Abstract
Experience with multiple odorants during early postnatal development increases the number of cells in the olfactory bulb of rats. In this study, we asked whether at least part of this increase was due to decreased cell death. We selected 30 natural odorants or synthetic odorant mixtures to stimulate a broad area of the bulb during postnatal days 1–15, and counted the number of cells with DNA damage associated with cell death in both the glomerular and the granule cell layers of the main olfactory bulb. Early olfactory enrichment significantly decreased cell death in both bulbar laminae. Thus, olfactory enrichment can spare bulbar cells during early development, possibly leading to increased efficacy in bulb function and enhanced bulbar responses.
Keywords: enriched environment, olfaction, olfactory bulb, TUNEL
Introduction
Enriched odor experience during the first 3 postnatal weeks results in significant morphological and metabolic changes in the rodent main olfactory bulb. Odor enrichment with multiple odorants for postnatal days (PNDs) 1–21 increased the number of granule cells present on PNDs 31 and 60 [1]. Enriched odor experience with concurrent somatosensory stimulation for only 10 min per day during the first 3 postnatal weeks was sufficient to increase the number of glomerular layer cells within the focal regions of the glomerular layer of the olfactory bulb that were activated by the familiar odorants, but not within areas distant to these regions [2]. In addition, enriched odor experience during the first 3 postnatal weeks significantly increased [14C]2-deoxyglucose uptake in focal regions of the glomerular layer in response to the familiar odorant [3–5]. Activity evoked in these odor-experienced animals by a novel odorant that stimulated a different region of the bulb was not enhanced [6].
Taken together, these data suggest that early olfactory enrichment leads to both enhanced neural activity in the glomerular layer of the olfactory bulb, as well as increased cell numbers in multiple laminae of the bulb. Cell death in the main olfactory bulb is a normally occurring process during the early postnatal period [7,8]. We therefore speculated that the increased neural activity that occurs during early olfactory enrichment could promote survival in these active cells. To this end, we examined whether early olfactory enrichment with multiple odorants during the early postnatal period could decrease the overall incidence of cell death across the main olfactory bulb, thus saving cells from an early death.
To examine the effects of odor enrichment on cell survival, animals were exposed to a battery of 30 different natural odorants or synthetic odorant mixtures from PNDs 1 to 15, and the incidence of cell death, as determined by the presence of DNA damage, was quantified on PND 16 by using the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL) assay.
Materials and methods
Odor enrichment for cell death analysis
Female Wistar rats obtained from Charles River (Boston, Massachusetts, USA) were bred in our laboratory, and litters were culled to six male and two female rats on PND 1. Beginning on PND 1, natural odorants or commercially available synthetic odorant mixtures were wrapped in cheesecloth, or applied onto cotton balls and then wrapped in cheesecloth, and suspended from the top of the housing cage in metal tea balls. Solid odorant sources were pulverized or shredded to increase the volatility of the odorants. Litters in the enriched group were exposed to two different odorants per day, changed daily for 15 days (see Table 1). Pairs of odorants were selected to increase the total number of odorants experienced during the exposure period. Individual tea balls contained only one odorant at a time, and the two tea balls were suspended in different areas of the cage. Control litters were exposed to two tea balls containing lab chow wrapped in cheesecloth. Control and enriched litters were housed in separate rooms to avoid odor contamination.
Table 1.
