Abstract
Colour vision in the majority of humans is trichromatic, relying on a comparison of the quantal absorption in three different types of cone photoreceptors. The first steps in this comparison process take place at an early level of the visual system, in the retina. This topical review will highlight recent experiments which have advanced our understanding of how cone signals are compared to generate cone-opponent responses in the primate retina.
In trichromatic humans, and in Old World monkeys such as the macaque, the cone photoreceptors have peak spectral sensitivity in the short wavelength (S or ‘blue’), medium wavelength (M or ‘green’) and long wavelength (L or ‘red’) range of the visible spectrum (Schnapf et al. 1988; Bowmaker et al. 1991; Jacobs, 1996). The majority of retinal ganglion cells and thalamocortical relay cells in the lateral geniculate nucleus (LGN) show cone-opponent behaviour: they are excited by some wavelengths of light and inhibited by others (De Valois, 1965; Wiesel & Hubel, 1966; De Monasterio & Gouras, 1975; Derrington et al. 1984; Lee et al. 1988). Red-green cells draw their opponent input from mversus L cones, and blue-yellow cells draw their opponent input from S versus some combination of the m and L cones. Three major lines of study have in recent years given new insights into the origin and nature of cone opponency. First, the development of in vitro preparations of primate retina (Cohen & Miller, 1994; Dacey & Lee, 1994; Dacey et al. 1996) has clarified the response properties and morphological identity of cone-opponent cells. Second, analyses of the microcircuitry of the primate retina with light microscopic (Boycott & Wässle, 1991; Ahnelt & Kolb, 1994), immunocytochemical (Kouyama & Marshak, 1992; Grünert et al. 1994; Ghosh et al. 1997) and electron microscope-based methods (Kolb & Dekorver, 1991; Grünert & Ghosh, 1997; Hopkins & Boycott, 1997; Calkins et al. 1998) have delineated the intra-retinal pathway devoted to processing cone signals. Third, analytic and computational advances have breathed new life into extracellular single cell recording studies in the primate retina and LGN (Reid & Shapley, 1992; Smith et al. 1992; Benardete & Kaplan, 1997; Lankheet et al. 1998b; Lee et al. 1998). Here I will describe some of the major discoveries and new questions that have arisen from these lines of inquiry.
Shedding new light on cone opponency: ganglion cells
The majority of ganglion cells which project to the parvocellular layers of the LGN (the ‘midget’ cells; Polyak, 1941) have small dendritic fields, whereas the main projection to the magnocellular layers is from large-field, ‘parasol’ morphology cells (Leventhal et al. 1981; Perry et al. 1984; Rodieck & Watanabe, 1993). It was long assumed that both blue-yellow and red-green opponent cells would have midget morphology. However, this view has changed. Dacey & Lee (1994) reported results obtained from an intact (whole-mount) in vitro preparation of the macaque retina, which allows long-lasting intracellular recordings from ganglion cells. In this preparation the choroid is kept intact, and the receptors remain attached to the pigment epithelium, which allows the process of regeneration of bleached photopigment to continue. The retina is stimulated by coloured lights delivered through the microscope objective lens; the wavelength and amplitude of the light is arranged to modulate the S, m or L cones, either alone or in combination.
The intracellular recordings showed that the cone-opponent cells are of two morphological types. Red-green opponent cells had the expected midget morphology (Fig. 1A and B), but one subgroup of blue-yellow opponent cells (‘blue-on’) turned out to be the recently discovered small-field bistratified (SBS) cells (Fig. 1D and E). The SBS ganglion cells, like midget ganglion cells, project to the LGN but occur at a much lower density than midget cells (Dacey, 1993; Rodieck & Watanabe, 1993). The SBS cells are the only ganglion cells recorded so far which show significant input from S cones (Fig. 1E). Curiously, none of the ganglion cells had blue-off receptive fields, although this type has been consistently identified (albeit much less frequently than blue-on cells) in extracellular recording studies (De Monasterio & Gouras, 1975; Derrington et al. 1984; Valberg et al. 1986).
Figure 1. Anatomy and physiology of opponent and non-opponent pathways in the primate retina.
