Abstract
Exercise reduces splanchnic blood flow, but the mesenteric contribution to this response is uncertain.
In nineteen humans, superior mesenteric and coeliac artery flows were determined by duplex ultrasonography during fasting and postprandial submaximal cycling and compared with the splanchnic blood flow as assessed by the Indocyanine Green dye-elimination technique.
Cycling increased arterial pressure, heart rate and cardiac output, while it reduced total vascular resistance. These responses were not altered in the postprandial state. During fasting, cycling increased mesenteric, coeliac and splanchnic resistances by 76, 165 and 126%, respectively, and it reduced corresponding blood flows by 32, 50 and 43% (by 0.18 ± 0.04, 0.42 ± 0.03 and 0.60 ± 0.04 l min−1). Postprandially, mesenteric and splanchnic vascular resistances decreased, thereby elevating regional blood flow, while the coeliac circulation was not influenced. Postprandial cycling did not influence the mesenteric resistance significantly, but its blood flow decreased by 22% (0.46 ± 0.28 l min−1). Coeliac and splanchnic resistance increased by 150 and 63%, respectively, and the corresponding regional blood flow decreased by 51 and 31% (0.49 ± 0.07 and 0.96 ± 0.28 l min−1). Splanchnic blood flow values assessed by duplex ultrasound and by dye-elimination techniques were correlated (r = 0.70; P < 0.01).
During submaximal exercise in humans, splanchnic resistance increases and blood flow is reduced following a 50% reduction in the hepato-splenic and a 25% reduction in the mesenteric blood flow.
In humans subjected to intense exercise, the splanchnic blood flow is reduced to about 40% (Kjær et al. 1993) or even as low as 20% of the resting value (Rowell, 1973). Such a substantial reduction in blood flow is not associated with symptoms of abdominal hypoperfusion, which contrasts with patients suffering abdominal angina in whom the splanchnic blood flow is reduced to only 84% of rest (0.98 vs. 1.17 l min−1 in a control group; Buchardt Hansen et al. 1977). Therefore, we hypothesized that the exercise-induced reduction in splanchnic blood flow primarily follows a decrease in the hepato-splenic flow, while the mesenteric circulation remains relatively stable. In contrast to our hypothesis, Qamar & Read (1987) noted a 43% reduction in the superior mesenteric artery (SMA) blood flow after treadmill exercise. However, Eriksen & Waaler (1994) reported a maintained SMA flow after fasting and postprandial short-duration (4 min) cycling, corroborating observations during submaximal exercise in the dog (Herrick et al. 1940; Van Citters & Franklin, 1969; Vatner, 1975).
To evaluate these contradictory results, we assessed splanchnic blood flow (SBF) during fasting and postprandial exercise in healthy volunteers by both Indocyanine Green dye-elimination (dye-elimination) and duplex ultrasound techniques, which permitted separation of the hepato-splenic from the mesenteric circulations.
METHODS
Nineteen healthy non-medicated and non-smoking volunteers aged 28 years (range, 24–35 years), 1.82 m (1.73–1.91 m) tall and weighing 81 kg (57–85 kg) participated in the study after informed consent and approval by the Ethics Committee for Medical Research in Copenhagen (Reg. no. KA 91192). The first ten subjects underwent fasting duplex ultrasound examination of the SMA and the coeliac artery (CA), while in the other subjects the ultrasound examination was performed after a meal and concomitantly with estimation of SBF by dye elimination.
After an overnight fast (8–10 h), the subjects were positioned supine and a liver venous catheter (Cournand, 7 Fr) was introduced via the right median cubital vein and positioned in a right hepatic vein guided by fluoroscopy. Indocyanine Green was infused in a dose of 0.18 ± 0.02 μmol min−1 into a peripheral vein by a peristaltic roller pump (type 104, Ole Dich, Hvidovre, Denmark) and a 45 min priming infusion was used to obtain a steady-state plasma concentration. A 1 mm i.d. (20 gauge) catheter was placed in the non-dominant brachial artery to record systolic, diastolic and mean (MAP) arterial pressures and to obtain blood samples. Eight disposable electrodes (Blue Sensor VL-00-S, Medicotest, Ølstykke, Denmark) were placed over the sternocleidomastoid muscle and in the mid-axillary line at the level of the umbilicus on both sides of the body for detection of thoracic electrical impedance by a cardiograph (CDM 3000 Hemodynamic Monitor, CardioDynamics International Corporation, San Diego, CA, USA).