Pairs of odorants to which animals were exposed
| 1. | Peppermint extract and all-spice |
| 2. | Oregano and grass (oil) |
| 3. | Floral musk (oil) and cinnamon sticks |
| 4. | Almond extract and cedar chip (oil) |
| 5. | Potting soil and fresh lemon peels |
| 6. | Rose (oil) and fresh eucalyptus leaves |
| 7. | Vanilla extract and peppercorns |
| 8. | Cumin and fresh ginger root |
| 9. | Herbal (oil) and fresh onion |
| 10. | Fennel and baby (oil) |
| 11. | Anise extract and rosemary |
| 12. | Marjoram and dark musk (oil) |
| 13. | Jasmine (oil) and fresh juniper |
| 14. | Coffee (oil) and fresh orange peels |
| 15. | Savory and fresh peach (oil) |
| 16. | Caraway and fresh garlic |
| 17. | Japanese flower (oil) and ground clove |
| 18. | Dill weed and fresh wood (oil) |
| 19. | Lavender (oil) and fresh banana peels |
| 20. | Basil and cucumber (oil) |
Tissue sectioning and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling assay
On PND 16, one male rat from each of the eight control and eight enriched litters was euthanized by a Nembutal overdose, and brains were removed and frozen in cold isopentane. Coronal sections (20 μm) were taken through the entire olfactory bulb using a cryostat, and every 14th section was thaw-mounted onto slides and processed using the TUNEL assay [7,9]. All slides were processed in the same reaction to obtain comparable staining on all tissues included in the analysis. Briefly, the tissue was incubated in Tris-HCl, pH 7.2, containing 140 mM sodium cacodylate, 1 mM cobalt chloride, 0.1 U/μl terminal deoxynucleotidyl transferase (United States Biochemical, Cleveland, Ohio, USA), 7 μM biotin-dUTP (Boehringer/Mannheim, Indianapolis, Indiana, USA), and 0.2 mg/ml bovine serum albumin in a humidified chamber for 3 h at 37°C. The slides were immersed in 300 mM sodium chloride and 30 mM sodium citrate, pH 8.1, to terminate the reaction. TUNEL-positive cells were labeled with Cy3-streptavidin (2 μg/ml) (Jackson Immunochemicals, West Grove, Pennsylvania, USA), and the sections were counterstained with bisbenzimide (Hoechst 33342; 10 μg/ml; Acros Brand (Fisher), Tustin, California, USA).
Granule cell counts
Slides were coded before cell count analyses to avoid experimenter bias. One bulb from each animal was analyzed (enriched group, n=8; control group, n=8). Initially, individual sections were saved at a 4 × magnification on an Olympus microscope (Melville, New York, USA) equipped for fluorescence using the Optronics program (Goleta, California, USA) with a computer interface. At this low magnification, the boundaries of the different laminae of the bulb were readily visible owing to background staining. Using NIH Image 1.61 (Bethesda, Maryland, USA), each layer of the olfactory bulb was outlined and the area of each lamina was calculated. As a large number of TUNEL-positive cells were present in the granule cell layer, a systematic random sampling technique was used to quantify these cells. Using NIH Image 1.61, labeled grids were overlaid onto each section along with the drawn outline of each layer, and printed in black and white as a merged image. Although the sections were mounted on the glass slides in a column, the exact orientation of each image varied from section to section. To allow for random sampling of the tissue, no image rotation was performed on either the grid or the image. It was determined that counts in every other box of the grid would give a reasonable estimate of the population; therefore, a grid was printed on an acetate sheet, and every other box was marked with red permanent marker. A grid box was randomly selected, and the acetate sheet was placed on top of the printed images such that the grid lines were aligned, and one of the marked boxes was lined up with the randomly selected box. The coordinates of each box that aligned with a red mark were recorded, thus setting up a means to perform random, systematic sampling. The area of each of the labeled boxes was measured. Boxes that were located on the borders of the granule cell layer were subdivided to include only the area within the granule cell layer.
Using stained cells or other landmarks that were visible on the printed copy of the 4 × magnification, each grid box to be analyzed was located at a 40 × magnification, and the image was labeled and saved on the computer if any fluorescent object was visible in the field. Each saved image was then examined in detail by two observers. To avoid overestimates of the total cell counts, cells that touched only two of the axis lines were counted while those touching the other two lines of the grid were not counted. To randomize the determination of which lines to use in the first section of the bulb, a coin toss determined whether to use the left and top lines of the grids, or the right and bottom lines of the grids, and the selection of the lines to use was alternated for all successive sections. Only labeled cells that were located within the grid box were counted. Counts were expressed as cell densities, and cell counts per section were extrapolated using the area of the granule cell layer within a section. The counts from two subjacent sections were averaged and multiplied by the number of sections not collected between the two subjacent sections (13 sections), and all numbers were summed to obtain full bulb totals. Statistical analyses were carried out using the Kolmogorov–Smirnov test for small samples.
