Abstract
Elevated levels of cytokines, especially interleukin (IL)-6 and IL-1ra, can be measured in the plasma of athletes after exhaustive long term exercise.
The present study investigates the kinetics of several cytokines and chemokines in ten male athletes before, during and after 2.5 h of treadmill running at 75% of maximal oxygen consumption (VO2,max). Blood was sampled before, every half-hour during running and every hour in the following 6 h recovery period.
The plasma concentration of IL-6 increased after 30 min of running, and peaked at the end of running with a 25-fold increase compared with the pre-exercise value. IL-1ra increased only after running, and peaked after 2 h of rest with an 18-fold increase compared with the pre-exercise value. No changes were found in the concentrations of IL-1β, tumour necrosis factor (TNF)α, IL-15 and macrophage inflammatory protein (MIP)-1β, and the concentrations of IL-8 and MIP-1α were below detection limits.
The results suggest that very early events in exercise trigger the release of IL-6, and that the cytokine response to exercise has similarities to that observed after trauma.
Intense and prolonged exercise induces high levels of circulating inflammatory cytokines, especially interleukin (IL)-6 (Drenth et al. 1995; Nehlsen-Cannarella et al. 1997; Castell et al. 1997; Ostrowski et al. 1998), and it has been suggested that the release of IL-6 in exercise is related to the occurrence of muscle damage (Bruunsgaard et al. 1997). In experimental models of meningitis and sepsis, endotoxins induce an increase in tumour necrosis factor (TNF)α, which is followed by an increase in IL-1β and later IL-6 (Waage et al. 1989; Mustafa et al. 1989). In trauma patients, however, the release of cytokines is different. A recent study by Martin et al. (1997), including twenty-five non-trauma patients with septic shock and sixty multiple trauma patients, found elevated levels of IL-6 in both groups of patients whereas elevated levels of TNFα were found only in the septic shock patients. A study by Svoboda et al. (1994) found elevated levels of IL-6 in multiple trauma patients less than 3 h after injury, at which time IL-6 correlated with the injury severity score. In the same study no or minor changes were found in plasma IL-1β concentration. Elevated levels of TNFα did occur, but usually much later concomitantly with multiple organ failure, or just before death. Other studies report similar findings which has led to the suggestion that elevated TNFα and IL-1β are connected to bacterial challenges, whereas elevations in IL-6 following trauma are associated with soft tissue damage (Roumen et al. 1993; Svoboda et al. 1994; Biffl et al. 1996; Martin et al. 1997).
Changes in the plasma concentration of various cytokines have been investigated post-exercise in multiple studies, but no information has previously been available regarding the time course of changes in plasma levels of cytokines during exercise. Such information could reveal important information concerning the nature of the acute phase response observed in exercise. The present study therefore investigates the kinetics of the change in plasma concentration of several cytokines during and after 2.5 h of treadmill running.
We hypothesize that the cytokine response to muscle-damaging exercise is similar to that observed in trauma patients. Thus, we expect an increase in plasma IL-6, which correlates with the amount of tissue damage, but no, or minor, changes in plasma TNFα and IL-1β. Since IL-6 induces the cytokine antagonist IL-1ra (Tilg et al. 1994; Jordan et al. 1995), we expect an increase in the concentration of IL-1ra to follow an increase in IL-6.
Exercise induces mobilization of neutrophils and lymphocytes into the circulation. This mobilization is probably mediated by hormones (e.g. adrenaline, cortisol and growth hormone), but it is possible that circulating chemoattractant cytokines, such as IL-8, macrophage inflammatory protein (MIP)-1α and MIP-1β are also involved in the mobilization of these leukocyte subpopulations. The present study therefore includes measurements of these chemokines.
METHODS
Subjects
Ten endurance trained male athletes 23–47 years old (mean, 38 years) with maximal oxygen consumption (VO2,max) of 3.7- 5.1 l min−1 (mean, 4.5 l min−1) corresponding to 56.2–65.5 ml kg−1 min−1 (mean, 59.7 ml kg−1 min−1) participated in the study. None of the subjects were taking any medication. The experimental protocol was approved by the local ethics committee for Copenhagen and Frederiksberg Communities (J.nr. 01-111/97). All subjects were informed about the purpose and risks of the study, before written informed consent was obtained.
