacey, 1996).
Nonetheless, stronger support for presynaptic facilitation of GABA release comes from two other electrophysiological studies in brain slices. The first is the report that the evoked GABAB (but not GABAA) receptor-mediated IPSP in midbrain dopamine neurones can be potentiated by D1-like receptor activation (Cameron & Williams, 1993). This GABAB IPSP, which is likely to emanate from striato-nigral terminals, can be evoked in a similar manner to the present study by stimulating the internal capsule or cerebral peduncles in sagittal rodent brain slices (Häusser & Yung, 1994). As there is yet no report of a GABAB synaptic event in SNr neurones, this may prove to be a distinctive feature of striato-nigral input to dopamine neurones alone. In another recent study, Radnikow & Misgeld (1998) reported potentiation by D1 receptor activation of GABAA IPSCs evoked by a minimal stimulation protocol in SNr neurones. In their study electrical stimulation was performed within the SNr, rather than in the cerebral peduncle, and in coronal slices, not sagittal. These methodological differences might increase the contribution to the IPSC from proximal inputs, relative to those from extrinsic inputs onto distal dendrites. Although there was no suggestion of a dual-component IPSC in the study of Radnikow & Misgeld (1998), direct comparisons of IPSC characteristics with the present study are confounded by other different experimental conditions, principally the relatively depolarized Cl− equilibrium potential (due to Cl− loading of the cells), loading of cells with Cs+, and the lower operating temperature. It is conceivable that under those conditions the evoked IPSC might have been a compound event comprising both the iIPSC and the oIPSC (in the terms of the present study), and that inhibition of the oIPSC component by dopamine could have been obscured by a resultant increase in iIPSC amplitude attributable to disinhibition by either relief of a dendritic shunt, or depolarization, reflecting increased lateral inhibition. However, the increase in spontaneous tetrodotoxin-insensitive ‘mini’ IPSC frequency seen with dopamine (Radnikow & Misgeld, 1998) cannot easily be accounted for in this manner. Clearly further study is required to resolve this apparent discrepancy.
Physiological significance
The selective depression by dopamine of an IPSC confined to the distal dendrites of SNr neurones is likely to be an important physiological substrate for dopamine release within the SN. It is a candidate cellular mechanism for many of the behavioural effects of intranigral dopamine and indirectly acting dopamine receptor agonists, particularly those involving D1-like receptors. A selective inhibition of GABA-ergic, striato-nigral input onto SNr neuronal distal dendrites would increase the relative contribution of other synaptic influences that impinge on dendrites of SNr neurones. These would include the glutamatergic inputs from the subthalamic nucleus, which synapse largely upon non-somatic regions (Bevan et al. 1994). Increased axon collateral activity resulting from this disinhibition of SNr neurones could reduce activity within certain ‘channels’ of basal ganglia information throughput via lateral inhibition (Deniau et al. 1982), while activity in other ‘channels’, perhaps driven in part by subthalamic input, would be correspondingly increased. This suggests a means whereby dopamine could reinforce parallel processing within discrete ‘channels’ of the cortico-striato-thalamo-cortical pathway (Alexander & Crutcher, 1990) by an action on SNr neurones, which are themselves a potentially critical nodal point for information convergence in the basal ganglia circuitry (Smith & Bolam, 1990; Smith & Bolam, 1991; Bevan et al. 1994). The breakdown of such parallel processing is thought to underlie several movement disorders of basal ganglia origin (Wichmann & Delong, 1996), some of which are attributable to dopamine system malfunction. In this respect the present results point to synaptic integration on the dendritic tree of SNr neurones as an important locus for the action of dopamine.
