Abstract
Acetylcholine (ACh)-activated currents and their interaction with ATP-activated currents were studied in primary cultures of myenteric neurons from guinea-pig small intestine using patch clamp techniques. Peak currents caused by co-application of ACh (1 mm) and ATP (300 μm) were 78 ± 2 % of the sum of currents activated by each agonist alone (P < 0.05, n = 29). Reversal potentials measured during co-application of ACh and ATP did not differ from those measured during application of ACh or ATP alone. Addition of BAPTA (10 mm) to the pipette solution or replacement of extracellular Ca2+ with Na+ did not prevent occlusion.
Responses caused by co-application of 5-HT (300 μm), acting at 5-HT3 receptors, and ACh (3 mm) or ATP (1 mm) were additive (94 ± 3 or 96 ± 4 %, respectively, of the sum of currents activated by 5-HT and ACh or ATP alone; P > 0.05). Currents caused by GABA (1 mm), acting at GABAA receptors, and ACh (3 mm) or ATP (1 mm) were also additive (105 ± 4 or 100 ± 3 %, respectively, of the sum of currents activated by GABA and ACh or GABA and ATP applied separately; P > 0.05).
Single channel currents caused by ACh and ATP in the same outside-out patches were less than additive (85 ± 10 % of the predicted sum, P < 0.05).
P2X receptors and nicotinic cholinergic receptors (nAChRs) are linked in a mutually inhibitory manner in guinea-pig myenteric neurons. The functional interaction does not involve ligand binding sites, Ca2+-dependent mechanisms, a change in the driving force for Na+ or cytoplasmic signalling mechanisms.
ATP and ACh can act as co-transmitters or co-activators of the same receptors in the peripheral nervous system and at the somatic neuromuscular junction. ACh acts at nicotinic cholinergic receptors (nAChRs) and ATP can act at P2X purine receptors or as an allosteric modulator of nAChRs. For example, ATP may activate nAChRs or potentiate the actions of ACh at nAChRs on skeletal muscle (Akasu et al. 1981; Igusa, 1988; Lu & Smith, 1991; Fu, 1994). In addition, P2X receptors and nAChRs are co-expressed by rat PC12 cells (Nakazawa et al. 1991), guinea-pig coeliac ganglion neurons (Silinsky & Gerzanich, 1993) and rat superior cervical ganglion neurons (Nakazawa, 1994). However, currents activated by ATP and ACh in these neurons are not additive suggesting that the nAChR and P2X receptors are not independent channels. To explain these data, it was proposed that ACh and ATP may act at separate binding sites on the same ion channel as the properties of single channel currents activated by ACh or ATP were similar (Nakazawa et al. 1991; Nakazawa, 1994). However, cloning of P2X receptors from different preparations (Brake et al. 1994; Valera et al. 1994; Chen et al. 1995; Collo et al. 1996) showed that there is no primary sequence homology between nAChRs and P2X receptors. P2X receptors are more closely related in proposed structure to epithelial Na+ channels (Surprenant et al. 1995). These results make it unlikely that ACh and ATP activate a common ion channel.
Although many neurons co-express nAChRs and P2X receptors, the myenteric plexus of guinea-pig intestine is one of a few examples where these receptors have been shown to be activated by synaptically released ACh or ATP. This conclusion is based on data obtained from electrophysiological experiments (Galligan & Bertrand, 1994; Zhou & Galligan, 1996; LePard et al. 1997) and from histochemical (Olson et al. 1976) and neurochemical (White & Leslie, 1982) assays demonstrating that ATP is contained in high concentrations in some myenteric nerves, ATP can be released from enteric nerve endings and ATP acting at P2X receptors contributes to fast excitatory neurotransmission in the myenteric plexus. The purpose of the present study was to study potential functional interactions between myenteric nAChRs and P2X receptors where ACh and ATP act as co-transmitters.
METHODS
Tissue culture
Myenteric neurons were cultured using a modification of techniques described previously (Zhou & Galligan, 1996). Weekly batches of neuronal cultures were prepared using tissues from two new born guinea-pigs (< 36 h old). Animals were killed by severing the major neck blood vessels and spinal cord following deep halothane anaesthesia. Halothane anaesthesia was induced by placing the animals in a sealed plexiglass chamber which was aerated with room air bubbled through a halothane solution (100 %). These animal use procedures were approved by the All University Committee on Animal Use and Care at Michigan State University. The small intestine was removed from the animals and placed in cold (4°C) sterile-filtered Krebs solution of the following composition (mm): NaCl, 117; KCl, 4.7; CaCl2, 2.5; MgCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 25; and glucose, 11. The longitudinal muscle myenteric plexus was removed from the entire length of the small intestine, and cut into 5 mm long pieces. The dissected tissues were divided into 4 aliquots and each aliquot was placed in 1 ml of Krebs solution containing 1600 U of trypsin (Sigma Chemical Company) for 25–30 min at 37°C. After trypsin incubation the tissues were triturated 30 times and then centrifuged at 900 g for 5 min using a bench-top centrifuge. The supernatant was discarded and the pellet was resuspended in sterile Krebs solution and incubated (25–30 min, 37°C) in 1 ml of Krebs solution containing 2000 U crab hepatopancreas collagenase (Calbiochem-Novabiochem, Corp., La Jolla, CA, USA). The suspension was triturated and then centrifuged again at 900 g for 5 min. The pellet was resuspended in Eagle's minimum essential medium (MEM) containing 10 % fetal bovine serum, gentamicin (10 μg ml−1), penicillin (100 units ml−1) and streptomycin (50 μg ml−1) (all from Sigma). Cells were plated on plastic dishes coated with poly-L-lysine and maintained in an incubator at 37°C in an atmosphere of 5 % CO2 for up to 2 weeks. After 2 days in culture, 10 μm cytosine arabinoside was added to the MEM to limit smooth muscle and fibroblast proliferation and the medium was changed twice weekly thereafter.
Electrophysiological recording
Whole-cell and outside-out patch clamp recordings were obtained at room temperature using standard methods. The pipette solution contained (mm): CsCl, 160; MgCl2, 2; EGTA, 1; Hepes, 10; ATP, 1; GTP, 0.25; the pH and osmolarity were adjusted to 7.4 (using CsOH) and 320 mosmol l−1 (using CsCl), respectively. Krebs solution (see above) was used as the extracellular solution. Series resistance (80 %) and capacitative currents were electronically compensated. All recordings were made using an Axopatch 200A amplifier (Axon Instruments). Data were acquired using Axotape 2.0 and pCLAMP 6.0 software (Axon Instruments). Currents were sampled at 2 kHz and were filtered at 1 kHz (4 pole Bessel filter, Warner Instruments, New Haven CT, USA) and stored on a computer hard drive.