Exercise protocol
For each subject VO2,max was determined prior to the experiment during an incremental exercise test on the same treadmill as used in the experiment. The experiment consisted of 2.5 h of treadmill running, and an incline of 2.5% was used. Subjects ran at the speed determined in the VO2,max test to give an oxygen consumption of 75% of VO2,max, and actual oxygen uptake was sampled every 15 min during running to control if deviations from this occurred.
Experimental protocol
The subjects reported to the laboratory at 08.00 h, after an overnight fast. Subjects were allowed to drink water ad libitum, but consumption of carbohydrates was not allowed until after running. Subjects had been instructed to arrive well rested and to refrain from exhaustive training in the days before the experiment. Before the 2.5 h of running, blood was sampled (see details below). During running a blood sample was taken every 30 min and after running a blood sample was taken every hour during a 6 h resting period. The speed of the treadmill was lowered to walking speed during blood sampling (average duration, 3 min (range, 2–4 min)). On days 2 and 5, the subjects reported back to the laboratory and a blood sample was taken.
Blood sampling
During the exercise blood samples were drawn from an in-dwelling intravenous catheter in the hand or forearm. The catheter was regularly flushed with 0.9% saline water to avoid clotting.
Clinical chemistry tests
Creatine kinase (CK) was measured in lithium heparinized plasma using automated enzyme reactions (automated analysis for Hitachi System 717, Boehringer Mannheim Diagnostica, Germany). Plasma lactate was measured in EDTA plasma using COBAS FARA autoanalyser (Roche).
Cytokine protein measurements
Blood samples for measurements of cytokines (IL-1β, IL-1ra, IL-6, IL-8, IL-15, MIP-1α, MIP-1β and TNFα) were drawn into precooled glass tubes containing EDTA and aprotinin (Trasylol; equivalent to 30 KIE (ml blood drawn)−1 (KIE is a kallikrein inactivator unit. Bayer Leverkusen, Germany)). Tubes were centrifuged within 30 min of sampling (2150 g, 15 min, 4°C). Plasma and serum for cytokine measurements were stored at −80°C until analysed by commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D systems, Minneapolis, MN, USA). All measurements were performed in duplicate. High sensitivity kits were used when available which was the case for IL-1β, IL-6 and TNFα. The ELISA used for measuring IL-6 and TNFα detects both soluble and receptor-bound cytokine. The detection limit (DL) and the intra-assay coefficients of variation (c.v.) for the ELISA measurements can be seen in Table 1.
Table 1.
Detection limit (DL) and intra-assay coefficient of variation (c.v.) for cytokine ELISA assays
| Assay | DL (pg ml−1) | c.v. (%) |
|---|---|---|
| IL-1β (HS) | < 0.1 | 7.8 |
| IL-1ra | < 14 | 4.9 |
| IL-6 (HS) | < 0.1 | 6.9 |
| IL-8 | < 10 | 5.8 |
| IL-15 | < 1 | 4.2 |
| MIP-1α | < 7 | 7.6 |
| MIP-1β | < 11 | 5.3 |
| TNFα (HS) | < 0.1 | 6.6 |
Correction for plasma volume shifts of runners
Changes in plasma volume were calculated from the measurements of haemoglobin and haematocrit according to the method described by Dill & Costill (1974), and cytokine measurements were corrected accordingly.
Statistics
Cytokine measurements were tested for effects of ‘time’ and ‘subject’ in a two-way analysis of variance (ANOVA). The model used was:
If the effect of ‘Time’ tested significant (P < 0.05) Tukey's test was used for comparison of the multiple measurements made during and after running with the pre-exercise value.
Before carrying out the statistical analysis the residuals in the ANOVA were examined for a normal distribution through investigation of a histogram and a normal-plot. If residuals were considered not to be normally distributed, data were log-transformed and residuals were investigated again. This was the case for IL-1ra and IL-6. After log-transformation, residuals were considered to be normally distributed and thus for these measurements log-transformed data were used in the subsequent statistical analysis. Statistical calculations were performed using SYSTAT 7.0 for Windows (SPSS Inc., Chicago, IL, USA). Data are plotted as the mean ±s.e.m.
RESULTS
The determination of oxygen uptake (VO2) for the subjects during running confirmed that the subjects ran at ∼75% of their individual VO2,max (average 74.3%, range 70–79%). A statistically significant difference was found among the different levels of ‘Time’ for the cytokines TNFα, IL-6 and IL-1ra (P < 0.001 for all), and for the muscle enzyme creatine kinase (P < 0.001).