The monitor calculates stroke volume and cardiac output from pulsatile changes in thoracic electrical impedance (Appel et al. 1986; Wong et al. 1990). Total systemic vascular resistance (TVR) was the ratio of MAP to cardiac output.
Ultrasonographic examination was performed with a HDI ATL Ultramark 9 high resolution sonography unit using a 2.5 MHz phased array probe (Advanced Technology Laboratories, Inc., Bothell, WA, USA). The coeliac and mesenteric arteries were examined at a depth of 4–8 cm, 1–3 cm from the aorta and at a 60 deg angle of insonation. Blood flow was calculated as TAMV×π× 4−1×d2, where TAMV is the time-averaged mean velocity of blood (mean velocity) and d is the diameter of the artery assessed during systole (Evans et al. 1989). The SBF was the sum of the CA and SMA blood flows. Accepting a difference between MAP in the brachial artery and in the splanchnic arteries, we estimated splanchnic, mesenteric and coeliac artery resistances as the ratio of MAP to the respective blood flow. Duplex scan examinations were videotape recorded for off-line measurements of five consecutive cardiac cycles selected from the period of blood sampling for SBF estimation. Due to the enhanced respiration and movement of the diaphragm during cycling, the subjects were instructed to stop breathing for several seconds during moderate inspiration to allow steady ultrasonographic samplings.
Arterial and hepatic venous blood samples for estimation of SBF by the dye-elimination method were collected simultaneously 5 times, with 3 min intervals between samples, from the 15th to 30th minute of semisupine rest, 25th to 40th minute of postprandial semisupine rest, and 15th to 30th minute of semisupine cycling. All five measurements were averaged to yield the reported values. Plasma Indocyanine Green (ICG) concentrations were determined by HPLC (Ott et al. 1993). SBF was calculated as:
where I is the infusion rate of Indocyanine Green; Caand Cvare the concentrations of the dye in the brachial artery and in the hepatic vein, respectively; dCa/dt×VdICG represents a correction for non-steady state; and Hct is the haematocrit. VdICG was estimated as 0.05 × body weight (kg) and dCa/dt was expressed as the linear regression between the five arterial samples (Wiegard et al. 1960; Skak & Keiding, 1979).
After 30 min of supine rest, the upper body was elevated to 35 deg and the subjects ingested a liquid meal of 1000 kcal (Nutridrink, NUTRICIA, Sweden) with 5 g protein (13%), 18 g carbohydrate (48%), 6.5 g fat (39%), minerals and vitamins. Afterwards, 40 min of semisupine rest was allowed to obtain maximal postprandial mesenteric hyperaemia (Perko et al. 1997). A metronome was used to maintain cycling at a pedalling frequency of 60 r.p.m. The ergometer was loaded to demand 75% of the maximal O2 uptake, which had been determined during semisupine cycling 2–4 days previously. During the first part of the study conducted on fasting subjects, we encountered significant motion artefacts on the Doppler presentation when heart rate exceeded 160 beats min−1. Therefore, during the postprandial cycling, the ergometer load was reduced in three subjects in whom the heart rate exceeded this value. The resulting O2 uptake of these subjects was about 70% of the maximal value. Power was calculated from applied load and the pedalling frequency.
Blood gas analyses (PCO2 and PO2) and haematocrit determinations were performed concurrently during experiments using an ABL-615 analyser (Radiometer, Copenhagen, Denmark). Blood samples for lactate concentration were centrifuged at 4°C, while blood for determination of catecholamines was stabilized with 5 μmol EDTA and 4 μmol reduced glutathione in 20 μl 0.6 n NaOH (ml blood)−1 and stored at −80°C for later analysis. Venous lactate was determined by an enzymatic fluorometric method and catecholamines using a single-isotope radioenzymatic method.
The Shapiro-Wilk W test revealed that all variables were normally distributed and the data were evaluated using one-way analysis of variance for repeated measures. Where a significant F ratio was found, pairwise differences were evaluated using the Newman- Keuls post hoc test. Correlation between the two estimates of SBF was performed using Pearson's correlation coefficient, and the degree of agreement is presented graphically (Bland & Altman, 1986). That a sufficient number of subjects were tested in the study was demonstrated by a power analysis relating minimal relevant difference to type 2 error. The results are presented as means ±s.e.m. and all differences were considered significant at the 95% confidence interval (P < 0.05).