Glomerular layer cell counts
A cell sampling technique was not needed in the glomerular layer, in which fewer TUNEL-positive cells were present. Images of all fluorescent objects in this layer were saved at a 40 × magnification, and examined in detail by two observers. The number of cells was recorded. The counts from two subjacent sections were averaged and multiplied by the number of sections not collected (13 sections), and all numbers were summed to obtain full bulb totals. Statistical analyses were carried out using the Kolmogorov–Smirnov test for small samples.
Results
Odor enrichment decreases the incidence of cell death in the granule cell layer
During PNDs 1–15, animals were systematically exposed to a battery of 30 odorants that included both natural odorants and synthetic odorant mixtures. Using TUNEL-labeling as a measure of cell death, the number of TUNEL-positive cells was compared in odor enriched and control animals on PND 16 (Fig. 1). Animals in the enriched group had 16% fewer TUNEL-positive cells in the granule cell layer of the main olfactory bulb than did control animals (enriched: 10526±620; control: 12490±960; mean±SEM; P<0.05; Kolmogorov–Smirnov test).
Fig. 1.
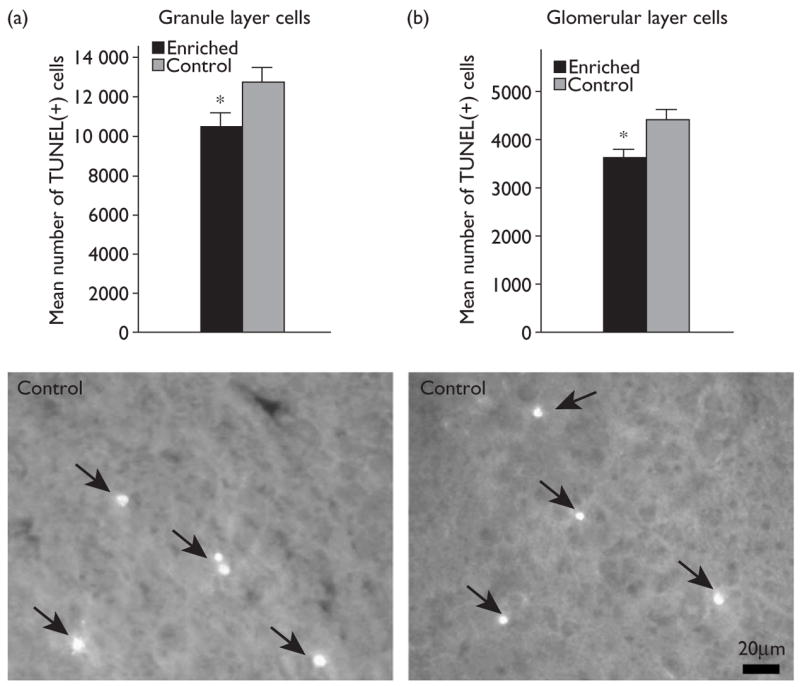
Bar graph illustrating the number of TUNEL-positive cells in the granule cell layer (a) and glomerular layer (b) of the main olfactory bulb in enriched (black bar) and control (gray bar) animals. Asterisks indicate P<0.05; Kolmogorov–Smirnov test. Below each graph is a representative example from control animals of TUNEL-positive cells (arrowheads) in the granule cell layer and glomerular layer, respectively. Error bars=SEM.