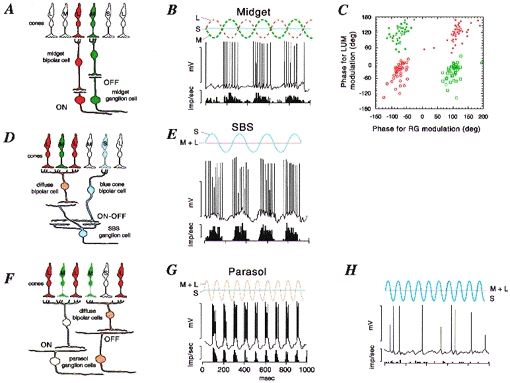
A, midget pathway. Schematic view of a vertical section through the parafoveal retina. Midget bipolar cells contact a single cone photoreceptor and provide excitatory input to midget ganglion cells. Each L and m cone makes contact with both an on-centre and an off-centre midget bipolar cell; only one example of each is drawn for clarity. B, response of a ‘green-on’ midget cell. The intracellular response and averaged spike discharge rate are shown below a schematic representation of the cone modulation due to the stimulus (red-green modulation). The cell responds to increases in m cone activation but is inhibited by L cones. C, 4 subclasses of red-green opponent cells can be distinguished on the basis of their response to achromatic (LUM) or red-green chromatic (RG) modulation at 4 Hz. Open red circles, red-on; filled red circles, red-off; open green squares, green-on; filled green squares, green-off. The phase of cell response is shown relative to the phase of the stimulus; negative phase representing increasing response lag relative to the stimulus. D and E, the SBS (blue-on) pathway. SBS cells receive excitatory (on) input from the blue cone-contacting bipolar cell, and excitatory (off) input from an off-centre diffuse bipolar cell. They respond vigorously to S cone modulation. F, parasol cells receive excitatory input from diffuse (on- or off-centre) bipolar cells, which contact both L and m cones and hence are non-opponent. The response of an off- parasol cell is shown in G. This cell responds to combined modulation of L and m cones, but is unaffected by a stimulus (shown in H) which modulates selectively the S cones. Panels B, E, G and H are modified from Dacey & Lee (1994). Panel C is modified from Lankheet et al. (1998). Calibration values for B, E, G and H are 50 mV and 400 impulses s−1.
The intracellular recordings from retina, and extracellular studies from macaque LGN (Wiesel & Hubel, 1966; Lee et al. 1987; Lankheet et al. 1998a) agree that four different functional types of red-green opponent ganglion cells can be distinguished. They all show some degree of red-green opponency, and differ in their excitatory synaptic drive (either from m or L cones), and by their response phase to luminance modulation (either on- or off- type response; Fig. 1C). In accord with the basic bauplan of the vertebrate retina, the dendrites of off-centre cells are located in the outer half of the inner plexiform layer (IPL), and the dendrites of on-centre cells are located in the inner half of the IPL. No morphological distinction between L or m centre cells can be made (Dacey & Lee, 1994). Parasol cells also come in on- and off- varieties (Fig. 1F). They respond best to achromatic stimuli presented at high temporal frequencies, but show little or no sign of M-L cone opponency, or of functional input from S cones (Fig. 1G and H). This basic anatomical and functional distinction of ‘S cone pathways’ and ‘M-L cone pathways’ is already present at the level of bipolar cells, as shown below.
Bipolar inputs to opponent cells: specificity and evolution
More than ten different morphological subtypes of bipolar cell have been distinguished in the primate retina (Polyak, 1941; Boycott & Dowling, 1969; Rodieck, 1988; Boycott & Wässle, 1991; Grünert et al. 1994). Midget bipolar cells connect individual L and m cones to midget ganglion cells (Polyak, 1941; Boycott & Dowling, 1969; Kolb & Dekorver, 1991; Calkins et al. 1994; Wässle et al. 1994). A different bipolar subtype, called the blue cone bipolar cell (Mariani, 1984) makes specific contact with S cones (Kouyama & Marshak, 1992; Wässle et al. 1994). The axon terminal system of the blue cone bipolar cell is costratified with, and makes synaptic contact with, the inner layer of dendritic terminals of the blue-on cells (Kouyama & Marshak, 1992; Dacey & Lee, 1994; Calkins et al. 1998). The same S cone pathway can also be defined in a New World primate, the marmoset (Ghosh et al. 1997; Grünert & Ghosh, 1997). This species shows a high proportion of dichromatic (red-green colour blind) individuals (Travis et al. 1988; Yeh et al. 1995). The S cone pathway is identical in dichromatic and trichromatic marmosets (Ghosh et al. 1997) suggesting that, in an evolutionary sense, it predated the development of trichromatic primate vision.
Horizontal cell connectivity and opponent responses
Horizontal cells are likely candidates to provide the inhibitory, opponent surround to bipolar cells and ganglion cells (Mangel, 1991; Wässle & Boycott, 1991), but their role in creating opponent responses has long been controversial. Horizontal cells in primate retina do show some chromatic selectivity, but this selectivity is different to that seen in lower vertebrates (Dacheux & Raviola, 1990; Kamermans & Spekreijse, 1995; Dacey et al. 1996). The two morphologically distinct horizontal cell classes, H1 and H2 (Kolb et al. 1980; Boycott et al. 1987; Wässle et al. 1989a) receive input from L and m cones, but have distinct patterns of input from S cones (Ahnelt & Kolb, 1994; Dacey et al. 1996; Goodchild et al. 1996). The H1 cells are very insensitive to S cone modulation and are only sparsely connected to S cones, but H2 cells always make substantial contact with, and receive depolarizing input from S cones as well as m and L cones (Fig. 2A, B and E). Furthermore, the same pattern of connectivity of the homologous H1 and H2 subtypes (Fig. 2E) is present in both dichromatic and trichromatic primates (Chan & Grünert, 1998) showing that the segregation of S cone signals to H2 horizontal cells is a basic feature of the organization of the primate retina.