RESULTS
In one subject, the CA main trunk was shorter than 3 mm, which made the duplex ultrasound examination unfeasible during cycling. In the other subjects, no anatomical variants or pathology of the vessels were detected. Due to significant abdominal gas in one volunteer, ultrasound examination was incomplete and his data were excluded from the analysis. In another subject, a low hepatic extraction of Indocyanine Green indicated dislocation of the hepatic vein catheter to the inferior cava vein during cycling and his data were also excluded. Thus, data from eight subjects were analysed in both the fasting and postprandial groups of subjects.
The subjects' maximal O2 uptake was 3.36 ± 0.14 l min−1 at a 4.6 ± 0.1 kg load on the ergometer corresponding to a power of 260 ± 6 W. During the experiment, O2 uptake was 2.17 ± 0.01 l min−1 at 2.3 ± 0.1 kg corresponding to 136 ± 5 W. The arterial O2 saturation (97.4 ± 0.1%), Pa,CO2 (5.4 ± 0.1 kPa) and Pa,O2 (13.6 ± 0.2 kPa) were not influenced significantly by cycling or by the meal.
Fasting cycling
Fasting cycling elevated heart rate, MAP and cardiac output, while TVR was reduced (Fig. 1). The SMA mean blood velocity did not change, while its diameter decreased thereby reducing the flow volume by 0.18 ± 0.04 l min−1 (32%; P < 0.01). The mesenteric resistance increased by 110 ± 14 mmHg min l−1 (76%; P < 0.01). The alterations in CA were more pronounced: its diameter and mean blood velocity decreased by 0.09 ± 0.03 cm and 24 ± 2 cm s−1 (17 and 48%), respectively, thereby reducing flow volume by 0.42 ± 0.04 l min−1 (50%; P < 0.01), while the resistance increased by 157 ± 24 mmHg min l−1 (165%; P < 0.01). In consequence, SBF decreased and splanchnic resistance increased by 0.60 ± 0.04 l min−1 and 71 ± 8 mmHg min l−1, respectively (43 and 126%; P < 0.01).
Figure 1. Cardiovascular variables during fasting semisupine rest, cycling and 2 min after termination of cycling.
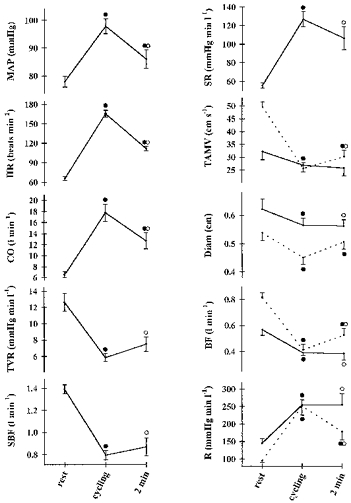
Values are means ±s.e.m. Continuous line, SMA; dotted line, CA. ^, different from rest, P < 0.05; •, different from the preceding value, P < 0.05. MAP, mean arterial pressure; CO, cardiac output; HR, heart rate; TVR, total systemic vascular resistance; SBF, splanchnic blood flow; SR, splanchnic vascular resistance; TAMV, time-averaged mean velocity; Diam, diameter; BF, blood flow; R, resistance.
MAP, cardiac output and TVR returned to the resting level within 6 min of recovery, while heart rate remained elevated for more than 15 min after cycling. All the SMA and CA duplex ultrasound variables stabilized within 6 min after cycling.
Postprandial semisupine rest
The ingestion of a meal did not influence plasma catecholamines or haemoglobin, but it elevated lactate, heart rate and cardiac output, and reduced MAP and TVR (Table 1, Fig. 2).
Table 1.
Influence of digestion and cycling on selected parameters
| Rest | Postprandial rest | Postprandial cycling | |
|---|---|---|---|
| SBF-DU (l min−1) | 1.46 ± 0.13 | 3.07 ± 0.31* | 2.11 ± 0.13* |
| SBF-ICG (l min−1) | 1.60 ± 0.09 | 2.80 ± 0.25* | 1.58 ± 0.26* |
| [Adrenaline] (nmol l−1) | 0.71 ± 0.21 | 0.55 ± 0.20 | 1.05 ± 0.18* |
| [Noradrenaline] (nmol l−1) | 1.33 ± 0.24 | 1.88 ± 0.30 | 9.13 ± 1.96* |
| [Haemoglobin] (mmol l−1) | 9.1 ± 0.3 | 9.1 ± 0.3 | 9.7 ± 0.3* |
| Haematocrit (%) | 45 ± 1.5 | 45 ± 1.5 | 48 ± 1.2* |
| [Lactate] (mmol l−1) | 0.46 ± 0.07 | 0.85 ± 0.10* | 2.79 ± 0.53* |
Different from preceding value, P < 0.05. SBF-DU and SBF-ICG, splanchnic blood flow by duplex ultrasound and dye-elimination technique, respectively.
