Abstract
A high-performance liquid chromatography assay with ultraviolet detection was developed for the simultaneous determination of the anti-epileptic drugs lamotrigine, carbamazepine, and zonisamide in human plasma and serum. Lamotrigine, carbamazepine, zonisamide, and the internal standard chloramphenicol were extracted from serum or plasma using liquid-liquid extraction under alkaline conditions into an organic solvent. The method was linear in the range 1–30 μg/mL for lamotrigine, 2–20 μg/mL for carbamazepine, and 1–40 μg/mL for zonisamide. Within- and between-run precision studies demonstrated coefficient of variation < 10% at all tested concentrations. Other anti-epileptic medications tested did not interfere with the assay. The method is appropriate for determining lamotrigine, carbamazepine, and zonisamide serum or plasma concentrations for therapeutic monitoring.
Keywords: anticonvulsants, lamotrigine, zonisamide, carbamazepine, HPLC, UV detection
INTRODUCTION
Lamotrigine (Lamictal®) is a broad-spectrum anti-epileptic drug of the phenyltriazine class chemically unrelated to other anti-convulsants (LaRoche and Helmers, 2004a,b). Lamotrigine has an average elimination half-life of 33 hours, although this can be influenced by concomitant therapy with other medications such as valproic acid. Lamotrigine is predominantly metabolized in the liver by glucuronidation (Bialer, 2005). Therapeutic drug monitoring of lamotrigine targets the parent drug as little clinical benefit has been found for monitoring the glucuronide metabolite (Bialer, 2005).
Zonisamide (Zonegran®) is an anti-epileptic drug licensed in the United States for the treatment of partial seizures in adults (LaRoche and Helmers, 2004a,b). Zonisamide is also used in West’s syndrome (a form of infantile epilepsy), Lennox-Gestaut syndrome (a refractory type of epilepsy), bipolar disorder, and migraine headaches. Zonisamide has an elimination half-life of approximately 50–70 hours in adults and is extensively metabolized by acetylation and conjugation. Zonisamide is a substrate of cytochrome P450 2C19 and 3A4 and is subject to drug-drug interactions with inhibitors or inducers of these hepatic enzymes (Bialer, 2005). Although several metabolites of zonisamide are generated following administration to humans, therapeutic drug monitoring of zonisamide has involved determination of serum/plasma concentrations of only the parent compound (Bialer, 2005).
Measuring lamotrigine and zonisamide concentrations in plasma is recommended for monitoring treatment adherence and for dose adjustment in patients with liver dysfunction or who are receiving other medications (Tomson and Johannessen, 2000; Bialer, 2005). Several methods have been used for determination of lamotrigine including high-performance liquid chromatography (HPLC) (Fraser et al.,, 1995; Forssblad et al.,, 1996; Lensmeyer et al.,, 1997; Torra et al.,, 2000; Contin et al.,, 2005), gas chromatography with a nitrogen-phosphorus detector (Watelle et al.,, 1997), and radioimmunoassay (Biddlecombe et al.,, 1990). Several HPLC methods have been reported for zonisamide (Shimoyama et al.,, 1999; Nakamura et al.,, 2001; Juenke et al.,, 2006). As yet, there are no commercially available assays for either drug, in contrast to multiple immunoassays available for the quantitation of more traditional anti-epileptic drugs such as carbamazepine, phenobarbital, phenytoin, and valproic acid. Given the expanding use of lamotrigine and zonisamide in our medical center, including co-administration of lamotrigine and zonisamide in some patients, we developed a rapid, simple HPLC method to measure the two drugs simultaneously. With a slightly longer run time, the assay can also be used to measure the concentrations of carbamazepine.
EXPERIMENTAL
Chemicals and apparatus
Lamotrigine was generously supplied by GlaxoSmithKline (Triangle Park, NC, USA). Carbamazepine, zonisamide, and the internal standard (IS) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile, methanol, and water were HPLC grade (Fisher, Pittsburgh, PA, USA). Ethylacetate, potassium phosphate, and phosphoric acid were analytical grade (Fisher). Drug-free human plasma was obtained from the University of Pittsburgh Medical Center Central Blood Bank. The HPLC system consisted of a Waters/Millipore 717 Plus Autosampler and Systems Controller.
Chromatographic conditions
The separation was performed at 22 °C with a μBondapak C-18 column. The mobile phase was a mixture of aqueous 30 mM potassium phosphate buffer (adjusted to pH 3.7 with 5% phosphoric acid) and acetonitrile (65:35) at a flow rate of 1.2 mL/min. Detection was monitored at 270 nm using a Waters 486 detector.