Figure 2. Connectivity and responses of horizontal cells.
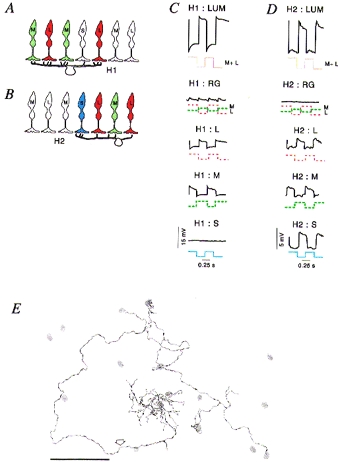
A and B, schematic views of vertical sections through primate retina showing the connection patterns of the dendrites of H1 and H2 horizontal cells. The H1 cells (A) make only very sparse contact with S cones within their dendritic field, but the H2 cells (B) contact all cone types, and make stronger connections with S cones. C and D, response of H1 and H2 cells in macaque retina, respectively. Each graph shows intracellular recordings of a single H1 or H2 cell to temporal square wave modulation of L, m or S cones types. Only the H2 cell shows measurable input from S cones. E, camera lucida drawing of an H2 cell in marmoset retina, showing the position of S cones (grey areas) within the dendritic and axonal field of the cell. The dendrites of the H2 cell make substantial contact with two S cones within the field. The axon (arrow) also makes contact with S cones which lie along its path. Panels C and D are modified from Dacey et al. (1996); panel E is modified from Chan & Grünert (1998). Scale bar in E, 50 μm.
The provenance of red-green opponency: could the midget system do the job?
A potential substrate for red-green opponent responses can be identified in the precise one-to-one connectivity of the fovea - a retinal specialization which (like red-green colour vision) is unique to primates among the mammals (Schein, 1988; Wässle et al. 1989b; Martin & Grünert, 1992; Calkins et al. 1994). This idea is encapsulated as the ‘random wiring’ hypothesis, where each midget ganglion cell in the fovea gets excitatory input (either on- or off-) to its receptive field centre from a single cone via a midget bipolar cell, and antagonistic input from a small number of neighbouring cones via H1 horizontal cells (Paulus & Kröger-Paulus, 1983; Shapley & Perry, 1986; Young & Marrocco, 1989; Lennie et al. 1991; De Valois & De Valois, 1993). Since the H1 cells get input from both L and m cones, their wavelength of peak spectral sensitivity (Fig. 3A) lies between that of m and L cones alone (Dacheux & Raviola, 1990; Lennie et al. 1991; Dacey et al. 1996). It should not really matter if the H1 cell-mediated inhibition is ‘mixed’ from m and L cones, because the opponent cone still contributes to the inhibitory mixture. But the attractiveness of an hypothesized state of affairs should not be confused with evidence for its existence, and recent electrophysiological studies have asked whether the behaviour of red-green opponent ganglion cells and horizontal cells really is consistent with the random wiring scheme.
Figure 3.
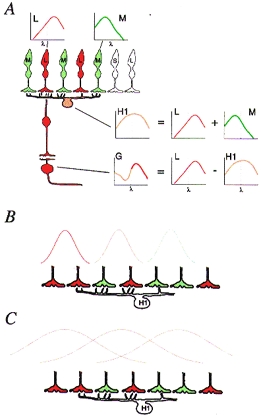
A, schematic view of the retina showing the key features of the ‘random wiring’ hypothesis for generation of spectral opponency. The small graphs show spectral sensitivity for the cone photoreceptors (L and M), an H1 horizontal cell and a midget ganglion cell (G). The H cell spectral sensitivity is a combination of m and L cones, and hence is broader than that of either cone alone. The G cell spectral response is the difference between the single cone response and the horizontal cell response. B and C, schematic representations of horizontal cell inputs to bipolar cell receptive fields. The hypothetical curves represent the relative efficacy of stimulation at progressively greater distances from reference points at different positions in the dendritic field of the horizontal cell. The curves in B and C have different space constants. If the space constant is small (B), the strongest effect at a given point of the horizontal cell will be due to activity in a small number of cones. This would lead to variation in spectral weighting of the inhibition for each local domain within the H cell field, according to the local distribution of m and L cones. If the space constant is larger (C) the spectral weighting would be closer to the average of the m and L cones.
The nature of the cone inputs to red-green opponent cells can be explored quantitatively with linear systems analysis. The pioneering studies of Gielen et al. (1982) and Derrington et al. (1984) first showed that opponent cell responses can be well predicted by linear combination of the responses of L and m cones. If the inhibitory surround of red-green opponent cells is combined from m and L cones (Fig. 3A), then the action of both cone types should be measurable for stimuli which activate the surround, or generate centre-surround interaction. One way to produce centre-surround interaction is to stimulate with spatially uniform, rapidly modulated (flickering) light. The distinct temporal properties of the centre and surround (the surround is ‘slower’) mean that centre and surround become synergistic at high temporal frequencies (Gouras & Zrenner, 1979; Frishman et al. 1987; Lee et al. 1989). A second way to search for the action of the surround is to use spatially discrete stimuli (such as gratings, bars or edges) to elicit centre-surround spatial antagonism (Lee et al. 1998).