Figure 2. Cardiovascular variables during fasting semisupine rest, postprandial (pp) semisupine rest, postprandial cycling and 2 min after termination of cycling.
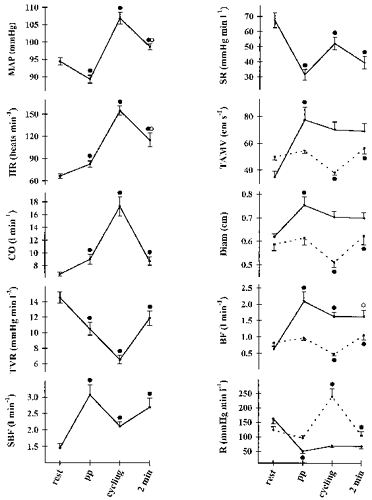
Values are means ±s.e.m. Continuous line, SMA; dotted line, CA. ^, different from pp, P < 0.05; •, different from the preceding value, P < 0.05. Abbreviations as for Fig. 1.
Neither the diameter nor the blood velocity of CA was affected significantly, while SMA duplex ultrasound variables increased, elevating blood flow by 1.46 ± 0.24 l min−1 (70%; P < 0.01). In consequence, SBF increased (52%; P < 0.01; Fig. 2, Table 1). The blood flow and the amplitude of postprandial change were comparable for the duplex ultrasound and dye-elimination techniques. Resistance in the CA did not change significantly, while in the mesenterium it decreased by 113 ± 17 mmHg min l−1 (69%; P < 0.01) and a reduction in splanchnic vascular resistance followed (Fig. 2, Table 1).
Postprandial cycling
Postprandial cycling elevated lactate, haemoglobin, haematocrit, catecholamines, MAP, heart rate and cardiac output, while TVR decreased (Fig. 2, Table 1). Non-significant reductions in the SMA mean blood velocity and diameter resulted in a 0.46 ± 0.28 l min−1 (22%; P = 0.03) decrease in flow volume (Fig. 2). However, in relation to rest before food intake, blood flow remained elevated by 1.0 ± 0.1 l min−1 (61%; P < 0.01). During cycling, the mesenteric vascular resistance was not affected significantly. The haemodynamic response of CA was comparable to that in the fasting state: its diameter and mean blood velocity decreased by 1.0 ± 0.02 cm and 17 ± 2 cm s−1 (16 and 31%), respectively, reducing flow volume by 0.49 ± 0.07 l min−1 (51%), and resistance increased by 144 ± 27 mmHg min l−1 (150%; P < 0.01). In consequence of these changes, SBF decreased and the difference between estimations by dye-elimination and duplex ultrasound methods did not differ significantly. Splanchnic vascular resistance increased (Fig. 2, Table 1).
Recovery
Within 2 min after termination of cycling, MAP, heart rate and cardiac output decreased and the latter reached the postprandial pre-cycling level. Accordingly, TVR increased and stabilized at the level before exercise. SMA blood flow decreased, while values of all other mesenteric, coeliac and splanchnic variables corresponded to the postprandial pre-cycling rest.
SBF
SBF measurements by the dye-elimination and duplex ultrasound methods were well correlated, reaching a coefficient of 0.70 (Fig. 3; P < 0.01). The mean difference in SBF estimation was −0.167 l min−1, and the 95% limits of agreement were −1.5 to 1.17 l min−1.
Figure 3. SBF measurements.