Whole-cell recordings were carried out using patch pipettes with tip resistances of 3–5 MΩ; seal resistances were greater than 5 GΩ. Current-voltage relationships for agonist-induced whole-cell currents were obtained using a ramp depolarization that changed the holding potential from −70 to 40, −80 to 30 or −50 to 50 mV in 100 ms. Interactions between agonist-induced responses were studied by measuring the amplitude of currents caused by a maximum agonist concentration alone and in combination with a second agonist. The difference between the predicted sum of the individual peak currents was compared with the current amplitude caused by co-application of two agonists.
Recordings from outside-out patches were obtained using low tip resistance pipettes (3–5 MΩ) when studying interactions between nAChRs and P2X receptors. In these experiments, responses in outside-out patches were quantified as total charge transfer calculated as the integral of the single channel currents occurring during agonist application. The integral of the single channel currents was calculated using the ‘integrate’ routine in Clampfit (pCLAMP 6.0, Axon Instruments). High tip resistance pipettes (15–20 MΩ) were used to study individual channels activated by ACh. The amplitude of single channel currents activated by ACh at different patch potentials was measured using on-screen cursors. The tips of pipettes used for single channel recordings were coated with Sylgard (Dow Corning).
Drugs
All drugs were purchased from Sigma Chemical Co.
When constructing steady-state concentration-response curves agonists were applied onto individual neurons by gravity flow from linear array quartz tubes (320 μm i.d. and 450 μm o.d., Polymicron Technologies, Phoenix, AZ, USA). The distance from the mouth of the tubes to the cell examined was around 200 μm and the position of the tubes containing different drug concentrations was controlled manually using a micromanipulator. In all other experiments, computer-controlled solenoid valves (General Valve Co., Fairfield, NJ, USA) were used to gate solution flow through the tubes. The pH of the ATP-containing solutions was 7.41–7.43. The pH of the ATP solutions was checked as the amplitude of ATP-induced currents caused by activation of recombinant ATP receptors is pH dependent (Wildman et al. 1997).
Statistics
All data are expressed as the mean ±s.e.m. Student's t test for paired data or analysis of variance were used to establish significant differences between control and treatment groups. Agonist concentration-response curves were fitted using the following logistic function:
where Ymin and Ymax are the minimum and maximum responses respectively, EC50 is the half-maximal effective concentration and nHis the slope. The time course of nAChR desenstization was fitted using the Simplex non-linear least-squares routine in Clampfit (pCLAMP 6). The part of the response to be fitted was identified manually begining at the peak of the inward current until the end of the ACh application period.
RESULTS
Whole-cell currents induced by ATP and ACh
ACh caused an inward current in > 98 % of myenteric neurons tested, while only 80 % of neurons tested responded to ATP (Zhou & Galligan, 1996). Figure 1A illustrates typical whole-cell currents activated by ACh (3 mm) and ATP (1 mm) in the same neuron. Neither ACh- nor ATP-induced currents required ATP or GTP in the pipette solution and both ACh- and ATP-evoked currents were associated with an increased conductance. Currents caused by ACh were due to activation of nAChRs as they were completely blocked by the nAChR antagonist hexamethonium (100 μm; Fig. 1B). The peak amplitude of the ACh-induced current was concentration dependent with a half-maximal effective concentration (EC50) of 112 ± 7 μm and the maximum response occuring at 1 mm (Fig. 1C). Whole-cell currents caused by ATP are mediated at P2X receptors as described previously (Zhou & Galligan, 1996). In the present study it was found that the EC50 for ATP was 34 ± 8 μm with the maximum current occurring at 300 μm (Fig. 1C). On average, the maxiumum responses induced by ACh (3 mm) were 1.4-fold larger than those caused by a maxiumum concentration of ATP (1 mm) (−1284 ± 115 pA for ACh versus−918 ± 108 pA for ATP at −60 mV, n = 30). At −60 mV, the 10–90 % rise time for ACh (1 mm)-induced currents was 79 ± 6 ms (n = 7). The ACh-induced current desensitized with a double exponential time course with τ1= 539 ± 62 ms and τ2= 4287 ± 574 ms (n = 10). The 10–90 % rise time for ATP-induced currents was 219 ± 31 ms and the ATP response desensitized much more slowly (τ1= 7 s) than the ACh response as reported previously (Zhou & Galligan, 1996).
Figure 1. ACh- and ATP-induced inward currents in myenteric neurons.
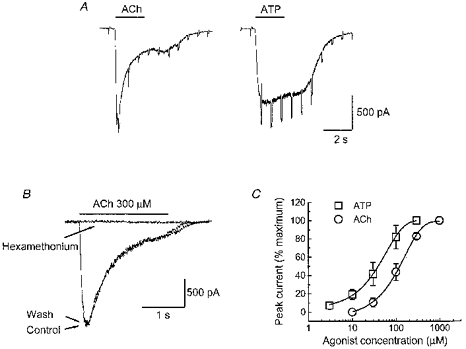
A, whole-cell currents elicited by acetylcholine (ACh, 3 mm) and ATP (1 mm). Downward deflections are current responses to 10 mV, 200 ms hyperpolarizing voltage commands. B, currents caused by ACh are reversibly blocked by hexamethonium (100 μm); holding potential, −60 mV. C, concentration-response curves for the peak currents elicited by ACh and ATP (holding potential, −60 mV). Each point is the mean ± s.e.m. of responses obtained from 4–6 cells per point; data points were fitted using a logistic function (see Methods). Data are normalized to the maximum ACh or ATP response recorded from each cell. The EC50 for ACh was 112 ± 7 μm (slope = 1.6 ± 0.1); the EC50 for ATP was 34 μm (slope = 1.3 ± 0.3).
Partial occlusion of ACh- and ATP-induced currents
As ACh and ATP activate separate classes of ligand-gated ion channels it would be expected that responses caused by these agonists would be independent and additive. Saturating concentrations of ACh (3 mm) or ATP (1 mm) caused inward currents that were stable in amplitude following repeated agonist application (Fig. 2Aa) but the current caused by combined application of ATP and ACh was only 80 ± 2 % (P < 0.05, n = 29) of the predicted sum of the currents activated by ACh and ATP alone (Fig. 2Ab). Responses caused by individual application of ACh and ATP obtained after agonist co-application were similar to the individual responses obtained before co-appliciation (Fig. 2Aa). Control ACh and ATP responses were −1374 ± 150 and −968 ± 155 pA, respectively. ACh and ATP responses obtained after agonist co-application were −1384 ± 149 and −844 ± 193 pA (P > 0.05, n = 17).