Sample preparation
Stock solutions of carbamazepine, lamotrigine, zonisamide, and the IS chloramphenicol were at 1000 μg/mL in HPLC grade methanol. To 250 μL of sample (calibrator, control, or patient sample) in a 16 × 125 mm glass culture tube, 100 μL of IS solution, 1.5 mL NaOH, and 4.0 mL ethylacetate were added. The samples were immediately vortexed for 1 min and centrifuged at 1,700 g for 5 min. The organic (upper) layer was transferred to a conical tube and dried completely at 40°C using a nitrogen evaporator. The sample was then reconstituted with 100 μL mobile phase, vortexed, and transferred to an autosampler vial for HPLC analysis.
Determination of recovery, intra-day and inter-day precision and accuracy
The absolute recovery was estimated by comparison with direct injection of aqueous drug solutions of corresponding concentrations. Intra-day precision and accuracy were evaluated by the analysis of spiked samples. The precision and accuracy for inter-day comparisons were assessed at the same concentration and summarized as coefficient of variation (CV%) and relative deviation (RD%), respectively.
RESULTS AND DISCUSSION
Specificity
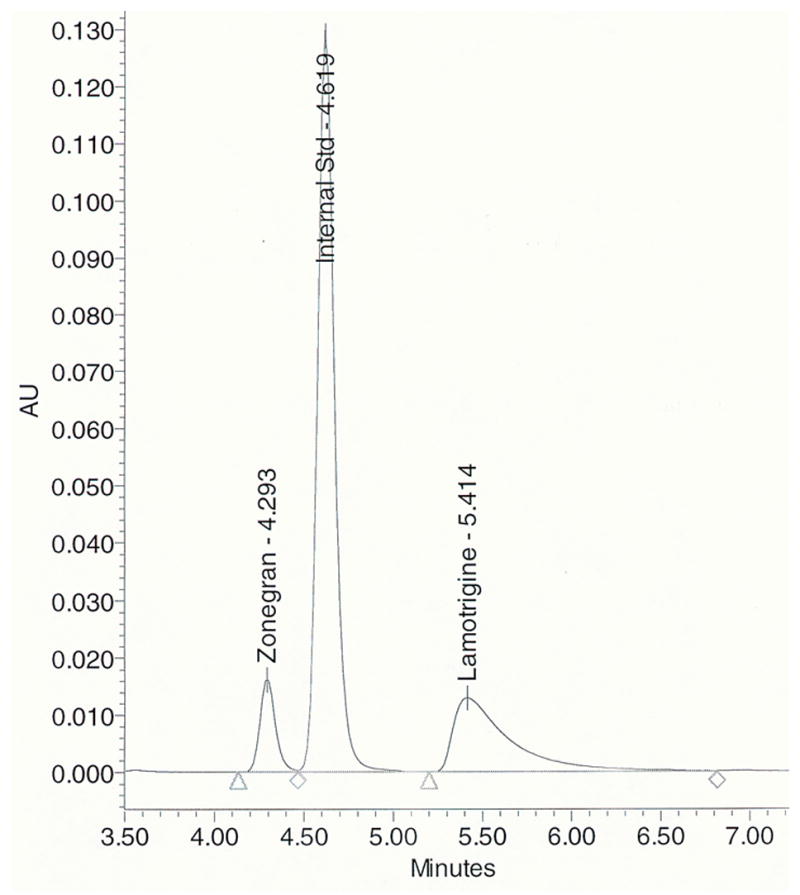
Under the described conditions, the retention times of zonisamide, IS, lamotrigine, and carbamazepine were 4.3, 4.7, 5.6, and 7.3 min, respectively (Fig. 1). A wide variety of therapeutic drugs were tested for interference, including other anti-epileptic medications (and in some cases their metabolites) such as ethosuximide, gabapentin, levetiracetam, oxcarbazepine, 10-hydroxycarbamazepine (active metabolite of oxcarbazepine), phenobarbital, phenytoin, primidone, topiramate, and valproic acid. None of the therapeutic drugs tested exhibited any interference. In addition, no peak interferences were found in any of the batches of drug-free plasma. The method has been used extensively in our clinical laboratory for therapeutic drug monitoring of lamotrigine and zonisamide. An example of a chromatogram from the plasma of a patient taking lamotrigine and zonisamide chronically is shown in Fig. 2.