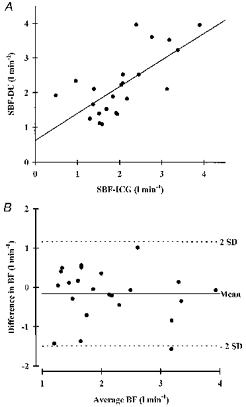
A, splanchnic blood flow obtained by the dye-elimination technique (SBF-ICG) and duplex ultrasound (SBF-DU) at rest, after a meal and during postprandial cycling (n = 8). B, agreement in blood flow assessment: difference between dye-elimination technique and duplex ultrasound values (SBF-ICG - SBF-DU) plotted against average SBF values ((SBF-ICG + SBF-DU)/2). s.d., standard deviation; BF, blood flow. Mean ± 2 s.d. defines the 95% limits of agreement.
DISCUSSION
This study demonstrates a more than a doubling of splanchnic vascular resistance with a 43% concomitant reduction in its blood flow during submaximal cycling in fasting subjects. The coeliac artery has the predominant influence upon this response, with a 165% increase in resistance and a 50% (0.42 l min−1) reduction in blood flow - more than twice the reduction in the superior mesenteric artery blood flow (0.18 l min−1). During postprandial cycling, the elevation in splanchnic resistance and reduction in blood flow were less pronounced in consequence of maintained mesenteric artery vasodilatation.
The effect of exercise on the splanchnic blood flow in humans was described more than forty years ago by Wade et al. (1956). However, the specific involvement of the mesenteric and coeliac circulation in humans was unknown until the introduction of duplex ultrasonography, which enabled non-invasive assessment of blood flow in the respective arteries. The coeliac artery response to exercise has not been investigated previously, while the conclusions from studies on the mesenteric circulation during exercise are conflicting. Qamar & Read (1987) noted a reduction in mesenteric blood flow, while Eriksen & Waaler (1994) observed no change in blood flow during postprandial cycling, with an increase in flow during fasting exercise. Both studies were based on duplex ultrasound, but they presented neither the diameter nor the blood velocity of the artery, which hinders interpretation of the results. One possible explanation of this divergence of outcome is a difference in the exercise procedure: treadmill walking vs. semisupine cycling. The former requires time to position a subject on an examination couch after exercise, then finding an artery on B-mode ultrasound presentation, adjustment of gate callipers and angle of insonation, measurement of the artery diameter, then switch to the Doppler mode and finally adjustment of the velocity scale. These procedures are time consuming and the evaluations by Qamar & Read (1987) must have been made rather late after exercise, which is of importance since significant changes in central haemodynamics (and in regional blood flow) take place within the first few minutes following the cessation of exercise (Figs 1 and 2). Cycling in a semisupine position of about 35 deg enables continuous visualization of the artery and Doppler sampling. Moreover, video recording of the ultrasound examination allows off-line processing and replication of the measurements, which is especially crucial for assessment of the artery diameter (Perko & Just, 1993).
The SMA and CA are connected by the gastroduodenal artery. Additionally, in as much as 1/4 of the population SMA has accessory branches to the liver and/or the pancreas (Kornblith et al. 1992). Increased ‘shunting’ through these pathways in some of the examined subjects might also contribute to dissimilar results.
During exercise, total vascular resistance is reduced (Gauer & Thron, 1965), but splanchnic and mesenteric resistance is elevated (Vatner et al. 1975). These observations are in line with the present results: TVR was reduced during fasting cycling and after a meal, and, furthermore, a significant reduction was observed during postprandial cycling. During cycling, splanchnic vascular resistance increased by 126% dominated by an elevation in coeliac artery tone. Alterations in CA resistance paralleled changes in arterial pressure, and blood flow was affected inversely. The cycling-induced increase in SMA resistance was less pronounced than that of the CA, and 2 min after termination of cycling the resistance was still elevated in spite of a reduced arterial pressure. A probable mechanism for the maintained mesenteric resistance is the vasoconstricting effect of elevated circulating catecholamine levels. However, mesenteric resistance did not increase during and 2 min after postprandial cycling in spite of a 5-fold increase in noradrenaline. Postprandially, functional vasodilatation in the mesenterium was reflected by a reduction in splanchnic resistance associated with a 70% increase in blood flow (2 l min−1; i.e. 23% of cardiac output vs. 9% at rest) in spite of a reduction in arterial pressure. Cycling-induced increases in blood pressure, cardiac output and catecholamines were associated with a stable postprandially reduced mesenteric resistance. Compared with the fasting state, cessation of cycling induced reductions in MAP and cardiac output; however, the SMA blood flow remained elevated by 1 l min−1 and its resistance was not affected. After the meal, elevation of mesenteric vascular tone was inhibited during cycling, while the CA response was the same as in the fasting state and resulted in a 150% increase in resistance. The splenic constriction in dogs during exercise has been suggested to increase the haematocrit and therefore to serve as a blood ‘reservoir’ (Vatner et al. 1974). However, the exercise-induced increases in haematocrit in splenectomized and non-splenectomized humans during exercise are comparable (Nielsen et al. 1997), thus other organs or mechanisms must be involved. CA gives rise to the splenic, left gastric and hepatic artery. Of the dependent organs the largest is the liver, which is likely to yield a dominant component to the splanchnic vascular response to exercise.