Figure 2. Occlusion of currents elicited by saturating concentrations of ACh (3 mm) and ATP (1 mm).
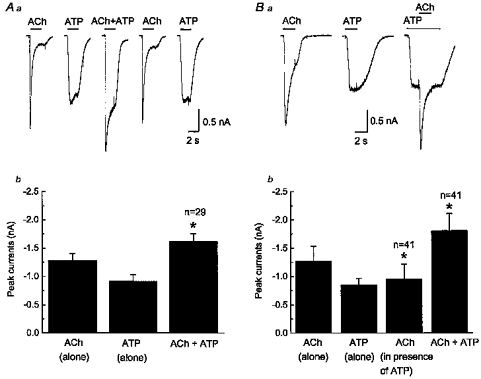
Aa, currents evoked by ACh and ATP are not additive. Combined agonist application causes a response smaller in amplitude (middle trace) than the predicted sum of the individual agonist responses obtained before agonist co-application (left traces). Amplitude of the individual agonist responses obtained after agonist co-application (right traces) is the same as that obtained before agonist co-application; holding potential, −60 mV. Ab, pooled data from experiments shown in Aa; data are the mean ± s.e.m. amplitude (n = 29 cells) of responses to ACh alone, ATP alone and ACh and ATP co-applied. * Significantly less than the predicted sum of the ACh and ATP response in each cell tested (P < 0.05). Ba, differences in the rise time of ACh- and ATP-induced currents do not account for lack of additivity. ACh and ATP responses were obtained sequentially; ATP responses desensitize slowly and ACh was applied during the steady-state peak of the ATP current. Bb, pooled data (n = 41 cells) from experiments shown in Ba; data are the mean ± s.e.m. amplitude of currents caused by ACh alone, ATP alone, the ACh current in the presence of a steady-state response to ATP and the peak amplitude of the current caused by ACh plus ATP. * Significantly different from the ACh current alone (ACh in presence of ATP) or significantly different from the predicted sum of the ACh- and ATP-induced currents (ACh + ATP).
The rate of activation of the nAChR and P2X receptor is different (see above), and differences in time to peak response for ACh and ATP could account for the lack of additivity observed during combined application of the two agonists (Rogers et al. 1997). To address this issue ACh and ATP were applied separately to the same neurons and peak currents caused by these agonists were measured. Then, as the ATP-induced current desensitizes slowly, ATP was applied and the evoked current was allowed to reach a peak before ACh application (Fig. 2Ba). Under these conditions, the amplitude of the ACh-activated current in the presence of ATP was only 65 ± 4 % (P < 0.05, n = 41) of the ACh-induced current measured in the absence of ATP (Fig. 2Bb). Furthermore, the total current activated during co-application of ATP and ACh was less than the predicted sum of the currents evoked by individual agonist application (Fig. 2Bb).
In myenteric neurons, the magnitude of the functional interaction between nAChRs and P2X receptors is related to the number of P2X receptors expressed by the neurons. When the amplitude of the ATP-induced current was plotted against the ratio of ACh-induced currents measured in the absence and presence of ATP it was found that there was an inverse correlation between the amplitudes of these responses (r = 0.6, P < 0.0001, n = 41). That is, with the large ATP-induced currents, there was a greater occlusion of the ACh-induced current in the same cells (Fig. 3). Neurons in which either ACh or ATP produced currents of less than 200 pA in amplitude were not included in the analysis.
Figure 3. Ratio of ACh current amplitudes in the presence and absence of ATP is negatively correlated with the amplitude of ATP currents in the same cell.
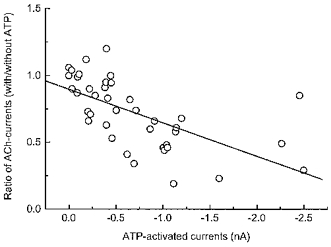
The amplitude of the ACh-induced current recorded in the presence of ATP is smaller when the amplitude of the ATP current is large. Points (n = 41) are the ratio of ACh currents recorded before and in the presence of a maximum concentration of ATP (1 mm) versus the amplitude of the ATP current in the same cell. The line is a best fit straight line (slope = −0.89, r = 0.62, P < 0.00001).
Cross desensitization
The next studies were designed to determine if non-additivity between nAChRs and P2X receptors persisted after receptor desensitization. Responses to ATP were obtained in the same neurons before and after nAChR desensitization (Fig. 4Aa). In neurons in which the nAChR had been desensitized by continuous application of ACh (3 mm), the ATP response was only 74 ± 5 % (P < 0.05, n = 6) of the control amplitude (Fig. 4Ab). Similar studies were done to determine the effect of P2X receptor desensitization on ACh responses. In these studies, a control ACh response was obtained before and after P2X receptor desensitization caused by continuous application of ATP (1 mm) (Fig. 4Ba). ACh responses after P2X receptor desensitization were only 80 ± 7 % (P < 0.05, n = 7, Fig. 4Bb) of the control ACh response.
Figure 4. Non-additivity of ACh- and ATP-induced currents persists after receptor desensitization.
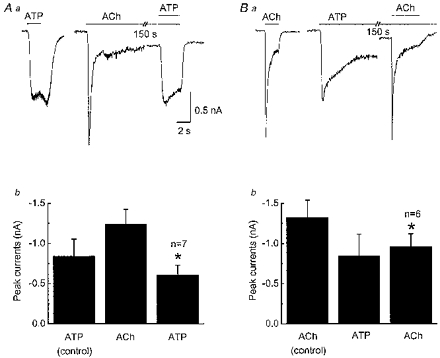
Aa, ATP (1 mm)-activated currents evoked in the absence (left) and presence of a desensitizing concentration of ACh (3 mm, right) applied for 150 s. The ATP response is smaller after nAChR desensitization; holding potential, −60 mV. Ab, pooled data from experiments shown in Aa; data are mean ±s.e.m. amplitude of ATP currents, ACh currents and the amplitude of the ATP current after nAChR desensitization. * Significantly different from ATP control (P < 0.05). Ba, ACh (3 mm)-evoked current evoked before and after P2X receptor desensitization caused by ATP (1 mm) applied for 150 s. The ACh response is smaller after P2X receptor desensitization. Bb, pooled data from experiments shown in Ba; data are the mean ±s.e.m. amplitude of ACh currents, ATP currents and the amplitude of the ACh current after P2X receptor desensitization. * Significantly different from ACh control (P < 0.05).