To determine whether the decrease in TUNEL-positive cells observed in the glomerular layer was localized to particular portions of the bulb, or distributed evenly across the rostro-caudal axis of the bulb, an analysis of variance was used to compare the incidence of TUNEL-positive cells through the full extent of the bulb. Comparisons of binned sections revealed a statistically significant decrease in the number of TUNEL-positive cells both across groups and across the rostro-caudal axis [F(1,126)=10.991; P<0.002; F(8,126)=54.686; P<0.0001]. The difference appeared to be distributed evenly throughout the rostro-caudal extent of the bulb (Fig. 2), and correlation analysis revealed a high correlation between the enriched and control groups across this axis (r2=0.9489).
Fig. 2.
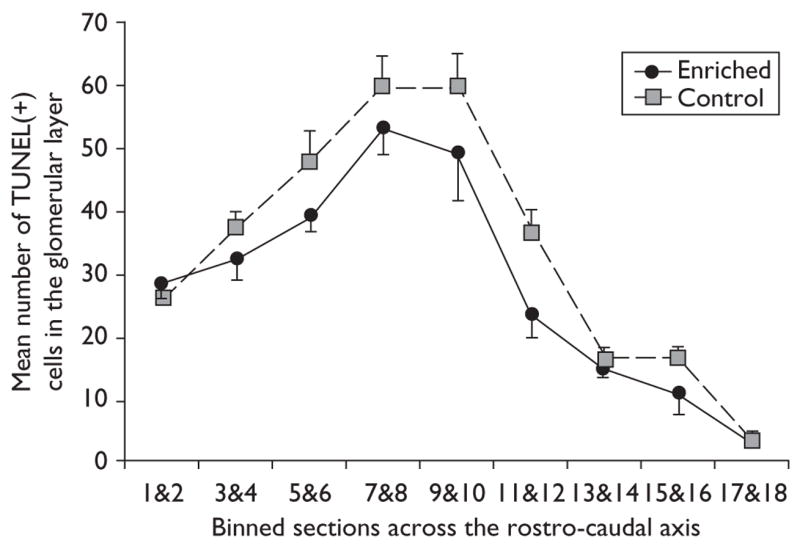
Mean number of TUNEL-positive cells across the rostro-caudal axis in the glomerular layer of enriched (black line) and control (gray line) animals. Individual values from two sections (binned sections) were summed then averaged across animals. The distance between each point on the x-axis is 560 μm. Error bars=SEM.
Odor enrichment decreases the incidence of cell death in the glomerular layer
Early odor enrichment during the first 2 postnatal weeks also resulted in decreased cell death in the glomerular layer on PND 16 (P<0.01; Kolmogorov–Smirnov test). A 16% decrease in the number of TUNEL-positive cells present in the glomerular layer was observed in odor-enriched animals compared with that in controls (3652±135 enriched; 4357±245 controls; mean±SEM; Fig. 1).
Other observations
Neither the area of the granule cell layer nor that of the glomerular layer changed relative to those of controls (P>0.05; Kolmogorov–Smirnov test; data not shown). In the granule cell layer, clusters of TUNEL-positive cells were observed fairly regularly in both the enriched and control groups. Often these clusters involved cells exhibiting different degrees of degradation. Similar clusters were also reported following olfactory deprivation by nares closure [7]. Clusters of TUNEL-positive cells were also observed in the glomerular layer of both enriched and control animals; however, their occurrence was rarer than that in the granule cell layer.
Discussion
During the early postnatal period, olfactory enrichment using a large number of natural and artificial odorants for as little as 2 weeks resulted in the saving of cells from an early death in both the glomerular and the granule cell layers of the main olfactory bulb. A striking similarity was noted in the percentage of cells saved from cell death in both the glomerular and the granule cell layers. In the glomerular layer, the decreased number of TUNEL-positive cells of enriched animals compared with that of controls was fairly evenly distributed throughout the rostro-caudal extent of the bulb, suggesting that either the entire bulb was similarly stimulated by the enriched odorant exposures or the olfactory enrichment affected the bulb in a global manner, perhaps resulting in the release of growth factors throughout the bulb, or a change in centrifugal input to the bulb.