Acknowledgments
We are grateful for support from The Wellcome Trust (grant number 046056/Z/95/Z) and to Dr Ian M. Stanford for his comments on the manuscript.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in Neurosciences. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. 10.1016/0166-2236(90)90107-L. [DOI] [PubMed] [Google Scholar]
- Arbilla S, Kamal LA, Langer SZ. Inhibition by apomorphine of the potassium-evoked release of [H3]-gamma-aminobutyric acid from the rat substantia nigra in vitro. British Journal of Pharmacology. 1981;74:389–397. doi: 10.1111/j.1476-5381.1981.tb09983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Bolam JP, Crossman AR. Convergent synaptic input from the neostriatum and the subthalamus onto identified nigrothalamic neurons in the rat. European Journal of Neuroscience. 1994;6:320–334. doi: 10.1111/j.1460-9568.1994.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- Della Corte L, Bolam JP, Clarke DJ, Parry DM, Smith AD. Sites of [3H]taurine uptake in the rat substantia nigra in relation to the release of taurine from the striatonigral pathway. European Journal of Neuroscience. 1990;2:50–61. doi: 10.1111/j.1460-9568.1990.tb00380.x. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Kitai ST, Donoghue JP, Grofova I. Neuronal interactions in the substantia nigra pars reticulata through axon collaterals of the projection neurons. Experimental Brain Research. 1982;47:105–113. doi: 10.1007/BF00235891. [DOI] [PubMed] [Google Scholar]
- Double KL, Crocker AD. Dopamine receptors in the substantia nigra are involved in the regulation of muscle tone. Proceedings of the National Academy of Sciences of the USA. 1995;92:1669–1673. doi: 10.1073/pnas.92.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchy C, Kemel ML, Desban M, Romo R, Glowinski J, Besson MJ. The role of dopamine released from distal and proximal dendrites of nigrostriatal dopaminergic neurons in the control of GABA transmission in the thalamic nucleus ventralis medialis in the cat. Neuroscience. 1987;22:935–946. doi: 10.1016/0306-4522(87)92971-x. 10.1016/0306-4522(87)92971-X. [DOI] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annual Review of Neuroscience. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Grofova I, Deniau JM, Kitai ST. Morphology of the substantia nigra pars reticulata projection neurons intracellularly labeled with HRP. Journal of Comparative Neurology. 1982;208:352–368. doi: 10.1002/cne.902080406. [DOI] [PubMed] [Google Scholar]
- Häusser MA, Yung WH. Inhibitory synaptic potentials in guinea-pig substantia nigra dopamine neurones in vitro. The Journal of Physiology. 1994;479:401–422. doi: 10.1113/jphysiol.1994.sp020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe EH, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. Journal of Neuroscience. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A, Pearce RA, Lytton WW, Haberly LB. GABAA-mediated IPSCs in piriform cortex have fast and slow components with different properties and locations on pyramidal cells. Journal of Neurophysiology. 1997;78:2531–2545. doi: 10.1152/jn.1997.78.5.2531. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. The Journal of Physiology. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LP, Waszczak BL. D1 agonist-induced excitation of substantia nigra pars reticulata neurons: Mediation by D1 receptors on striatonigral terminals via a pertussis toxin-sensitive coupling pathway. Journal of Neuroscience. 1994;14:4494–4506. doi: 10.1523/JNEUROSCI.14-07-04494.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Lacey MG. Presynaptic inhibition of transmitter release in rat substantia nigra pars reticulata by dopamine. Society for Neuroscience Abstracts. 1997;23:358. [Google Scholar]
- Momiyama T, Sim JA. Modulation of inhibitory transmission by dopamine in rat basal forebrain nuclei: Activation of presynaptic D1-like dopaminergic receptors. Journal of Neuroscience. 1996;16:7505–7512. doi: 10.1523/JNEUROSCI.16-23-07505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. Journal of Neuroscience. 1997;17:5697–5710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong E, Jones RSG. Interactions of dopamine with glutamate-mediated and GABA-mediated synaptic transmission in the rat entorhinal cortex in vitro. European Journal of Neuroscience. 1993;5:760–767. doi: 10.1111/j.1460-9568.1993.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Quik M, Emson PC, Joyce E. Dissociation between the presynaptic dopamine-sensitive adenylate cyclase and [3H]spiperone binding sites in rat substantia nigra. Brain Research. 1979;167:355–365. doi: 10.1016/0006-8993(79)90829-1. 10.1016/0006-8993(79)90829-1. [DOI] [PubMed] [Google Scholar]