Smith et al. (1992) and Lankheet et al. (1998b) recorded from ganglion cells in macaque retina and relay cells in macaque LGN, using flickering stimuli to modulate specifically the m and L cones. Both studies found that the behaviour of many of the red-green opponent cells was consistent with input from both m and L cones to the receptive field surround (random wiring), but Smith et al. (1992) also found some cells which were better accounted for by a selective surround model where, say, an L centre cell has only m cones in the surround. More recently, Lee et al. (1998) used a discrete spatial stimulus (a bipartite field with a straight edge) and likewise found many red-green opponent ganglion cells where the inhibition was opponent and specific, again supporting a ‘selective surround’ model. This result was similar to one obtained previously using the reverse correlation technique (Reid & Shapley, 1992), but the receptive field sizes reported by Lee et al. (1998) are much closer to other reports from the literature (De Monasterio & Gouras, 1975; Derrington & Lennie, 1984; Crook et al. 1988).
Where does this leave the random wiring model? On one hand, the above studies show that some red-green opponent cells have pure cone inputs to the surround, as proposed over 30 years ago (De Valois et al. 1966; Wiesel & Hubel, 1966). On the other hand, in the studies of both Lankheet et al. (1998b) and Lee et al. (1998), the responses of most cells were always compatible with the presence of at least a weak input from the ‘wrong’ cone type in the surround. In other words, even if some cells do have specific surrounds, they need not be specific in order to produce a perfectly respectable degree of red-green opponency. So the critical issue returns to the question of whether horizontal cells could provide a surround which is sometimes specific, and sometimes not.
Chromatic processing in horizontal cells: a beautiful day in the neighbourhood?
Horizontal cells are coupled together by gap junctions to form a functional network. When they have been measured in non-primate mammals, the receptive fields of horizontal cells have been much larger than their anatomically measured dendritic fields (Nelson, 1977; Dacheux & Raviola, 1982; Bloomfield et al. 1995). If a similar state of affairs holds in the primate retina, then the surround of foveal midget cells would be at least 1 deg in diameter, which is over 20 times larger than contemporary estimates of the surround diameter of red-green opponent cells (Derrington & Lennie, 1984; Croner & Kaplan, 1995; Lee et al. 1998). However, there may be a way out of this dilemma. From the point of view of a midget bipolar cell, the important feature of the receptive field of the horizontal cell is not its total diameter as measured at the soma for high contrast stimuli, but the efficacy with which changes in the horizontal cell membrane potential are translated to changes in bipolar cell membrane potential. This will determine the extent to which any one cone will influence bipolar cells in its neighbourhood, and may well be subject to non-linear effects such as thresholds and/or shunting inhibition (Smith, 1995).
If this ‘effective space constant’ in horizontal cells is small (Fig. 3B), then the inhibitory input to a bipolar cell will be dominated by the cones within its immediate neighbourhood. By contrast, if the effective space constant of the cell is large (Fig. 3C), then the inhibitory effect of the horizontal cell will reflect the average of a larger number of cones, both in the spatial and in the chromatic domains. Now, the chromatic effect will obviously depend on the local distribution of m and L cones. If, as can be assumed from psychophysical and neurochemical evidence, the m and L cones are randomly arranged (Marc & Sperling, 1977; Roorda & Williams, 1998), then some of the surrounds will be predominantly due to m cones, some due to L cones, and most surrounds will have a mixture of the two cone types (Paulus & Kröger-Paulus, 1983; Lennie et al. 1991). This is substantially compatible with the results from ganglion cell recordings. In fact, a small space constant for horizontal cells is compatible with other data from the primate literature. Firstly, it would account for the variability in spectral weighting for the surrounds of both midget and parasol cells (Lankheet et al. 1998b; Lee et al. 1998). Secondly, it is consistent with surround space constants reported from the literature for red-green opponent cells (Derrington & Lennie, 1984; Lennie et al. 1991; Croner & Kaplan, 1995; Lee et al. 1998). Thirdly, it is consistent with the recent finding that there is relative independence of adaptation of m and L cone mechanisms in horizontal cells (Lee et al. 1997), under the assumption that each cone would have only a small effect (via feedback) on its neighbours. Fourthly, it is consistent with the fact that the anatomically demonstrated sparse S cone input to H1 cells (Goodchild et al. 1996; Chan & Grünert, 1998) is not functionally detectable at the horizontal cell soma (Dacey et al. 1996). The receptive field diameter of primate bipolar cells has not been measured, but such measurements could be made using the in vitro retinal preparation, which is bound to become the method of choice for addressing these kinds of functional questions.