Non-additivity is not mediated by Ca2+-dependent mechanisms
Neuronal nAChRs and P2X receptors are Ca2+-permeable cation channels (Fieber & Adams, 1991a, b; Trouslard et al. 1993; Rogers et al. 1997). Therefore, the effects of removing extracellular Ca2+ on ACh- and ATP-induced currents were examined (Fig. 5A). ACh-evoked responses evoked in Ca2+-free extracellular solution were only 57 ± 6 % (P < 0.05, n = 7) of those caused by ACh in normal (2.5 mm Ca2+) solution while currents activated by ATP in Ca2+-free solution were 95 ± 5 % (P > 0.05, n = 7) of those elicited in normal Ca2+-containing solution (Fig. 5A). These data suggest that Ca2+ is an important charge carrier for nAChRs in myenteric neurons and that entry through nAChRs could modulate the activity of current flow through P2X receptors. To address this possibility, the effect of changes in extracellular or intracellular Ca2+ concentration on the negative interaction between nAChRs and P2X receptors was tested. The first set of experiments was done with normal (2.5 mm) extracellular Ca2+ but with no Ca2+ and the Ca2+ chelators EGTA (1 mm) and BAPTA (10 mm) added to the pipette solution. In these studies, responses caused by combined application of ACh and ATP were only 73 ± 5 % of the predicted sum of responses caused by application of each agonist individually (n = 8, P < 0.05, Fig. 5B). Similar data were obtained when recordings were obtained with EGTA and BAPTA in the pipette solution and when Ca2+ was omitted from the extracellular solution; the response to combined application of ACh and ATP was only 80 ± 4 % (P < 0.05, n = 10) of the predicted sum of the amplitude of the two individual responses (Fig. 5B).
Figure 5. Non-additivity of currents elicited by ACh and ATP in the absence and presence of intra- and extracellular Ca2+.
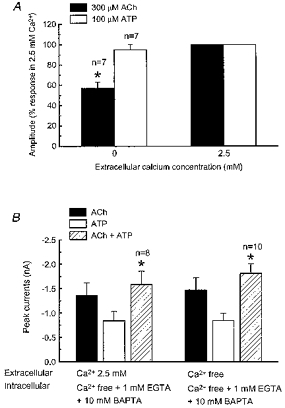
A, ACh- but not ATP-evoked responses are reduced in the absence of extracellular calcium, suggesting that calcium is an important charge carrier for the nAChR. The Ca2+ concentration of normal Krebs solution (2.5 mm) was used as the 100% control current amplitude. Bars represent the mean of 5–7 cells. *Significantly different from control (P < 0.01). B, left bars, currents activated by 3 mm ACh (▪), 1 mm ATP (□) and their combination ( ) with 2.5 mm Ca2+ in the extracellular solution and 1 mm EGTA and 10 mm BAPTA in the Ca2+-free pipette solution; * indicates that the amplitude of the current evoked by combined application of ACh and ATP was significantly less than the predicted sum of the individual currents. Right bars, amplitude of currents caused by ACh, ATP and co-application of ACh and ATP using a Ca2+-free extracellular solution and a Ca2+-free plus 10 mm BAPTA pipette solution; amplitude for ACh plus ATP was 80.1 ± 4 % (n = 10) of the sum of currents activated by ACh and ATP alone. * indicates that the amplitude of currents evoked by combined application of ACh and ATP were significantly less than the predicted sum of the individual currents.
) with 2.5 mm Ca2+ in the extracellular solution and 1 mm EGTA and 10 mm BAPTA in the Ca2+-free pipette solution; * indicates that the amplitude of the current evoked by combined application of ACh and ATP was significantly less than the predicted sum of the individual currents. Right bars, amplitude of currents caused by ACh, ATP and co-application of ACh and ATP using a Ca2+-free extracellular solution and a Ca2+-free plus 10 mm BAPTA pipette solution; amplitude for ACh plus ATP was 80.1 ± 4 % (n = 10) of the sum of currents activated by ACh and ATP alone. * indicates that the amplitude of currents evoked by combined application of ACh and ATP were significantly less than the predicted sum of the individual currents.
Co-application of ACh and ATP did not alter the Na+ gradient
Na+ is a charge carrier for nAChRs and P2X receptors and the reversal potential for currents carried by these receptors is near 0 mV (Fieber & Adams, 1991a, b). It is possible that co-application of high concentrations of ACh and ATP changes the driving force for Na+. In order to address this possibility, the reversal potential of currents caused by ACh, ATP and ACh plus ATP was measured using 100 ms ramp depolarizations from an initial holding potential of −70 mV to 40 mV. Using this protocol, the reversal potential of currents caused by co-application of ACh and ATP was not different from the reveral potential of currents caused by either agonist alone (Fig. 6A and B). Reversal potentials for currents caused by ACh alone and ACh plus ATP were 2.0 ± 0.7 and 2.4 ± 0.7 mV, respectively (P > 0.05, n = 4). Similarly, the average reversal potential for ATP alone and ATP plus ACh was 1.9 ± 1.6 and 2.1 ± 2.3 mV, respectively (P > 0.05, n = 4).
Figure 6. Reversal potentials of currents caused by ACh, ATP and co-application of ACh and ATP.
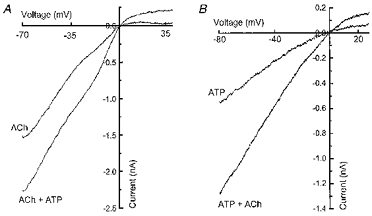
A, current-voltage relationship for currents activated by ACh and ACh plus ATP. Data were obtained using a voltage ramp which changed the holding potential of the neuron from −70 to 40 mV in 100 ms. Records show agonist-induced currents after subtraction of the resting current-voltage curve. B, current- voltage relationships for ATP-induced current and currents caused by co-application of ATP plus ACh. Reversal potentials for currents caused by agonist co-application were not different from those currents caused by separate agonist treatment. The voltage ramp changed the holding potential from −80 to 30 mV in 100 ms.
These studies also allowed measurement of outward currents caused by individual and co-application of ACh and ATP. At 50 mV, the amplitude of the ACh current was 17 ± 7 pA while the ATP current was 156 ± 59 pA. The amplitude of the current (at 50 mV) caused by co-application of ACh and ATP was 174 ± 63 pA. This was not different from the predicted sum of the currents caused by individual agonist application (P > 0.05, n = 9).