Resistance drawn from the pressure-to-flow ratio incorporates two potential sources of error: the difference between the brachial and splanchnic blood pressures and changes in venous pressure. In the semisupine position, however, the magnitudes of both are of little significance (Gauer & Thron, 1965; Rowell, 1993).
The indicator dye-elimination technique for blood flow estimation is subject to several limitations and inconveniences, e.g. flow can be measured only intermittently, multiple sampling is necessary and the data collection and analysis are time consuming (Perry & Parker, 1981). Additionally, during cycling we experienced displacement of the liver vein catheter to the inferior vena cava in one subject.
The only factor limiting duplex ultrasound measurements during submaximal cycling was extensive tachypnoea, which produced artefacts in Doppler presentation deteriorating the signal-to-noise ratio. To avoid the respiration-induced movements, the subjects stopped breathing for 4–5 s during moderate inspiration, which was sufficient to collect representative data. In spite of a correlation between the indicator extraction and the duplex methods, there was a dispersion of blood flow measurements reaching 1.5 l min−1.
The alterations in central haemodynamics, blood concentration of catecholamines, lactate, and gas variables during the present study were comparable to those obtained by others (Kjær et al. 1993; Nielsen et al. 1997).
Conclusion
After a meal, splanchnic arterial resistance decreases and blood flow is elevated in consequence of analogous changes in the superior mesenteric artery vascular bed, while the coeliac circulation is not affected. Submaximal dynamic exercise in humans increases splanchnic vascular resistance with a consequent reduction in blood flow. The dominant influence on this response is the coeliac artery vascular bed, while the haemodynamic changes in the mesenteric artery are less pronounced. Exercise has no effect on superior mesenteric artery resistance during digestion-induced vasodilatation.
Acknowledgments
The impedance electrodes were kindly provided by Medicotest A/S, Ølstykke, Denmark. This study was supported by The Danish Heart Foundation, Danish Foundation for the Advancement of Medical Science, The Danish Medical Association Research Fund and Lily Benthine Lunds Fond.
References
- Appel PL, Kram HB, Mackabee J, Fleming AW, Shoemaker WC. Comparison of measurements of cardiac output by bioimpedance and thermodilution in severely ill surgical patients. Critical Care Medicine. 1986;14:933–935. doi: 10.1097/00003246-198611000-00004. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i:307–310. [PubMed] [Google Scholar]
- Buchardt Hansen HJ, Engell HC, Ring-Larsen H, Ranek L. Splanchnic blood flow in patients with abdominal angina before and after arterial reconstruction. Annals of Surgery. 1977;186:216–220. doi: 10.1097/00000658-197708000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen M, Waaler BA. Priority of blood flow to splanchnic organs in humans during pre- and post-meal exercise. Acta Physiologica Scandinavica. 1994;150:363–372. doi: 10.1111/j.1748-1716.1994.tb09700.x. [DOI] [PubMed] [Google Scholar]
- Evans DH, McDicken WN, Skidmore R, Woodcock JP. Doppler Ultrasound. Physics, Instrumentation, and Clinical Applications. New York: John Wiley & Sons; 1989. pp. 162–205. [Google Scholar]
- Gauer OH, Thron HL. Postural changes in the circulation. In: Hamilton WF, Dow P, editors. Handbook of Physiology, Postural Changes in the Circulation, section 2, Circulation. III. Bethesda, MD, USA: American Physiological Society; 1965. pp. 2409–2439. chap. 67. [Google Scholar]
- Herrick JF, Grindlay JH, Baldes EJ, Mann FC. Effect of exercise on the blood flow in the superior mesenteric, renal and common iliac arteries. American Journal of Physiology. 1940;128:338–344. [Google Scholar]