A unified view of subcortical chromatic mechanisms, and some unanswered questions
A scheme for the basis of chromatic signal transfer in the primate subcortical visual pathway can be summarized as follows. The midget ganglion cells, whose general function is to carry high spatial resolution signals at high contrast levels in the photopic range, also carry a red-green opponent signal for central vision in trichromatic primates. Since both excitatory and inhibitory inputs to midget cells draw from a larger number of cones outside the fovea, the quality of the red-green opponent signal would be expected to decline with increasing visual angle. The small bistratified cells carry a blue-yellow opponent signal. The midget ganglion cell and small bistratified (blue-on) ganglion cell types have been identified in all diurnal primates studied so far (Ghosh et al. 1996, 1997; Yamada et al. 1996), so these pathways are presumed to be common to (red-green colour blind) dichromatic and trichromatic primates. The red-green and blue-yellow opponent signals travel through distinct subdivisions of the lateral geniculate nucleus to the primary visual cortex (Martin et al. 1997). The main attraction of this scheme is that it is compatible with known anatomical and physiological properties of the subcortical visual system in both dichromatic and trichromatic primates, but there remain unanswered questions, some of which are summarized below.
The random wiring hypothesis predicts that all the red-green opponent cells should have type I centre- surround receptive field structure, rather than type II overlapping centre and surround. In retrospect, it is obvious from the literature that the clear majority of type II cells is blue-on (De Valois et al. 1966; Wiesel & Hubel, 1966; Dreher et al. 1976; Derrington et al. 1984) and the existence of red-green opponent type II cells was questioned from the beginning (Wiesel & Hubel, 1966, p. 1128; Derrington et al. 1984). That amacrine cells could be mediators of specific red-green opponent interactions is now also thought to be unlikely (Calkins & Sterling, 1996), but there is still, quite reasonably, reluctance to give up any search for potential type II red-green circuitry in the retina (Rodieck, 1991; Calkins & Sterling, 1996). The random wiring hypothesis also predicts that some foveal midget cells would have surrounds with the same cone type as the centre cone. Such cells would be only a small proportion of midget cells and, because their action spectrum would be the same as that of either the m or L cone, would fall into one of the clusters shown in Fig. 1C rather than performing like parasol cells (which sum m and L cones). A parametric study of a large number of midget cells might reveal such a population, and give a better understanding of how the responses underlying red-green opponency change with retinal eccentricity. Finally, it could be argued that the largest gap in our knowledge of colour processing mechanisms in the primate retina is the morphological correlate of the elusive blue-off cell. It is impressive to look back at the progress which has been made since 1966, and encouraging to realise that this will remain an active field of study into the next millennium.
Acknowledgments
I thank U. Grünert, J. Kremers, B. Lee and R. Smith for discussion and many helpful comments on the manuscript; also T. Chan, N. Gilroy, A. White and A. Lara for help with illustrations and preparation of the manuscript.
References
- Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in human retina: A Golgi-electron microscopic study of spectral connectivity. Journal of Comparative Neurology. 1994;343:406–427. doi: 10.1002/cne.903430306. [DOI] [PubMed] [Google Scholar]
- Benardete EA, Kaplan E. The receptive field of the primate P retinal ganglion cell, II: Nonlinear dynamics. Visual Neuroscience. 1997;14:187–205. doi: 10.1017/s0952523800008865. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, Persky SE. A comparison of receptive field and tracer coupling size of horizontal cells in the rabbit retina. Visual Neuroscience. 1995;12:985–999. doi: 10.1017/s0952523800009524. [DOI] [PubMed] [Google Scholar]
- Bowmaker JK, Anstell S, Hunt DM, Mollon JD. Photosensitive and photostable pigments in the retinae of Old World monkeys. Journal of Experimental Biology. 1991;156:1–19. doi: 10.1242/jeb.156.1.1. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Dowling JE. Organization of the primate retina: light microscopy. Philosophical Transactions of the Royal Society B. 1969;255:109–176. [Google Scholar]
