Abstract
The pregnane X receptor (PXR; NR1I2) is a nuclear hormone receptor (NR) that transcriptionally regulates genes encoding transporters and drug-metabolizing enzymes in the liver and intestine. PXR activation leads to enhanced metabolism and elimination of xenobiotics and endogenous compounds such as hormones and bile salts. Relative to other vertebrate NRs, PXR has the broadest specificity for ligand activators by virtue of a large, flexible ligand-binding cavity. In addition, PXR has the most extensive sequence diversity across vertebrate species in the ligand-binding domain of any NR, with significant pharmacologic differences between humans and rodent PXRs and especially marked divergence between mammalian and non-mammalian PXRs. The unusual properties of PXR complicate the use of in silico and animal models to predict in vivo human PXR pharmacology. Research into the evolutionary history of the PXR gene has also provided insight into the function of PXR in humans and other animals.
1. Introduction
The pregnane X receptor (PXR; NR1I2) is a key regulator of xenobiotic, steroid hormone, and bile salt metabolism and excretion. PXR is a member of the nuclear hormone receptor (NR) superfamily, a diverse group of transcription factors found throughout the animal kingdom that regulate gene expression, often in response to binding of small molecules such as hormones, vitamins, and lipids. Genome sequencing projects have revealed 48 NRs in humans [1,2], 49 in mice [1], and 47 in rats [1]. Teleost (bony) fish have a somewhat larger complement of NR genes due to gene duplication [3–5], illustrated by the 68 NR genes in the pufferfish Fugu rubripes [6]. NRs share a characteristic multi-domain structure, which includes, from N-terminus to C-terminus, a modulatory A/B domain, the DNA-binding domain (DBD; C domain), the hinge D domain, the ligand-binding domain (LBD; E domain) and a variable F domain. Sequence-specific binding to ‘response elements’ in target genes is mediated by the DBD. The LBD mediates ligand activation, dimerization (to other NRs or homodimerization), and ligand-independent repression.
Insights into PXR function have been greatly aided by in vitro studies, mouse models, comparative genomics, and multiple high-resolution, crystallographic structures of human PXR bound to various ligands. These studies have revealed that PXR has a number of properties that are unusual in the NR superfamily. Three major features distinguish PXR from other NRs:
First, mammalian PXRs, particularly human PXR, have the broadest specificity for ligands of any functionally characterized NR, a consequence of a large and flexible ligand-binding pocket. The broad specificity of the PXR LBD makes it difficult to develop in silico models that can accurately predict ligand activity at human PXR. In addition, while the PXR LBD shares some structural features with other NRs, the crystal structures of human PXR bound to ligands reveal a mode of ligand binding different than that used by other NRs.
Second, PXRs show the greatest sequence and functional differences across species of any vertebrate NR, with substantial divergence even between mammalian PXRs. The high cross-species variation of PXR complicates the relevance of animal models to human pharmacology and physiology, a situation partially addressed by mice genetically altered to express human PXR in place of the endogenous (mouse) PXR. PXRs from the zebrafish (Danio rerio) and the African clawed frog (Xenopus laevis), two animals commonly used in high-throughout drug discovery and toxicology research, are especially divergent from mammalian PXRs. The frog PXRs, in fact, show completely different pharmacology and tissue expression patterns from mammalian PXRs. Paradoxically, in contrast to the high degree of sequence divergence across animal species, the PXR gene actually shows less inter-individual variation between humans than the genome-wide average for other genes. The documented genetic variation of the human PXR gene accounts for little of the observed phenotypic variation in liver and intestinal metabolism such as that carried out by cytochrome P450 (CYP) 3A4.
Third, PXRs show the strongest evidence for evolutionary selection in the LBD of any vertebrate NR, raising interesting questions on the evolution and biological functions of this receptor. The PXR LBD has likely adapted to cross-species differences in important ligands, although so far evidence exists only for biliary bile salts as ligands that have shaped PXR evolution. Evidence for evolutionarily relevant dietary or environmental activators is currently limited but it seems likely that exogenous ligands have also shaped the evolution of PXR.
This article reviews the insights that experimental and evolutionary analyses provide into the functions of PXR. This unusual NR presents many challenges for in vitro, in silico, and in vivo animal models to predict human PXR function.
2. Discovery and functional characterization of PXRs
2.1 Discovery and cloning of PXRs
PXR is part of the NR1I subfamily, which also includes the 1,25-(OH)2-vitamin D3 (calcitriol) receptor (VDR; NR1I1) and constitutive androstane receptor (CAR; NR1I3). Mouse and human PXRs were first cloned in 1998 and found to be highly expressed in liver and intestine [7–9]. Functional expression of human and mouse PXRs revealed activation by an impressive array of structurally diverse molecules, ranging from xenobiotics to steroids [7–11]. These studies showed that human PXR mediated the ability of the tuberculosis drug rifampin and the herbal antidepressant St. John’s wort to ‘induce’ (upregulate) the expression of drug-metabolizing enzymes such as CYP3A4 [7–9,12–14].