- Radnikow G, Misgeld U. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. Journal of Neuroscience. 1998;18:2009–2016. doi: 10.1523/JNEUROSCI.18-06-02009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi JC, Iversen LL, Jessell TM. Dopamine selectively increases 3H-GABA release from slices of rat substantia nigra in vitro. Nature. 1977;268:652–654. doi: 10.1038/268652a0. [DOI] [PubMed] [Google Scholar]
- Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. 10.1016/S0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Rick CE, Lacey MG. Rat substantia nigra pars reticulata neurones are tonically inhibited via GABAA, but not GABAB, receptors in vitro. Brain Research. 1994;659:133–137. doi: 10.1016/0006-8993(94)90872-9. 10.1016/0006-8993(94)90872-9. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Robertson HA. Evidence that L-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. Journal of Neuroscience. 1989;9:3326–3331. doi: 10.1523/JNEUROSCI.09-09-03326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffieux A, Schultz W. Dopaminergic activation of reticulata neurones in the substantia nigra. Nature. 1980;285:240–241. doi: 10.1038/285240a0. [DOI] [PubMed] [Google Scholar]
- Seeman P, Van Tol HHM. Dopamine receptor pharmacology. Trends in Pharmacological Sciences. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Presynaptic GABAB and adenosine A1 receptors regulate synaptic transmission to rat substantia nigra reticulata neurones. The Journal of Physiology. 1997;505:153–163. doi: 10.1111/j.1469-7793.1997.153bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends in Neurosciences. 1990;13:259–266. doi: 10.1016/0166-2236(90)90106-k. 10.1016/0166-2236(90)90106-K. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. Convergence of synaptic inputs from the striatum and the globus pallidus onto identified nigrocollicular cells in the rat: a double anterograde labelling study. Neuroscience. 1991;44:45–73. doi: 10.1016/0306-4522(91)90250-r. 10.1016/0306-4522(91)90250-R. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra reticulata neurons in vitro. Journal of Neuroscience. 1996;16:7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr MS. Opposing roles of dopamine D1 and D2 receptors in nigral gamma-[3H]aminobutyric acid release? Journal of Neurochemistry. 1987;49:1042–1049. doi: 10.1111/j.1471-4159.1987.tb09992.x. [DOI] [PubMed] [Google Scholar]
- Starr MS. The role of dopamine in epilepsy. Synapse. 1996;22:159–194. doi: 10.1002/(SICI)1098-2396(199602)22:2<159::AID-SYN8>3.0.CO;2-C. 10.1002/(SICI)1098-2396(199602)22:2<159::AID-SYN8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Stewart J, Vezina P. Microinjections of Sch-23390 into the ventral tegmental area and substantia nigra pars reticulata attenuate the development of sensitization to the locomotor activating effects of systemic amphetamine. Brain Research. 1989;495:401–406. doi: 10.1016/0006-8993(89)90236-9. 10.1016/0006-8993(89)90236-9. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Abercrombie ED. Amphetamine-induced release of dendritic dopamine in substantia nigra pars reticulata: D1-mediated behavioral and electrophysiological effects. Synapse. 1996;23:280–291. doi: 10.1002/(SICI)1098-2396(199608)23:4<280::AID-SYN6>3.0.CO;2-3. 10.1002/(SICI)1098-2396(199608)23:4<280::AID-SYN6>3.3.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BHC. Extracellular gamma-aminobutyric acid in the substantia nigra reticulata measured by microdialysis in awake rats: Effects of various stimulants. Neuroscience Letters. 1995;197:21–24. doi: 10.1016/0304-3940(95)11888-4. 10.1016/0304-3940(95)11888-4. [DOI] [PubMed] [Google Scholar]
- van der Heyden JA, Venema K, Korf J. Biphasic and opposite effects of dopamine and apomorphine on endogenous GABA release in the rat substantia nigra. Journal of Neurochemistry. 1980;34:119–125. doi: 10.1111/j.1471-4159.1980.tb04629.x. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Delong MR. Functional and pathophysiological models of the basal ganglia. Current Opinion in Neurobiology. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. 10.1016/S0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Yung KKL, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: Light and electron microscopy. Neuroscience. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-e. 10.1016/0306-4522(94)00536-E. [DOI] [PubMed] [Google Scholar]