- Boycott BB, Hopkins JM, Sperling HG. Cone connections of the horizontal cells of the rhesus monkey's retina. Proceedings of the Royal Society B. 1987;229:345–379. doi: 10.1098/rspb.1987.0001. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. Morphological classification of bipolar cells of the primate retina. European Journal of Neuroscience. 1991;3:1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Schein SJ, Tsukamoto Y, Sterling P. M and L cones in macaque fovea connect to midget ganglion cells by different numbers of excitatory synapses. Nature. 1994;371:70–72. doi: 10.1038/371070a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Sterling P. Absence of spectrally specific lateral inputs to midget ganglion cells in primate retina. Nature. 1996;381:613–615. doi: 10.1038/381613a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Tsukamoto Y, Sterling P. Microcircuitry and mosaic of a blue-yellow ganglion cell in the primate retina. Journal of Neuroscience. 1998;18:3373–3385. doi: 10.1523/JNEUROSCI.18-09-03373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TL, Grünert U. Horizontal cell connections with short wavelength-sensitive cones in the retina: a comparison between New World and Old World primates. Journal of Comparative Neurology. 1998;393:196–209. [PubMed] [Google Scholar]
- Cohen ED, Miller RF. The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Visual Neuroscience. 1994;11:317–332. doi: 10.1017/s0952523800001668. [DOI] [PubMed] [Google Scholar]
- Croner LJ, Kaplan E. Receptive fields of P and M ganglion cells across the primate retina. Vision Research. 1995;35:7–24. doi: 10.1016/0042-6989(94)e0066-t. [DOI] [PubMed] [Google Scholar]
- Crook JM, Lange-Malecki B, Lee BB, Valberg A. Visual resolution of macaque retinal ganglion cells. The Journal of Physiology. 1988;396:205–224. doi: 10.1113/jphysiol.1988.sp016959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. Morphology of a small-field bistratified ganglion cell type in the macaque and human retina. Visual Neuroscience. 1993;10:1081–1098. doi: 10.1017/s0952523800010191. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB, Stafford DK, Pokorny J, Smith VC. Horizontal cells of the primate retina: cone specificity without spectral opponency. Science. 1996;271:656–659. doi: 10.1126/science.271.5249.656. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. Horizontal cells in the retina of the rabbit. Journal of Neuroscience. 1982;2:1486–1493. doi: 10.1523/JNEUROSCI.02-10-01486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. Physiology of H1 horizontal cells in the primate retina. Proceedings of the Royal Society B. 1990;239:213–230. doi: 10.1098/rspb.1990.0014. [DOI] [PubMed] [Google Scholar]
- De Monasterio FM, Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. The Journal of Physiology. 1975;251:167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. The Journal of Physiology. 1984;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. The Journal of Physiology. 1984;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois RL. Analysis and coding of color vision in the primate visual system. Cold Spring Harbor Symposia on Quantitative Biology. 1965;30:567–579. doi: 10.1101/sqb.1965.030.01.055. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Abramov I, Jacobs GH. Analysis of response patterns of LGN cells. Journal of the Optical Society of America. 1966;56:966–977. doi: 10.1364/josa.56.000966. [DOI] [PubMed] [Google Scholar]
- De Valois RL, De Valois KK. A multi-stage color model. Vision Research. 1993;33:1053–1065. doi: 10.1016/0042-6989(93)90240-w. 10.1016/0042-6989(93)90240-W. [DOI] [PubMed] [Google Scholar]
- Dreher B, Fukada Y, Rodieck RW. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of Old-World primates. The Journal of Physiology. 1976;258:433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman LJ, Freeman AW, Troy JB, Schweitzer-Tong DE, Enroth-Cugell C. Spatiotemporal frequency responses of cat retinal ganglion cells. Journal of General Physiology. 1987;89:599–628. doi: 10.1085/jgp.89.4.599. 10.1085/jgp.89.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Goodchild AK, Sefton AE, Martin PR. Morphology of retinal ganglion cells in a New World monkey, the marmoset Callithrix jacchus. Journal of Comparative Neurology. 1996;366:76–92. doi: 10.1002/(SICI)1096-9861(19960226)366:1<76::AID-CNE6>3.0.CO;2-H. 10.1002/(SICI)1096-9861(19960226)366:1<76::AID-CNE6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Martin PR, Grünert U. Morphological analysis of the blue cone pathway in the retina of a New World monkey, the marmoset Callithrix jacchus. Journal of Comparative Neurology. 1997;379:211–225. 10.1002/(SICI)1096-9861(19970310)379:2<211::AID-CNE4>3.3.CO;2-V. [PubMed] [Google Scholar]
- Gielen CCAM, van Gisbergen JAM, Vendrik AJH. Reconstruction of cone-system contributions to responses of colour-opponent neurones in monkey lateral geniculate. Biological Cybernetics. 1982;44:211–221. doi: 10.1007/BF00344277. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Chan TL, Grünert U. Horizontal cell connections with short wavelength sensitive cones in macaque monkey retina. Visual Neuroscience. 1996;13:833–845. doi: 10.1017/s0952523800009093. [DOI] [PubMed] [Google Scholar]