The name ‘pregnane’ X receptor derives from activation of the receptor by pregnane (21-carbon or C21) steroids such as progesterone or 5β-pregnan-3,20-dione [8,9], although estrane (C18) and androstane (C19) steroids also activate PXRs (note that another name for PXR is the ‘steroid and xenobiotic receptor’ or SXR). In contrast to the ‘classic’ steroid hormone receptors (e.g., estrogen and androgen receptors), high-affinity (subnanomolar) ligands for PXR have not been discovered. The lowest EC50 values of steroids for activating human PXR in reporter gene assays are low micromolar or barely submicromolar, generally at least two to three orders of magnitude higher than concentrations found circulating in plasma [7–10,13,15–18]. The highest affinity ligands for human PXR (e.g., hyperforin, the active component of St. John’s wort) only have binding affinities in the tens of nanomolar range [11,19]. PXR has been cloned and functionally expressed from zebrafish, frog, chicken, and multiple mammalian species (human, rhesus monkey, mouse, rat, rabbit, dog, and pig) [7–11,18,20–22].
2.2 Transcriptional targets of PXR
PXR activation induces the expression of broad-specificity hepatic and intestinal phase I enzymes such as CYP2C9 [23,24] and CYP3A4 [7,13,14,25]. PXR also upregulates the expression of phase II conjugating enzymes (e.g., uridine diphosphate glucuronosyltransferases or UGTs) [25,26] as well as ‘phase III’ transporters such as P-glycoprotein (MDR1; ABCB1) [14,25,27–29]. VDR, PXR, and CAR have overlapping functions and share transcriptional targets in regulating metabolism and elimination of endogenous compounds [30,31].
PXR-mediated upregulation of phase I enzymes, phase II enzymes, and transporters enhances the metabolism and elimination of a broad range of endogenous and exogenous compounds, including bile salts, steroid hormones, and xenobiotics [8,9,13,25,32–35]. In an evolutionary sense, PXR activation would serve a ‘chemical defense’ function by mediating a coordinated response to exposure to potentially toxic compounds [36]. In humans, certain xenobiotics cause clinically important drug-drug or drug-hormone interactions by virtue of PXR activation. For example, the efficacious PXR activators rifampin and St. John’s wort increase clearance of the immunosuppressant cyclosporine by inducing CYP3A4 and MDR1 expression, possibly leading to organ rejection in an allograft recipient [37]. Similarly, PXR activators increase metabolism of the estrogen component of combined oral contraceptives, potentially leading to unintended pregnancy [38,39].
3. Ligand specificity of PXRs
3.1 Broad ligand specificity of mammalian PXRs
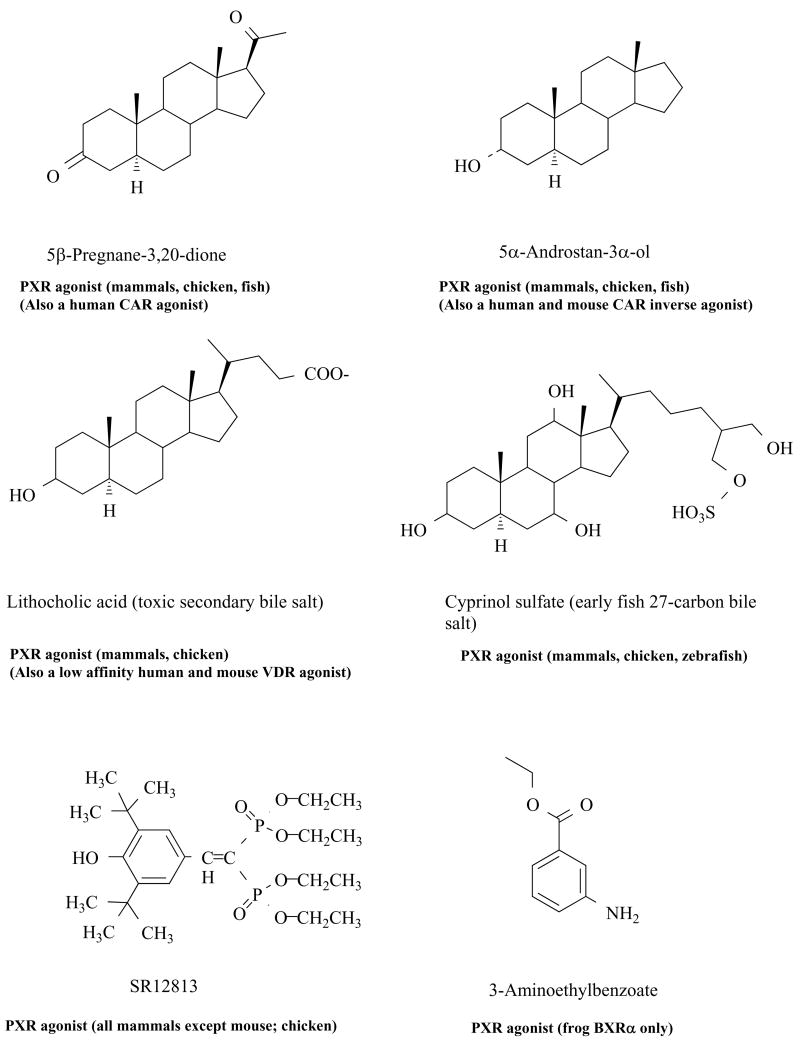
Human PXR has the broadest ligand specificity of any human NR, consistent with its flexible and large ligand-binding cavity [40–44]. In general, mammalian PXRs are remarkably promiscuous with respect to ligand specificity [45–47]. Human, rabbit, pig, and dog PXRs have especially broad specificity for activating compounds [10]. The ability of PXR to be activated by structurally diverse ligands parallels the broad substrate specificity of two important transcriptional targets of PXR: the CYP3A subfamily (e.g., CYP3A4 and 3A7 in humans; CYP3A11 in mice) [48–52] and P-glycoprotein [53–56]. A variety of ligands are capable of activating human PXR, including prescription drugs (rifampin, nifedipine, indinavir), herbal compounds (St. John’s wort), steroids (androstane, pregnane, and estrane), environmental contaminants, endocrine disruptors, and bile salts [7–11,18,19,57–60]. Some endogenous and exogenous ligands of PXR are shown in Figure 1.