- Kjær M, Engfred K, Fernandes A, Secher NH, Galbo H. Regulation of hepatic glucose production during exercise in humans: role of sympathoadrenergic activity. American Journal of Physiology. 1993;265:E275–283. doi: 10.1152/ajpendo.1993.265.2.E275. [DOI] [PubMed] [Google Scholar]
- Kornblith PL, Boley SJ, Whitehouse BS. Anatomy of the splanchnic circulation. Surgical Clinics of North America. 1992;72:1–30. doi: 10.1016/s0039-6109(16)45625-2. [DOI] [PubMed] [Google Scholar]
- Nielsen HB, Secher NH, Kristensen JH, Christensen NJ, Espersen K, Pedersen BK. Splenectomy impairs lymphocytosis during maximal exercise. American Journal of Physiology. 1997;272:R1847–1852. doi: 10.1152/ajpregu.1997.272.6.R1847. [DOI] [PubMed] [Google Scholar]
- Ott P, Keiding S, Bass L. Plasma elimination of indocyanine green in the intact pig after bolus injection and during constant infusion: Comparison of spectrophotometry and high-pressure liquid chromatography for concentration analysis. Hepatology. 1993;18:1504–1515. [PubMed] [Google Scholar]
- Perko MJ, Just S. Duplex ultrasound of superior mesenteric artery: Interobserver variability. Journal of Ultrasound in Medicine. 1993;5:259–263. doi: 10.7863/jum.1993.12.5.259. [DOI] [PubMed] [Google Scholar]
- Perko MJ, Madsen P, Perko G, Schroeder TV, Secher NH. Mesenteric artery response to head-up tilt-induced central hypovolaemia and hypotension. Clinical Physiology. 1997;17:487–496. doi: 10.1046/j.1365-2281.1997.05252.x. [DOI] [PubMed] [Google Scholar]
- Perry MA, Parker JC. Indicator dilution measurements of splanchnic blood flow. In: Granger DN, Bulkley GB, editors. Measurement of Blood Flow, Applications to the Splanchnic Circulation. Baltimore: Williams & Wilkins; 1981. pp. 161–176. [Google Scholar]
- Qamar MI, Read AE. Effects of exercise on mesenteric blood flow in man. Gut. 1987;28:583–587. doi: 10.1136/gut.28.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Regulation of splanchnic blood flow in man. The Physiologist. 1973;16:127–142. [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. Oxford: Oxford University Press; 1993. [Google Scholar]
- Skak C, Keiding S. Methodological problems in the use of indocyanine green to estimate hepatic blood flow and ICG clearance in man. Liver. 1979;7:155–162. doi: 10.1111/j.1600-0676.1987.tb00336.x. [DOI] [PubMed] [Google Scholar]
- van Citters RL, Franklin DL. Cardiovascular performance of Alaska sled dogs during exercise. Circulatory Research. 1969;24:33–38. doi: 10.1161/01.res.24.1.33. [DOI] [PubMed] [Google Scholar]
- Vatner SF. Effects of exercise on distribution of regional blood flow and resistance. In: Zelis R, editor. The Peripheral Circulations. New York: Grune & Stratton; 1975. pp. 211–233. [Google Scholar]
- Vatner SF, Higgins CB, Millard RW, Franklin D. Role of the spleen in the peripheral vascular response to severe exercise in untethered dogs. Cardiovascular Research. 1974;8:276–282. doi: 10.1093/cvr/8.2.276. [DOI] [PubMed] [Google Scholar]
- Wade OL, Combes B, Childs AW, Wheeler HO, Cournand A, Bradley SE. The effect of exercise on the splanchnic blood flow and splanchnic blood volume in normal man. Clinical Science. 1956;15:457–463. [PubMed] [Google Scholar]
- Wiegard BD, Ketterer SG, Papaport E. The use of indocyanine green for the evaluation of hepatic function and blood flow in man. American Journal of Digestive Diseases. 1960;5:427–436. doi: 10.1007/BF02232628. [DOI] [PubMed] [Google Scholar]
- Wong DH, Tremper KK, Stemmer EA, O'Connor D, Wilbur S, Zuccari J, Reeves C, Weidoff P, Trujillo RJ. Noninvasive cardiac output: simultaneous comparison of two different methods with thermodilution. Anesthesiology. 1990;72:4–12. doi: 10.1097/00000542-199005000-00002. [DOI] [PubMed] [Google Scholar]