IL-6
The mean plasma concentration of IL-6 was increased (4-fold) after only 30 min of running and continued to increase until the end of running (Fig. 1A). At this time IL-6 peaked and was 25-fold above the pre-exercise value. After running the level of IL-6 in plasma declined steadily, but after 6 h of rest it was still 6-fold higher than the pre-exercise value. All measurements of IL-6, except on day 2 and 5 after running, were significantly increased compared with the pre-exercise value (P < 0.001, Tukey's test).
Figure 1. Average of plasma cytokine concentrations for 10 male subjects measured before, every half-hour during 2.5 h of treadmill running and every hour in the 6 h resting period after running (means +s.e.m.).
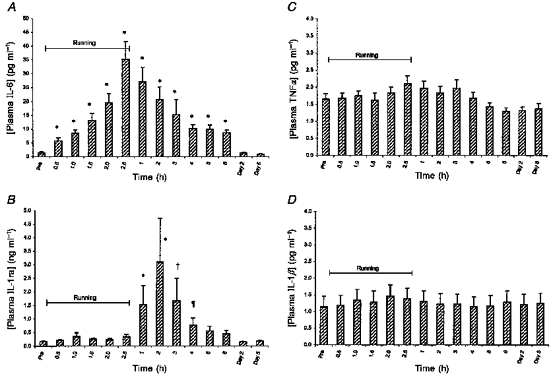
*, † and ¶: difference from pre-exercise value (P < 0.001, P < 0.01 and P < 0.05, respectively).
IL-1ra
The mean plasma IL-1ra concentration remained largely at the pre-exercise level during the 2.5 h of running (Fig. 1B). At 1 h post-running plasma IL-1ra had increased 9-fold and at 2 h post-exercise it peaked (18-fold increase). After 4 h of resting recovery the level had declined, now being 4-fold increased compared with the pre-exercise value. Only the four samples taken from 1 to 4 h post-exercise were significantly different from the pre-exercise sample (P < 0.001, P < 0.001, P < 0.01 and P < 0.05, respectively, Tukey's test).
TNFα
A minor elevation in mean plasma TNFα was seen towards the end of running, and the level remained approximately 25% increased until 3 h after the end of running (Fig. 1C). At 6 h post-running, and on day 2 and 5, TNFα was approximately 15% below the resting level. In the Tukey's multiple comparison, however, no differences were found between the pre-exercise sample and any of the later samples.
IL-1β
No changes were found in the mean plasma concentration of IL-1β during or after running (Fig. 1D).
IL-8, IL-15, MIP-1α and MIP-1β
These were measured in two of the subjects. The concentrations of IL-8 and MIP-1α were below the detection limits (31 and 23 pg ml−1, respectively) of the ELISA assays. The levels of IL-15 and MIP-1β were close to the lowest standard (3.9 and 31 pg ml−1, respectively) and no systematic variation was found.
Creatine kinase
The plasma creatine kinase (CK) activity was significantly increased after the 2.5 h of treadmill running compared with the pre-exercise value (P < 0.05, Tukey's test), and remained elevated until day 5 (Fig. 2). No significant difference was found between any of the samples taken from the end of running to day 5 (Tukey's test), but Fig. 2 suggests that the plasma CK activity was highest on day 2.
Figure 2. Average plasma creatine kinase of 10 male subjects measured before and after 2.5 h of treadmill running (means +s.e.m.).
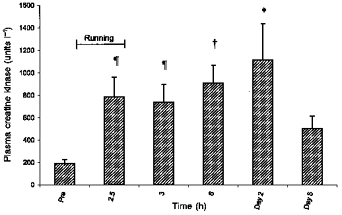
*, † and ¶: difference from pre-exercise value (P < 0.001, P < 0.01 and P < 0.05, respectively).