Figure 1.
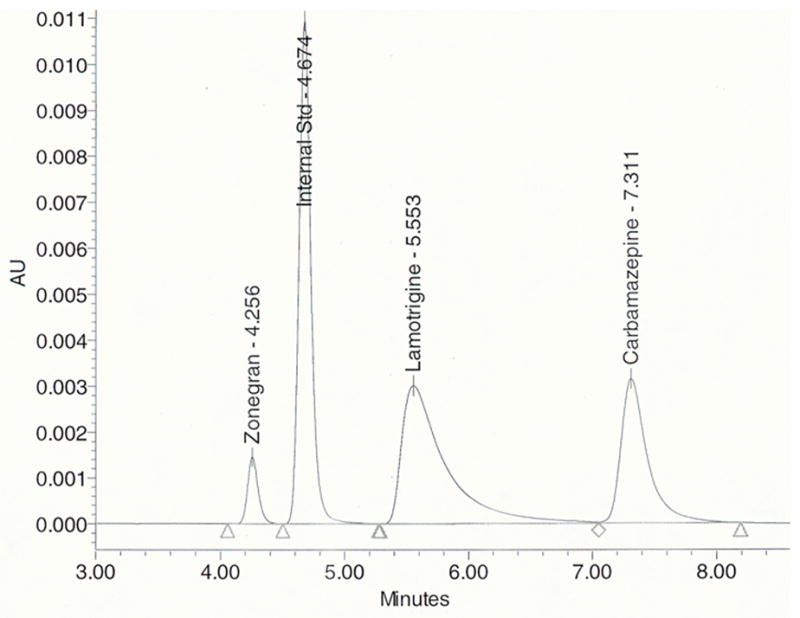
Figure 2.
Linearity, accuracy, precision, and detection limits
The method exhibited a good linearity over a concentration range of 1–30 μg/mL for lamotrigine, 2–20 μg/mL for carbamazepine, and 1–40 μg/mL for zonisamide. A representative regression line for lamotrigine was y = 1.0014 x − 0.024 (r2=0.9992), with correlation coefficient greater than 0.99 on five different days. Results of intra- and inter-day accuracy and precision are shown in Table 1. The lower limit of detection was 0.5 μg/mL for lamotrigine, 0.5 μg/mL for zonisamide, and 0.25 μg/mL for carbamazepine.
Table 1.
Intra-day and inter-day accuracy and precision for lamotrigine and zonisamide determination in human plasma/serum (all concentrations in μg/mL)
| Lamotrigine | ||
| Intra-day (n=5) | ||
| Nominal concentration | 10.5 | |
| Mean | 10.66 ± 0.14a | |
| Accuracy as RD (%) | 1.6 | |
| Precision as CV (%) | 1.3 | |
| Inter-day (n=10) | ||
| Nominal concentration | 7.0 | 12 |
| Mean | 7.57 ± 0.55 | 12.25 ± 0.91 |
| Accuracy as RD (%) | 8.1 | 2.1 |
| Precision as CV (%) | 7.3 | 7.5 |
|
| ||
| Zonisamide | ||
| Intra-day (n=5) | ||
| Nominal concentration | 21.5 | |
| Mean | 21.74 ± 0.14 | |
| Accuracy as RD (%) | 1.1 | |
| Precision as CV (%) | 0.6 | |
| Inter-day (n=10) | ||
| Nominal concentration | 20 | 30 |
| Mean | 20.94 ± 1.85 | 32.72 ± 2.38 |
| Accuracy as RD (%) | 4.7 | 9.1 |
| Precision as CV (%) | 8.8 | 7.3 |
Values are reported as mean ± SD.
Recovery
The average absolute recovery values for lamotrigine were 96% for 2.5 μg/mL, 96% for 7 μg/mL, 97% for 12 μg/mL, and 97% for 20 μg/mL. The average absolute recovery values for zonisamide were 97% for 10 μg/mL, 94% for 15 μg/mL, 95% for 20 μg/mL, and 98% for 30 μg/mL. The mean percentage recovery of the IS was 94%. The results indicate that the extraction efficiency of the method is consistent and reproducible.
CONCLUSIONS
The HPLC method described here is simple, sensitive, and specific and allows for the simultaneous determination of three commonly prescribed anti-epileptic drugs. Using this methodology, we now analyze over 1000 patient plasma or serum samples per year for lamotrigine and/or zonisamide concentrations, drugs for which reliable immunoassays have yet to be made available commercially.