In adult rodents, enriched odor exposure increased the survival of cells that had newly migrated to the bulb via the rostral migratory stream [10,11], and the olfactory-enriched animals performed better than controls in an olfactory memory task [10], although both these observations were reported to be short-lived, and enriched animals were comparable to controls 1 month after being returned to a normal cage [11]. In addition, in contrast to that seen in young rats, odor enrichment in adult mice affected the rostral bulb to a greater degree than the caudal bulb. The different results found in these studies were possibly due to different odorants used in the enrichment paradigm, or the species and age difference of the animals.
The locations of the TUNEL-positive cells in the glomerular and granule cell layers suggest that early olfactory enrichment may increase the survival of inhibitory interneurons. It seems possible that the increase in the number of inhibitory interneurons could contribute to an increase in lateral inhibition within the bulb. These data are consistent with the observation that early olfactory learning results in a decreased number of excitatory mitral cell responses to the learned odorant [12]. Similarly, short, daily exposure to an odorant for 6 days resulted in a decreased number of excitatory mitral cell responses not only to the familiar odorant but also to four other novel odorants [13].
Much data support the notion that an enriched environment can affect cell number or enhanced neural activity in other systems. In the visual system, an enriched environment results in acceleration of the visual system’s development in mouse pups by using both behavioral and electrophysiological measures [14]. Continuous exposure to an enriched environment, starting at PND 0, results in improved learning and enhanced visual acuity at 12 months of age [15]. In the somatosensory cortex, exposing adult rats to a ‘naturalistic’ environment leads to the reorganization of cortical sensory maps [16]. In the auditory system, an enriched environment results in increased responses, increased neural sensitivity, and decreased firing latencies in adult rats [17]. In the cricket, visual, auditory, and olfactory enrichment leads to increased neurogenesis in the mushroom body, a brain structure that integrates sensory information [18] and, in crayfish, an enriched environment results in increased proliferation and survival of neurons in the crayfish brain [19]. In many of these systems, increased levels of neurotrophic factors, specifically nerve growth factor or brain-derived neurotrophic factor, have been observed following environmental enrichment [14,20–23] and it would not be surprising if such factors contributed to the decrease in cell mortality that we have observed.
In the present study, early olfactory enrichment to a large battery of odorants during the first 2 postnatal weeks resulted in decreased cell death in both the glomerular and the granule cell layers of the bulb. It seems plausible that decreased cell mortality during this critical growth period would greatly influence the circuitry of the bulb and result in both long-lasting changes in the functioning and efficacy of the bulb, as well as increased sensitivity and responsiveness of bulbar neurons to odorants.
Conclusion
Early olfactory enrichment during the first 2 postnatal weeks using a battery of 30 different natural or synthetic odorants results in the sparing of olfactory bulb cells from an early death in both the glomerular and granule cell layers of the main olfactory bulb of the rat.
Acknowledgments
Sponsorship: Supported by grant DC03840 from the NIDCD to M.L.