ACh/ATP- and 5-hydroxytryptamine (5-HT)- or GABA-activated currents are additive
5-HT3 receptors are ligand-gated cation channels (Derkach et al. 1989). It is possible that the negative interaction between nAChRs and P2X receptors is a general property shared by many ligand-gated cation channels. To test this possibility, the amplitude of responses caused by 5-HT in the absence and presence of ACh or ATP was measured. 5-HT caused an inward current in all cells tested (n = 37) and the EC50 value for 5-HT was 8.0 ± 0.6 μm (Fig. 7A, n = 7). 5-HT-activated currents were inhibited by 1 μm ondansetron, a 5-HT3 receptor antagonist (Fig. 7B, n = 3). These experiments suggest that myenteric neurons express 5-HT3 receptors. The mean amplitude of 5-HT-activated currents was −420 ± 47 pA (holding potential, −60 mV) and was 33 % of the mean amplitude of ACh-activated currents and 46 % of the mean amplitude of ATP-activated currents recorded from the same cells. Responses caused by simultaneous application of 5-HT (0.3 mm) and ACh (3 mm) were 94 ± 3 % (n = 10, P > 0.05) of the predicted sum of the current amplitude caused by individual agonist application (Fig. 7C and D). Responses caused by simultaneous application of 5-HT (0.3 mm) and ATP (1 mm) were 96 ± 4 % (n = 8, P > 0.05) of the predicted sum of the current amplitude caused by individual agonist application (Fig. 7D).
Figure 7. Currents caused by 5-hydroxytryptamine (5-HT) and ACh or ATP are additive.
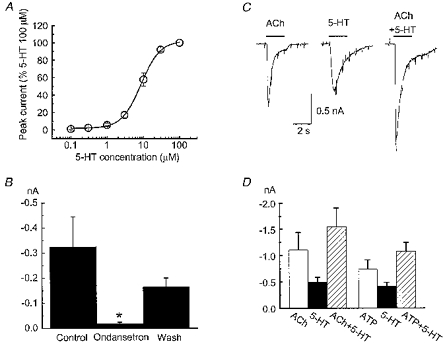
A, concentration-response curve for 5-HT. Responses were normalized to that caused by 100 μm 5-HT in each neuron (holding potential, −60 mV; data are the mean ±s.e.m. of 7 cells). Data were fitted using a logistic function (see Methods). The EC50 for 5-HT was 8 ± 0.6 μm and the slope of the curve was 1.6 ± 0.2. B, 5-HT-activated currents were reversibly inhibited by ondansetron (1 μm). * Significantly different from control (P < 0.05, n = 3). C, inward currents were evoked by ACh (3 mm), 5-HT (0.3 mm) and ACh plus 5-HT, respectively. The amplitude current activated by ACh plus 5-HT (right) was equal to the sum of currents evoked by ACh and 5-HT applied individually. D, pooled data from experiments shown in C. Currents activated by 5-HT plus ACh (n = 10) or 5-HT plus ATP (n = 8) were 94 ± 3 or 96 ± 4 %, respectively, of the sum of currents activated by 5-HT and ACh (P > 0.05) or ATP (P > 0.05) alone.
GABAA receptors are ligand-gated chloride channels (Unwin, 1993). GABA caused a concentration-dependent inward current which achieved peak amplitude within 100 ms and desensitized slowly (Fig. 8A and C). The EC50 value for GABA responses was 17 ± 2 μm (n = 6). Bicuculline (10 μm), a GABAA receptor antagonist, reversibly blocked the GABA-activated current (Fig. 8B, n = 7). GABA (1 mm) caused an inward current in 29/37 (78 %) of myenteric neurons tested with a mean amplitude of −1854 ± 583 pA (holding potential, −60 mV, n = 29). GABA-induced currents were 44 and 102 % larger than those caused by ACh (3 mm) and ATP (1 mm), respectively. The amplitude of the current activated by co-application of ACh and GABA was 105 ± 4 % (n = 8, P > 0.05) of the sum of currents evoked by ACh and GABA alone (Fig. 8C and D). The amplitude of currents activated by co-application of ATP (1 mm) and GABA was 100 ± 3 % of the predicted sum of the currents caused by individual application of ATP and GABA (Fig. 8D).
Figure 8. Currents caused by ACh or ATP and γ-aminobutyric acid (GABA) are additive.
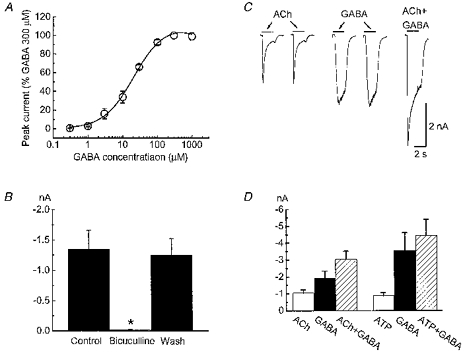
A, GABA concentration-response curve. Responses were normalized to those caused by 300 μm GABA in each neuron (holding potential, −60 mV; data are mean ±s.e.m. of 3–6 cells). Data were fitted by a logistic function (see Methods). The EC50 for GABA was 17 ± 2 μm; the slope of the curve was 1.2 ± 0.1. B, GABA-activated currents were blocked by bicuculline (10 μm, P < 0.05, n = 7). C, currents caused by ACh, GABA and ACh plus GABA (left to right). The amplitude of currents activated by ACh plus GABA (right) equalled the sum of currents evoked by ACh and GABA alone (left and middle). D, pooled data from experiments in C. Currents caused by co-application of ACh and GABA were 105 ± 4 % of the sum of the currents activated by GABA and ACh alone (P > 0.05). Currents caused by co-application of ATP and GABA were 100 ± 3 % of the sum of the currents activated by ATP and GABA alone (P > 0.05).
Single channel currents activated by ATP and ACh in outside-out patches
In outside-out patches studied using high resistance pipettes (> 15 MΩ), ACh (100 μm) activated single channel currents which had an amplitude of −1.7 ± 0.1 pA at −70 mV (Fig. 9A and B). No channel activity was recorded in these patches prior to ACh application. Single channel currents activated by ACh had a linear I–V relationship with a mean single channel conductance of 25.0 ± 0.9 pS (n = 8) (Fig. 9B). Hexamethonium (100 μm) completely prevented activation of ACh-induced single channel currents in four patches tested.
Figure 9. ACh- and ATP-activated currents in outside-out patches.