Acknowledgments
M.D.K. is supported by a Clinical-Scientist development award K08-GM074238-01A1 from the National Institutes of Health and a Competitive Medical Research Fund (CMRF) grant from University of Pittsburgh.
References
- Bialer M. The pharmacokinetics and interactions of new antiepileptic drugs: an overview. Therapeutic Drug Monitoring. 2005;27:722–726. doi: 10.1097/01.ftd.0000179854.16846.67. [DOI] [PubMed] [Google Scholar]
- Biddlecombe RA, Dean KL, Smith CD, Jeal SC. Validation of a radioimmunoassay for the determination of human plasma concentration of lamotrigine. Journal of Pharmaceutical and Biomedical Analysis. 1990;8:691–694. doi: 10.1016/0731-7085(90)80104-w. [DOI] [PubMed] [Google Scholar]
- Contin M, Balboni M, Callegati E, Candela C, Albani F, Riva R, Baruzzi A. Simultaneous liquid chromatographic determination of lamotrigine, oxcarbazepine monohydroxy derivative and felbamate in plasma of patients with epilepsy. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2005;828:113–117. doi: 10.1016/j.jchromb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Forssblad E, Eriksson AS, Beck O. Liquid chromatographic determination of plasma lamotrigine in pediatric samples. Journal of Pharmaceutical and Biomedical Analysis. 1996;14:755–758. doi: 10.1016/0731-7085(95)01669-4. [DOI] [PubMed] [Google Scholar]
- Fraser AD, MacNeil W, Isner AF, Camfield PR. Lamotrigine analysis in serum by high-performance liquid chromatography. Therapeutic Drug Monitoring. 1995;17:174–178. doi: 10.1097/00007691-199504000-00012. [DOI] [PubMed] [Google Scholar]
- Juenke JM, Brown PI, Urry FM, McMillin GA. Drug monitoring and toxicology: a procedure for the monitoring of levetiracetam and zonisamide by HPLC-UV. Journal of Analytical Toxicology. 2006;30:27–30. doi: 10.1093/jat/30.1.27. [DOI] [PubMed] [Google Scholar]
- LaRoche SM, Helmers SL. The new antiepileptic drugs: clinical applications. JAMA. 2004a;291:615–620. doi: 10.1001/jama.291.5.615. [DOI] [PubMed] [Google Scholar]
- LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004b;291:605–614. doi: 10.1001/jama.291.5.605. [DOI] [PubMed] [Google Scholar]
- Lensmeyer GL, Gidal BE, Wiebe DA. Optimized high-performance liquid chromatographic method for determination of lamotrigine in serum with concomitant determination of phenytoin, carbamazepine, and carbamazepine epoxide. Therapeutic Drug Monitoring. 1997;19:292–300. doi: 10.1097/00007691-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Hirade K, Sugiyama T, Katagiri Y. High-performance liquid chromatographic assay of zonisamide in human plasma using a non-porous silica column. Journal of Chromatography B, Biomedical Sciences and Applications. 2001;755:337–341. doi: 10.1016/s0378-4347(01)00062-7. [DOI] [PubMed] [Google Scholar]
- Shimoyama R, Ohkubo T, Sugawara K. Monitoring of zonisamide in human breast milk and maternal plasma by solid-phase extraction HPLC method. Biomedical Chromatography. 1999;13:370–372. doi: 10.1002/(SICI)1099-0801(199908)13:5<370::AID-BMC900>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tomson T, Johannessen SI. Therapeutic monitoring of the new antiepileptic drugs. European Journal of Clinical Pharmacology. 2000;55:697–705. doi: 10.1007/s002280050001. [DOI] [PubMed] [Google Scholar]
- Torra M, Rodamilans M, Arroyo S, Corbella J. Optimized procedure for lamotrigine analysis in serum by high-performance liquid chromatography without interferences from other frequently coadministered anticonvulsants. Therapeutic Drug Monitoring. 2000;22:621–625. doi: 10.1097/00007691-200010000-00019. [DOI] [PubMed] [Google Scholar]
- Watelle M, Demedts P, Franck F, De Deyn PP, Wauters A, Neels H. Analysis of the antiepileptic phenyltriazine compound lamotrigine using gas chromatography with nitrogen phosphorus detection. Therapeutic Drug Monitoring. 1997;19:460–464. doi: 10.1097/00007691-199708000-00016. [DOI] [PubMed] [Google Scholar]