If the IL-6 response for each individual is plotted as in Fig. 3A, an apparent subdivision into two groups is revealed. To investigate this difference in response further, we chose to divide subjects into two groups: IL-6 high responders (IL6HR) and IL-6 low responders (IL6LR) based on whether their peak plasma IL-6 level was above or below 25 pg ml−1, respectively. Based on this criterion, the subjects were divided into six IL6HR and four IL6LR. When the IL-1ra data were separated into these two groups, it became apparent that the IL6HR also had high IL-1ra levels, whereas the IL6LR had only small changes in IL-1ra (Fig. 3B). The peak in IL-6 correlated with the peak in IL-1ra (Spearman correlation coefficient: rs = 0.79, P < 0.005). Furthermore the heart rate of the subjects during running was correlated to the peak in IL-6 (rs = 0.69, P < 0.05); thus the IL6HR all had a heart rate above the IL6LR during running (Fig. 3C). The high heart rate during running was in general not related to a faster pace (data not shown). There was no correlation between peak IL-6 levels and peak CK (data not shown). Plasma lactate at the end of running correlated to peak IL-6 (rs = 0.76, P < 0.01).
Figure 3. Division of subjects into two groups according to IL6HR and IL6LR criteria, i.e. peak plasma IL-6 above or below 25 pg ml−1, respectively.
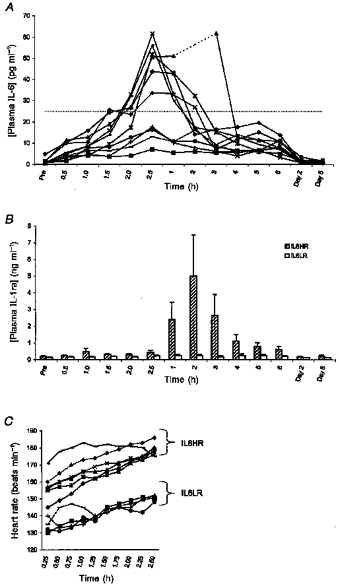
A, plasma IL-6 concentration of each subject measured before, during and after 2.5 h of treadmill running (n = 10). B, IL-1ra data arranged according to IL6HR and IL6LR criteria. C, heart rate for all subjects (n = 10) measured during running.
DISCUSSION
The main finding of this study is that an increase in plasma IL-6 can be detected after only 30 min of treadmill running. The plasma IL-6 concentration increased steadily during running and a steady decline was evident immediately after running. Experiments by Bruunsgaard et al. (1997) demonstrated an association between muscle damage and plasma IL-6 levels when comparing eccentric and concentric cycle exercise. In recent work from our group we found increased levels of IL-6 mRNA in muscle biopsies obtained from runners immediately after a marathon run (Ostrowski et al. 1998), which supports the hypothesis of IL-6 production being connected with muscle damage. Lieber et al. (1996) demonstrated that muscle damage was present already after 5 min of eccentric contractions. Taken together this evidence gives further support to the idea that the induction of IL-6 is associated with the initial disruption of muscle fibres.
Another main finding is that plasma IL-1ra remains at pre-exercise values until after the end of running, and then starts to increase. This is surprising, since IL-1ra is induced by IL-6 (Tilg et al. 1994; Jordan et al. 1995), and an increase in IL-1ra would be expected to follow immediately after the increase in IL-6. The peak IL-6 and IL-1ra responses were correlated, which is in accordance with the findings by Nehlsen-Cannarella et al. (1997).
For IL-1β and TNFα no differences were found between the pre-exercise sample and any of the later samples. This supports our hypothesis that the exercise-induced changes in plasma cytokines are similar to those seen after trauma.
It has been suggested that the changes in plasma IL-1β during or after exercise might be too transient to be detected (Sprenger et al. 1992; Ostrowski et al. 1998), but this option should be ruled out through the frequent sampling in the present study. However, since the ELISA assay used for the detection of IL-1β in the present study does not detect receptor-bound IL-1β, it is still possible that IL-1β is produced and then rapidly bound to soluble IL-1 receptors and therefore not detected. This possibility might explain the findings of IL-1β mRNA in skeletal muscle after strenuous exercise (Ostrowski et al. 1998) and IL-1β in the urine of runners after a 20 km run (Sprenger et al. 1992).
To understand the mechanisms underlying the cytokine response to exercise it is important to identify the cells that are producing IL-6 in the muscle and to understand the mechanisms leading to this production. Infusion of adrenaline has been shown to induce an increase in plasma IL-6 in rats (DeRijk et al. 1994). Other evidence, however, indicates that adrenaline is not the only important factor in the induction of IL-6. A study by Bruunsgaard et al. (1997) compared eccentric and concentric cycle exercise at identical plasma catecholamine levels, and found that IL-6 increased significantly only after eccentric exercise. This indicates that, although adrenaline may play some role in the induction of pro-inflammatory cytokines, there is also another factor (or factors) involved which is related to eccentric exercise.