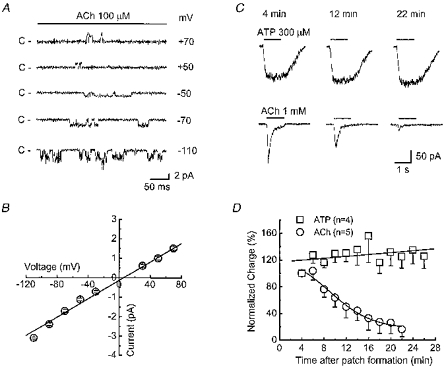
A, single channel currents activated by ACh in an outside-out patch. Recordings were obtained at the indicated patch potentials; C indicates closed level. B, current-voltage relationship for single channel currents activated by ACh. Data are mean ± s.e.m. of recordings from patches. C (upper), ATP-induced currents evoked by application of ATP at the indicated time intervals after patch formation (patch potential, −60 mV). C (lower), records of nAChR currents in an outside-out patch in response to application of 1 mm ACh (continuous line above traces) at the indicated time intervals after patch formation (patch potential, −60 mV). D, time course of ACh (1 mm, ○) but not ATP (300 μm, □) current run-down. Data are expessed as a percentage of the charge transfer measured 2 min after patch formation.
In outside-out patches obtained with low resistance pipettes (< 5 MΩ), ATP (300 μm) acting at P2X purine receptors elicited single channel currents (Zhou & Galligan, 1996) and the ATP response did not desensitize during a 1 s ATP application. Furthermore, the amplitude of P2X receptor channel activity remained stable during repeated ATP applications up to 30 min after patch formation (Fig. 9C and D). In the same patches, ACh (100 μm) also elicited single channel currents and channel activity in these patches exhibited marked desensitization in the continued presence of ACh (Fig. 9C). In addition to acute desensitization during a single ACh application, nAChRs ‘ran down’ when ACh was applied at 2 min intervals over a period of 20 min (Fig. 9C and D). ACh-induced channel activity did not recover after run-down.
Interaction of P2X receptors and nAChRs in outside-out patches was studied by first applying ATP followed by co-application of ATP and ACh and then applying ACh alone (Fig. 10A). This protocol was used in an attempt to account for run-down of the ACh-induced channel activity. The amplitude of the current caused by co-application of ATP and ACh was only 85 ± 10 % (P < 0.05; n = 8) of the predicted sum of the currents caused by individual application of ATP and ACh (Fig. 10B). In a subset of these patches (n = 5), ATP responses were recorded after ACh-induced channel activity had fully run-down (Fig. 10C). The amplitude of the single channel currents caused by ATP alone was not different from the amplitude of the currents caused by ATP in the presence of ACh (Fig. 10D). These data indicate that nAChR activation is neccessary for the negative interaction between nAChRs and P2X receptors.
Figure 10. Non-additivity of ACh- and ATP-activated currents in outside-out patches.
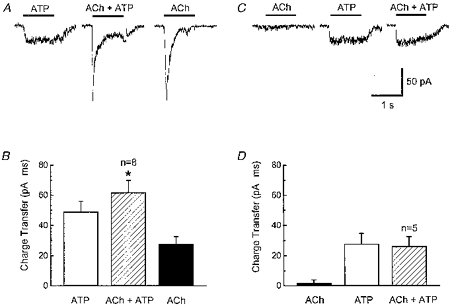
A, records of currents caused by sequential application of ATP (1 mm), ACh (3 mm) and ATP and ACh alone to the same patch. Currents caused by ACh plus ATP were less than the predicted sum of currents activated by ATP and ACh alone. B, pooled data from experiments shown in A. Data are expressed as mean ± s.e.m. charge transfer during 1 s agonist application. * Significantly different from the predicted sum of currents activated by ATP and ACh applied individually. C, records of currents caused by sequential application of ACh (3 mm), ATP (1 mm) and ACh plus ATP obtained after currents caused by ACh had run down. Currents caused by ATP plus ACh were similar to those caused by ATP alone. D, pooled data from experiements in C. Data are expressed as mean ± s.e.m. total charge transfer during 1 s agonist application. Currents caused by ATP alone were not different from those caused by co-application of ATP plus ACh.
DISCUSSION
Interaction between AChRs and P2X receptors
ACh and ATP are fast excitatory neurotransmitters in guinea-pig myenteric plexus with ATP acting on P2X purine receptors to cause fast excitatory postsynaptic potentials (Galligan & Bertrand, 1994; Zhou & Galligan, 1996, LePard et al. 1997). It has been suggested that, as ACh and ATP are co-released from the same nerve terminals (White & Leslie, 1982), these transmitters may activate a common set of ligand-gated ion channels on postsynaptic or postjunctional cells (Bean, 1992). This conclusion is based on the observations that the properties of the single channel currents activated by ACh and ATP are nearly identical and that responses caused by co-application of ACh and ATP are saturable and not additive (Igusa, 1988; Nakazawa et al. 1991; Silinksky & Gerzanich, 1993; Nakazawa, 1994). Similar data were obtained in the present study where it was found that responses elicited by co-application of maximum concentrations of ACh and ATP were less than the predicted sum of the responses obtained by individual application of the two agonists. However, P2X receptors and nAChRs have markedly different pharmacological properties including sensitivity to open channel blocking drugs (Rogers et al. 1997). Furthermore molecular biological studies have demonstrated that P2X receptors are structurally unrelated to nAChRs (Valera et al. 1994; Brake et al. 1994; Chen et al. 1995; Surprenant et al. 1995; Seguela et al. 1996). Finally, data from the present study show that nAChR single channel currents in outside-out patches exhibited rapid and irreversible run-down following repeated agonist application while P2X receptor single channel currents were stable for up to 30 min. These data indicate that the activity of channels activated by ACh and ATP are regulated differently. Therefore it is unlikely that ACh and ATP activate a common set of ion channels in myenteric neurons and that other mechanisms must be responsible for response saturability.
P2X receptors and nAChRs are ligand-gated cation channels that are permeable to Na+ and K+ and to a variable degree Ca2+. It is possible that intracellular accumulation of Na+ during co-application of high agonist concentrations could decrease the driving force for Na+ to attenuate response amplitude. This possibility was evaluated by comparing the reversal potentials of responses caused by separate application of ACh and ATP and by co-application of ACh and ATP. ACh-activated whole-cell currents desensitize rapidly. In order to measure reversal potentials near the peak response, current-voltage relationships for agonist-induced currents were obtained using fast ramp depolarizations. Reversal potentials measured during co-application of ACh and ATP were not different from those measured during application of either ACh or ATP alone. These observations suggest that changes in the driving force for Na+ were not responsible for occlusion. A similar conclusion was reached in a previous study (Nakazawa, 1994) in which glucosamine was used as the external permeable cation. P2X receptors but not nAChRs are glucosamine permeable. In these studies ACh reduced the response to ATP even though ACh did not elicit a detectable inward current.