References
- 1.Roselli-Austin L, Williams J. Enriched neonatal odor exposure leads to increased number of olfactory bulb mitral and granule cells. Dev Br Res. 1990;51:135–137. doi: 10.1016/0165-3806(90)90267-3. [DOI] [PubMed] [Google Scholar]
- 2.Woo CC, Leon M. Increase in a focal population of juxtaglomerular cells in the olfactory bulb associated with early learning. J Comp Neurol. 1991;305:49–56. doi: 10.1002/cne.903050106. [DOI] [PubMed] [Google Scholar]
- 3.Coopersmith R, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;4664:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- 4.Johnson BA, Woo CC, Duong H, Nguyen V, Leon M. A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Br Res. 1995;699:192–200. doi: 10.1016/0006-8993(95)00896-x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson BA, Leon M. Spatial distribution of [14C]2-deoxyglucose uptake in the glomerular layer of the rat olfactory bulb following early odor preference learning. J Comp Neurol. 1996;376:557–566. doi: 10.1002/(SICI)1096-9861(19961223)376:4<557::AID-CNE5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Coopersmith R, Henderson SR, Leon M. Odor specificity of the enhanced neural response following early odor experience in rats. Br Res. 1986;393:191–197. doi: 10.1016/0165-3806(86)90245-2. [DOI] [PubMed] [Google Scholar]
- 7.Najbauer J, Leon M. Olfactory experience modulated apoptosis in the developing olfactory bulb. Br Res. 1995;674:245–251. doi: 10.1016/0006-8993(94)01448-q. [DOI] [PubMed] [Google Scholar]
- 8.Fiske BK, Brunjes PC. Cell death in the developing and sensory-deprived rat olfactory bulb. J Comp Neurol. 2001;431:311–319. [PubMed] [Google Scholar]
- 9.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rochefort C, Lledo PM. Short-term survival of newborn neurons in the adult olfactory bulb after exposure to a complex odor environment. Eur J Neurosci. 2005;22:2863–2870. doi: 10.1111/j.1460-9568.2005.04486.x. [DOI] [PubMed] [Google Scholar]
- 12.Wilson DA, Leon M. Spatial patterns of olfactory bulb single-unit responses to learned olfactory cues in young rats. J Neurophysiol. 1988;59:1770–1782. doi: 10.1152/jn.1988.59.6.1770. [DOI] [PubMed] [Google Scholar]
- 13.Buonviso N, Gervais R, Chalansonnet M, Chaput M. Short-lasting exposure to one odour decreases general reactivity in the olfactory bulb of adult rats. Eur J Neurosci. 1998;10:2472–2475. doi: 10.1046/j.1460-9568.1998.00266.x. [DOI] [PubMed] [Google Scholar]
- 14.Cancedda L, Putignano E, Sale A, Viegi A, Berardi N, Maffei L. Acceleration of visual system development by environmental enrichment. J Neurosci. 2004;24:4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sale A, Putignano E, Cancedda L, Landi S, Cirulli F, Berardi N, Maffei L. Enriched environment and acceleration of visual system development. Neuropharmacology. 2004;47:649–660. doi: 10.1016/j.neuropharm.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Polley DB, Kvasnak E, Frostig RD. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature. 2004;429:67–71. doi: 10.1038/nature02469. [DOI] [PubMed] [Google Scholar]
- 17.Engineer ND, Percaccio CR, Pandya PK, Moucha R, Rathbun DL, Kelgard MP. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. J Neurophysiol. 2004;92:73–82. doi: 10.1152/jn.00059.2004. [DOI] [PubMed] [Google Scholar]
- 18.Scotto-Lomassese S, Strambi C, Strambi A, Charpin P, Augier R, Aouane A, Cayre M. Influence of environmental stimulation on neurogenesis in the adult insect brain. J Neurobiol. 2000;45:162–171. [PubMed] [Google Scholar]
- 19.Sandeman R, Sandeman D. ‘Impoverished’ and ‘enriched’ living conditions influence the proliferation and survival of neurons in crayfish brain. J Neurobiol. 2000;45:215–226. [PubMed] [Google Scholar]
- 20.Branchi I, Francia N, Alleva E. Epigenetic control of neurobehavioural plasticity: the role of neurotrophins. Behav Pharmacol. 2004;15:353–362. doi: 10.1097/00008877-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Gobbo OL, O’Mara SM. Impact of enriched-environment housing on brain-derived neurotrophic factor and on cognitive performance after a transient global ischemia. Behav Brain Res. 2004;152:231–241. doi: 10.1016/j.bbr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Pham TM, Soderstrom S, Winblad B, Mohammed AH. Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav Brain Res. 1999;103:63–70. doi: 10.1016/s0166-4328(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 23.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]