The finding that the level of IL-6 correlated with heart rate needs to be addressed in a future study. It should be noticed, however, that heart rate was not related to running speed. From this it appears that training status is involved, i.e. well-trained runners having a low IL-6 response. IL-6 low responders were not in general subjects with a higher VO2,max, but the impression is supported by the fact that peak IL-6 correlated with plasma lactate.
In conclusion, the inflammatory response observed after exercise-induced muscle damage is similar to that seen after trauma. Thus, no changes were found in IL-1β and TNFα plasma levels, whereas marked increases in IL-6 and IL-1ra were found.
Acknowledgments
The excellent technical assistance of Ruth Rousing and Hanne Villumsen is acknowledged. This study was supported by TEAM DK, Idrættens Forskningsråd and The Danish National Research Foundation (504-14).
References
- Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Annals of Surgery. 1996;224:647–664. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Galbo H, Halkjaer KJ, Johansen TL, Maclean DA, Pedersen BK. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. The Journal of Physiology. 1997;499:833–841. doi: 10.1113/jphysiol.1997.sp021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell LM, Poortmans JR, Leclercq R, Brasseur M, Duchateau J, Newsholme EA. Some aspects of the acute phase response after a marathon race, and the effects of glutamine supplementation. European Journal of Applied Physiology and Occupational Physiology. 1997;75:47–53. doi: 10.1007/s004210050125. [DOI] [PubMed] [Google Scholar]
- DeRijk RH, Boelen A, Tilders FJ, Berkenbosch F. Induction of plasma interleukin-6 by circulating adrenaline in the rat. Psychoneuroendocrinology. 1994;19:155–163. doi: 10.1016/0306-4530(94)90005-1. 10.1016/0306-4530(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. Journal of Applied Physiology. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Drenth JP, Van Uum SH, Van Deuren M, Pesman GJ, Van Der Ven Jongekrijg J, Van Meer JW. Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-alpha and IL-1 beta production. Journal of Applied Physiology. 1995;79:1497–1503. doi: 10.1152/jappl.1995.79.5.1497. [DOI] [PubMed] [Google Scholar]
- Jordan M, Otterness IG, Ng R, Gessner A, Rollinghoff M, Beuscher HU. Neutralization of endogenous IL-6 suppresses induction of IL-1 receptor antagonist. Journal of Immunology. 1995;154:4081–4090. [PubMed] [Google Scholar]
- Lieber RL, Thornell LE, Friden J. Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. Journal of Applied Physiology. 1996;80:278–284. doi: 10.1152/jappl.1996.80.1.278. [DOI] [PubMed] [Google Scholar]
- Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Critical Care Medicine. 1997;25:1813–1819. doi: 10.1097/00003246-199711000-00018. 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- Mustafa MM, Ramilo O, Olsen KD, Franklin PS, Hansen EJ, Beutler B, McCracken GH., Jr Tumor necrosis factor in mediating experimental Haemophilus influenzae type B meningitis. Journal of Clinical Investigation. 1989;84:1253–1259. doi: 10.1172/JCI114292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Schmitt RL, Bailey EM, Warren BJ, Utter A, Davis JM. Carbohydrate and the cytokine response to 2.5 h of running. Journal of Applied Physiology. 1997;82:1662–1667. doi: 10.1152/jappl.1997.82.5.1662. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. The Journal of Physiology. 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumen RM, Hendriks T, Van Der Ven Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, Van Der Meer J, Goris RJ. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Annals of Surgery. 1993;218:769–776. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger H, Jacobs C, Nain M, Gressner AM, Prinz H, Wesemann W, Gemsa D. Enhanced release of cytokines, interleukin-2 receptors, and neopterin after long-distance running. Clinical Immunology and Immunopathology. 1992;63:188–195. doi: 10.1016/0090-1229(92)90012-d. 10.1016/0090-1229(92)90012-D. [DOI] [PubMed] [Google Scholar]
- Svoboda P, Kantorova I, Ochmann J. Dynamics of interleukin 1, 2, and 6 and tumor necrosis factor alpha in multiple trauma patients. Journal of Trauma. 1994;36:336–340. doi: 10.1097/00005373-199403000-00009. [DOI] [PubMed] [Google Scholar]
- Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- Waage A, Halstensen A, Shalaby R, Brandtzaeg P, Kierulf P, Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. Journal of Experimental Medicine. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]