As both P2X receptors and nAChRs are Ca2+ permeable (Fieber & Adams, 1991a, b; Trouslard et al. 1993; Rogers et al. 1997), the rise in intracellular Ca2+ occurring during responses caused by co-application of ACh and ATP could alter the activity of nAChRs and P2X receptors either through a direct effect on the channels or indirectly through a Ca2+-activated intracellular mechanism. However, when the Ca2+ chelator BAPTA (10 mm) was added to the intracellular solution or when Ca2+ was omitted from both the intra- and extracellular solutions, responses caused by co-application of ATP and ACh were still significantly less than the predicted sum of responses caused by separate application of the two agonists. Therefore, a Ca2+-dependent mechanism does not contribute to the non-additivity responses mediated by nAChRs and P2X receptors.
It has been suggested that a difference in rise time of responses mediated at nAChRs and P2X receptors can account for the apparent lack of additivity between ACh and ATP-induced currents when the agonists are co-applied simultaneously (Rogers et al. 1997). To address this possibility, we took advantage of the slow rate of desensitization of P2X receptors by allowing ATP responses to reach a stable peak at which point a maximum concentration of ACh was also applied. It was found that the amplitude of the combined agonist-induced current was less than the predicted sum of the individual currents. Furthermore, the amplitude of the ACh current in the presence of ATP was significantly less than the amplitude of the current caused by ACh alone. Under these experimental conditions, differences in response rise time would not contribute to the non-additivity of ACh and ATP-induced currents.
In addition to nAChR and P2X receptors, myenteric neurons also express 5-HT3 and GABAA receptors. 5-HT3 receptors are ligand-gated cation channels while GABAA receptors are ligand-gated chloride channels (Derkach et al. 1989; Unwin, 1993; Ortells & Lunt, 1995). Both 5-HT3 and GABAA receptors are structurally similar to nAChRs and it is possible that 5-HT3- or GABAA-mediated responses may not be additive with those mediated at nAChRs or P2X receptors. In the present study, 5-HT caused an inward current in every myenteric neuron tested. The 5-HT-induced response had a short latency, was associated with a conductance increase and was blocked by the 5-HT3 receptor antagonist ondansetron, indicating that these responses were mediated at 5-HT3 receptors. Inward currents caused by co-application of 5-HT and ACh or 5-HT and ATP were completely additive, as would be expected for independent channels. GABA also caused an inward current in most myenteric neurons and these responses had a brief latency and were blocked by the GABAA receptor antagonist bicuculine, indicating that they were mediated at GABAA receptors. Inward currents caused by ACh and GABA or ATP and GABA were completely additive as expected for currents carried by independent channels. Taken together these data indicate that non-additivity is not a general property of ligand-gated ion channels expressed by myenteric neurons but that there is some specificity for the negative interaction between nAChRs and P2X receptors.
Mechanisms of functional interaction between nAChRs and P2X receptors
Biochemical studies of nAChRs isolated from Torpedo have shown that the extracellular domain of the β-subunit contains an ATP binding site (Schrattenholz et al. 1997). This observation suggests that ATP could directly modify nAChR activity by binding to the nAChR rather by binding to the P2X receptor. Indeed, it has been shown that ATP enhances responses mediated at nAChRs at the somatic neuronmuscular junction (Akasu et al. 1981; Igusa, 1988; Lu & Smith, 1991; Fu, 1994) and in bullfrog sympathetic ganglia (Akasu & Koketsu, 1985). However, a positive allosteric effect of ATP on nAChR receptor function is inconsistent with the negative interaction between nAChRs and P2X receptors reported here. In addition, pretreatment of neurons with ACh to allow activation and desensitization of the nAChR results in a smaller than predicted response to ATP suggesting that there is a mutual negative interaction between ACh- and ATP-activated mechanisms. Allosteric modulation of the activity of nAChRs by ATP cannot account for the non-additivity of ACh- and ATP-induced currents.
There was a positive correlation between the amplitude of ATP-induced responses and the amount of occlusion of ACh-induced current in myenteric neurons. This observation suggests that the number of P2X receptors expressed by a neuron is related to the apparent negative interaction between P2X receptors and nAChRs. At −70 mV, the single channel current for nAChRs was 1.7 pA and for P2X receptors was 1.5 pA, while the average maximum whole-cell currents caused by ACh and ATP were 1167 and 855 pA, respectively. Based on these measurements, there is an average of 686 nAChRs and 570 P2X receptors per neuron with an approximate 1 : 1 ratio of nAChR : P2X receptors. If nAChRs and P2X receptors are not diffusely distributed on the neuronal surface but are clustered in synapses in a 1 : 1 ratio, simultaneous activation of nAChRs and P2X receptors may hinder current flow through adjacent channels. In neurons expressing greater numbers of receptors this interaction would be more prominent. Interaction between closely positioned channels could modify channel conductance or gating. This would occur when nAChRs and P2X receptors are in the ligand-bound, active state as well as in the ligand-bound, desensitized state. This conclusion is supported by the data derived from the studies done in outside-out patches where it was found that currents in these patches elicited by co-application of ATP and ACh are less than additive. These data indicate that cytoplasmic signalling mechanisms are not essential for the negative interaction between nAChRs and P2X receptors. However, in outside-out patches in which the nAChR had run down, ATP-evoked responses in the presence of ACh were the same as those caused by ATP alone. These data indicate that the nAChR must be in either the active or desensitized state to inhibit currents carried by P2X receptors. Outward currents (at a holding potential of 50 mV) evoked by co-application of ACh and ATP were completely additive suggesting that the mechanism of the interaction between nAChRs and P2X receptors is voltage dependent. The open probability of nAChRs (Mathie et al. 1990; Fieber & Adams, 1991a) and P2X receptors (Fieber & Adams, 1991b; Cloues, 1995) in autonomic neurons is voltage dependent and decreases as the membrane potential becomes less negative. At 50 mV, the probability that nAChR and P2X receptors open simultaneously would be lower than at −60 mV. Therefore, the observation that outward currents carried by nAChRs and P2X receptors are additive is consistent with the proposal that these receptors must be in the active (or desensitized) state for a negative interaction to occur.