- Gouras P, Zrenner E. Enhancement of luminance flicker by color-opponent mechanisms. Science. 1979;205:587–589. doi: 10.1126/science.109925. [DOI] [PubMed] [Google Scholar]
- Grünert U, Ghosh KK. Synaptic input to the small bistratified cell in a new world primate. Investigative Ophthalmology and Visual Science. 1997;38:S708. (ARVO abstracts) [Google Scholar]
- Grünert U, Martin PR, Wässle H. Immunocytochemical analysis of bipolar cells in the macaque monkey retina. Journal of Comparative Neurology. 1994;348:607–627. doi: 10.1002/cne.903480410. [DOI] [PubMed] [Google Scholar]
- Hopkins JM, Boycott BB. The cone synapses of cone bipolar cells of primate retina. Journal of Neurocytology. 1997;26:313–325. doi: 10.1023/a:1018504718282. 10.1023/A:1018504718282. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Primate photopigments and primate color vision. Proceedings of the National Academy of Sciences of the USA. 1996;93:577–581. doi: 10.1073/pnas.93.2.577. 10.1073/pnas.93.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M, Spekreijse H. Spectral behavior of cone-driven horizontal cells in teleost retina. Progress in Retinal and Eye Research. 1995;14:313–360. 10.1016/1350-9462(94)00003-2. [Google Scholar]
- Kolb H, Dekorver L. Midget ganglion cells of the parafovea of the human retina: A study by electron microscopy and serial section reconstructions. Journal of Comparative Neurology. 1991;303:617–636. doi: 10.1002/cne.903030408. [DOI] [PubMed] [Google Scholar]
- Kolb H, Mariani A, Gallego A. A second type of horizontal cell in the monkey retina. Journal of Comparative Neurology. 1980;189:31–44. doi: 10.1002/cne.901890103. [DOI] [PubMed] [Google Scholar]
- Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. Journal of Neuroscience. 1992;12:1233–1252. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankheet MJM, Lennie P, Krauskopf J. Distinctive characteristics of subclasses of red-green P-cells in LGN of macaque. Visual Neuroscience. 1998a;15:37–46. doi: 10.1017/s0952523898151027. 10.1017/S0952523898151027. [DOI] [PubMed] [Google Scholar]
- Lankheet MJM, Lennie P, Krauskopf J. Temporal-chromatic interactions in LGN P-cells. Visual Neuroscience. 1998b;15:47–54. doi: 10.1017/s0952523898151015. 10.1017/S0952523898151015. [DOI] [PubMed] [Google Scholar]
- Lee BB, Dacey DM, Smith VC, Pokorny J. Time course and cone specificity of adaptation in primate outer retina. Investigative Ophthalmology and Visual Science. 1997;38:1163. (ARVO abstracts) [Google Scholar]
- Lee BB, Kremers J, Yeh T. Receptive fields of primate retinal ganglion cells studied with a novel technique. Visual Neuroscience. 1998;15:161–175. doi: 10.1017/s095252389815112x. 10.1017/S095252389815112X. [DOI] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. The physiological basis of heterochromatic flicker photometry demonstrated in the ganglion cells of the macaque retina. The Journal of Physiology. 1988;404:323–347. doi: 10.1113/jphysiol.1988.sp017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. Amplitude and phase of responses of macaque retinal ganglion cells to flickering stimuli. The Journal of Physiology. 1989;414:245–263. doi: 10.1113/jphysiol.1989.sp017686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Valberg A, Tigwell DA, Tryti J. An account of responses of spectrally opponent neurons in macaque lateral geniculate nucleus to successive contrast. Proceedings of the Royal Society B. 1987;230:293–314. doi: 10.1098/rspb.1987.0021. [DOI] [PubMed] [Google Scholar]
- Lennie P, Haake PW, Williams DR. The design of chromatically opponent receptive fields. In: Landy MS, Movshon JA, editors. Computational Models of Visual Processing. Cambridge, MA, USA: MIT Press; 1991. pp. 71–82. [Google Scholar]
- Leventhal AG, Rodieck RW, Dreher B. Retinal ganglion cell classes in the Old World monkey: morphology and central projections. Science. 1981;213:1139–1142. doi: 10.1126/science.7268423. [DOI] [PubMed] [Google Scholar]
- Mangel SC. Analysis of the horizontal cell contribution to the receptive field surround of ganglion cells in the rabbit retina. The Journal of Physiology. 1991;442:211–234. doi: 10.1113/jphysiol.1991.sp018790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Sperling HG. Chromatic organization of primate cones. Science. 1977;196:454–456. doi: 10.1126/science.403607. [DOI] [PubMed] [Google Scholar]
- Mariani AP. Bipolar cells in monkey retina selective for the cones likely to be blue-sensitive. Nature. 1984;308:184–186. doi: 10.1038/308184a0. [DOI] [PubMed] [Google Scholar]
- Martin PR, Grünert U. Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. Journal of Comparative Neurology. 1992;323:269–287. doi: 10.1002/cne.903230210. [DOI] [PubMed] [Google Scholar]
- Martin PR, White AJR, Goodchild AK, Wilder HD, Sefton AE. Evidence that blue-on cells are part of the third geniculocortical pathway in primates. European Journal of Neuroscience. 1997;9:1536–1541. doi: 10.1111/j.1460-9568.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Nelson R. Cat cones have rod input: A comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. Journal of Comparative Neurology. 1977;172:109–136. doi: 10.1002/cne.901720106. [DOI] [PubMed] [Google Scholar]
- Paulus W, Kröger-Paulus A. A new concept of retinal colour coding. Vision Research. 1983;23:529–540. doi: 10.1016/0042-6989(83)90128-1. 10.1016/0042-6989(83)90128-1. [DOI] [PubMed] [Google Scholar]
- Perry VH, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12:1101–1123. doi: 10.1016/0306-4522(84)90006-x. 10.1016/0306-4522(84)90006-X. [DOI] [PubMed] [Google Scholar]
- Polyak SL. The Retina. Chicago: University of Chicago Press; 1941. [Google Scholar]
- Reid RC, Shapley RM. Spatial structure of cone inputs to receptive fields in primate lateral geniculate nucleus. Nature. 1992;356:716–718. doi: 10.1038/356716a0. 10.1038/356716a0. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. The primate retina. In: Steklis HD, Erwin J, editors. Comparative Primate Biology, Neurosciences. Vol. 4. New York: Alan R. Liss; 1988. pp. 203–278. [Google Scholar]
- Rodieck RW. Which cells code for color? In: Valberg A, Lee BB, editors. From Pigments to Perception: Advances in Understanding Visual Processes. London: Plenum Press; 1991. pp. 83–93. [Google Scholar]
- Rodieck RW, Watanabe M. Survey of the morphology of macaque retinal ganglion cells that project to the pretectum, superior colliculus, and parvicellular laminae of the lateral geniculate nucleus. Journal of Comparative Neurology. 1993;338:289–303. doi: 10.1002/cne.903380211. [DOI] [PubMed] [Google Scholar]
- Roorda A, Williams DR. Objective identification of M and L cones in the living human eye. Investigative Ophthalmology and Visual Science. 1998;39:204. (ARVO abstracts) [Google Scholar]
- Schein SJ. Anatomy of macaque fovea and spatial densities of neurons in foveal representation. Journal of Comparative Neurology. 1988;269:479–505. doi: 10.1002/cne.902690403. [DOI] [PubMed] [Google Scholar]
- Schnapf JL, Kraft TW, Nunn BJ, Baylor DA. Spectral sensitivity of primate photoreceptors. Visual Neuroscience. 1988;1:255–261. doi: 10.1017/s0952523800001917. [DOI] [PubMed] [Google Scholar]
- Shapley R, Perry VH. Cat and monkey retinal ganglion cells and their visual functional roles. Trends in Neurosciences. 1986;9:229–235. 10.1016/0166-2236(86)90064-0. [Google Scholar]
- Smith RG. Simulation of an anatomically defined local circuit: the cone-horizontal cell network in cat retina. Visual Neuroscience. 1995;12:545–561. doi: 10.1017/s0952523800008440. [DOI] [PubMed] [Google Scholar]
- Smith VC, Lee BB, Pokorny J, Martin PR, Valberg A. Responses of macaque ganglion cells to the relative phase of heterochromatically modulated lights. The Journal of Physiology. 1992;458:191–221. doi: 10.1113/jphysiol.1992.sp019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis DS, Bowmaker JK, Mollon JD. Polymorphism of visual pigments in a callitrichid monkey. Vision Research. 1988;28:481–490. doi: 10.1016/0042-6989(88)90170-8. 10.1016/0042-6989(88)90170-8. [DOI] [PubMed] [Google Scholar]
- Valberg A, Lee BB, Tigwell DA. Neurones with strong inhibitory s-cone inputs in the macaque lateral geniculate nucleus. Vision Research. 1986;26:1061–1064. doi: 10.1016/0042-6989(86)90040-4. 10.1016/0042-6989(86)90040-4. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiological Reviews. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB, Röhrenbeck J. Horizontal cells in the monkey retina: Cone connections and dendritic network. European Journal of Neuroscience. 1989a;1:421–435. doi: 10.1111/j.1460-9568.1989.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Martin PR, Boycott BB. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vision Research. 1994;34:561–579. doi: 10.1016/0042-6989(94)90013-2. 10.1016/0042-6989(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Röhrenbeck J, Boycott BB. Cortical magnification factor and the ganglion cell density of the primate retina. Nature. 1989b;341:643–646. doi: 10.1038/341643a0. 10.1038/341643a0. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. Journal of Neurophysiology. 1966;29:1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Yamada ES, Silveira LCL, Gomes FL, Lee BB. The retinal ganglion cell classes of New World primates. Revista Brasileira de Biologia. 1996;56:381–396. [PubMed] [Google Scholar]
- Yeh T, Lee BB, Kremers J, Cowing JA, Hunt DM, Martin PR, Troy JB. Visual responses in the lateral geniculate nucleus of dichromatic and trichromatic marmosets (Callithrix jacchus) Journal of Neuroscience. 1995;15:7892–7904. doi: 10.1523/JNEUROSCI.15-12-07892.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RA, Marrocco RT. Predictions about chromatic opponent receptive fields assuming random cone connections. Journal of Theoretical Biology. 1989;141:23–40. doi: 10.1016/s0022-5193(89)80005-0. [DOI] [PubMed] [Google Scholar]