The negative interaction between nAChRs and P2X receptors could play an important role in modifying the strength of neurotransmission at synapses where ATP and ACh are co-transmitters. The nature of the functional interaction between nAChRs and P2X receptors may also depend on the specific subunit composition of these receptors. Neuronal nAChRs can be composed of combinations of eight different α-subnits and three different β-subunits (Sargent, 1993). There are also seven different P2X receptor subunits which can combine to form receptors with different functional and pharmacological properties (Surprenant et al. 1995; Collo et al. 1996; Seguela et al. 1996). Interactions between nAChRs and P2X receptors may only occur between receptors of specific subunit composition. The subunit composition of nAChRs and P2X receptors in myenteric neurons remains to be determined.
Acknowledgments
This work was supported by grants NS33289 and NS01738 from the National Institutes of Health.
References
- Akasu T, Hirai K, Koketsu K. Increase of acetylcholine-receptor sensitivity by adenosine triphosphate: a novel action of ATP on ACh-sensitivity. British Journal of Pharmacology. 1981;74:505–507. doi: 10.1111/j.1476-5381.1981.tb09997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T, Koketsu K. Effect of adenosine triphoshpate on the sensitivity of the nicotinic acetylcholine receptor in the bullfrog sympathetic ganglion cell. British Journal of Pharmacology. 1985;84:525–531. doi: 10.1111/j.1476-5381.1985.tb12937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. Pharmacology and electrophysiology of ATP-activated ion channels. Trends in Pharmacological Sciences. 1992;13:87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channel defined by an inotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Akoplan AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Cloues R. Properties of ATP-gated channels recorded from rat sympathetic neurones: voltage-dependence and regulation by Zn2+ ions. Journal of Neurophysiology. 1995;73:312–319. doi: 10.1152/jn.1995.73.1.312. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–708. doi: 10.1038/339706a0. 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- Fieber LA, Adams DJ. Acetylcholine-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. The Journal of Physiology. 1991a;434:215–237. doi: 10.1113/jphysiol.1991.sp018466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber LA, Adams DJ. Adenosine triphosphate-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. The Journal of Physiology. 1991b;434:239–256. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W-M. Potentiation of the postsynaptic acetylcholine response at developing neuromuscular synapses in Xenopus cultures. The Journal of Physiology. 1994;477:449–458. doi: 10.1113/jphysiol.1994.sp020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. Journal of Neuroscience. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y. Adenosine 5′-triphosphate activates acetylcholine receptor channels in cultured Xenopus myotomal muscle cells. The Journal of Physiology. 1988;405:169–185. doi: 10.1113/jphysiol.1988.sp017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePard KJ, Messori E, Galligan JJ. Purinergic fast excitatory postsynaptic potentials in myenteric neurons of guinea pig: distribution and pharmacology. Gastroenterology. 1997;113:1522–1534. doi: 10.1053/gast.1997.v113.pm9352854. [DOI] [PubMed] [Google Scholar]
- Lu Z, Smith DO. Adenosine 5′-triphosphate increases acetylcholine channel opening frequency in rat skeletal muscle. The Journal of Physiology. 1991;436:45–56. doi: 10.1113/jphysiol.1991.sp018538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A, Colquhoun D, Cull-Candy SG. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. The Journal of Physiology. 1990;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K. ATP-activated current and its interaction with acetylcholine-activated currents in rat sympathetic neurons. Journal of Neuroscience. 1994;14:740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Fujimori K, Takanaka A, Inoue K. Comparison of adenosine triphosphate- and nicotine-activated inward currents in rat pheochromocytoma cells. The Journal of Physiology. 1991;434:647–660. doi: 10.1113/jphysiol.1991.sp018491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L, Alund M, Norberg K-A. Fluorescence-microscopical demonstration of a population of gastro-intestinal nerve fibers with a selective affinity for quinacrine. Cell Tissue Research. 1976;171:407–423. doi: 10.1007/BF00220234. [DOI] [PubMed] [Google Scholar]
- Ortells MO, Lunt GG. Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends in Neurosciences. 1995;18:121–127. doi: 10.1016/0166-2236(95)93887-4. 10.1016/0166-2236(95)93887-4. [DOI] [PubMed] [Google Scholar]
- Rogers M, Colquhoun LM, Patrick JW, Dani JA. Calcium flux through predominantly independent purinergic ATP and nicotinic acetylcholine receptors. Journal of Neurophysiology. 1997;77:1407–1417. doi: 10.1152/jn.1997.77.3.1407. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annual Review of Neuroscience. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Schrattenholz A, Roth U, Godovac-Zimmermann J, Maelicke A. Mapping of a binding site for ATP within the extracellular region of the Torpedo nicotinic acetylcholine receptor β-subunit. Biochemistry. 1997;36:13333–13340. doi: 10.1021/bi9706024. [DOI] [PubMed] [Google Scholar]
- Seguela P, Haghighi A, Soghomonian JJ, Cooper E. A novel neuronal P2X ATP receptor ion channel with widespread distribution in the brain. Journal of Neuroscience. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM, Gerzanich V. On the excitatory effects of ATP and its role as a neurotransmitter in coeliac neurons of the guinea-pig. The Journal of Physiology. 1993;464:197–211. doi: 10.1113/jphysiol.1993.sp019630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Buell G, North RA. P2X receptors bring new structure to ligand-gated ion channels. Trends in Neurosciences. 1995;18:224–229. doi: 10.1016/0166-2236(95)93907-f. 10.1016/0166-2236(95)93907-F. [DOI] [PubMed] [Google Scholar]
- Trouslard J, Marsh SJ, Brown DA. Calcium entry through nicotinic receptor channels and calcium channels in cultured rat superior cervical ganglion cells. The Journal of Physiology. 1993;468:53–71. doi: 10.1113/jphysiol.1993.sp019759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Neurotransmitter action: opening of ligand-gated ion channels. Cell. 1993;72(suppl.):31–41. doi: 10.1016/s0092-8674(05)80026-1. [DOI] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- White TF, Leslie RA. Depolarization-induced release of adenosine 5′-triphosphate from isolated varicosities derived from the myenteric plexus of the guinea pig small intestine. Journal of Neuroscience. 1982;2:206–215. doi: 10.1523/JNEUROSCI.02-02-00206.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Potentiation of ATP-responses at a recombinant P2X2 receptor by neurotransmitters and related substances. British Journal of Pharmacology. 1997;120:221–224. doi: 10.1038/sj.bjp.0700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Galligan JJ. P2X purinoceptors in cultured myenteric neurons of guinea-pig small intestine. The Journal of Physiology. 1996;496:719–729. doi: 10.1113/jphysiol.1996.sp021722. [DOI] [PMC free article] [PubMed] [Google Scholar]