Figure 1. Ligands for PXR.
Chemical structures of ligands for PXR including pregnane and androstane steroid hormones, bile salts (including an ‘early’ bile salt typical of the earliest bile salts to evolve in vertebrates), the experimental cholesterol-lowering drug SR12813, and the putative endogenous benzoate ligand of the Xenopus laevis BXRα. Note that some of the PXR activators are also agonists of VDR or CAR or, in the case of 5α-androstanol, inverse agonists of human and mouse CAR.
3.2 Structural basis of human PXR ligand specificity
The structural basis of the ligand promiscuity of human PXR has been studied in several high-resolution crystal structures of human PXR, including structures of human PXR bound to three different ligands – rifampin [44], SR12813 (experimental cholesterol-lowering drug) [40,43], and hyperforin [41]. The human PXR LBD shares a number of structural features with other NRs, including the ligand-binding cavity in one hemisphere and an ‘α-helical sandwich’ of helices α1/α3, α4/α5/α8, and α7/α10 in the other hemisphere [40–44]. However, the ligand-binding cavity of human PXR is large, smooth, and hydrophobic, which contrasts with ‘typical endocrine’ NRs (including VDR) that have compact ligand-binding cavities that approximate the shape of their specific ligands [42]. The human PXR ligand-binding cavity also shows considerable flexibility, expanding by 250 Ǻ3 to accommodate the ligand hyperforin [41]. There are a number of features of the human PXR LBD that are not found in other NRs and which contribute to its broad ligand specificity: a variable four-residue turn between helices α1 and α3, replacement of α6 by a large, flexible loop, and two additional β strands not observed in other NRs [40–44]. No other NR has been documented to bind such large and diverse ligands [42,46].
3.3 Cross-species difference in PXR activators
There are considerable differences between species in terms of PXR activators. Human, dog, pig, rabbit, and chicken PXRs have very broad ligand specificity, accommodating large ligands such as rifampin while mouse PXR has a narrower ligand specificity [10,11,58]. Pregnenolone 16α-carbonitrile activates mouse but not human PXR, whereas human PXR is more sensitive to hyperforin and rifampin than mouse PXR [7,8,10,11,13,61,62]. The PXRs from the African clawed frog Xenopus laevis deserve special mention in that these receptors completely lack the broad ligand specificity of other PXRs and have a tissue expression pattern different from other PXRs, being found not in drug-metabolizing organs like liver or intestine but mostly in gonadal tissue and brain [10,20,63–65]. The frog PXRs are not activated by xenobiotics, steroids, or bile salts, but essentially only by benzoates (see Figure 1), endogenous compounds with unique roles in frog development (hence the alternative term of benzoate X receptors, or BXRs, for the frog PXRs) [10,20,63,66]. The zebrafish PXR shares a number of steroid and bile salt activators with mammalian PXRs but is only activated by a handful of xenobiotics [10,66,67].
4. Sequence variation of PXRs
4.1 Cross-species variation in PXR amino acid sequences
Vertebrate NR genes typically show tight sequence conservation between species. For example, amino acid sequence identities between orthologous human and mouse NR genes are typically greater than 95% in the DBD and greater than 85% in the LBD [1]. Not surprisingly, a study comparing human, mouse, and rat genomes and another study comparing human, mouse, and chimpanzee genomes revealed that genes in the NR superfamily have been subjected to negative evolutionary selection (i.e., selection against changes in protein amino acid sequence) [1,68]. The only two clear exceptions in the vertebrate NR superfamily were the LBDs of PXR and CAR [1,66,67,69].
In contrast to other NRs, a striking feature of PXR is high cross-species sequence divergence in the LBD (Figure 2). The LBD of PXR shares amino acid identities of only 75% between human and rodent sequences and only 50% between human, zebrafish, and chicken sequences [1,10]. The sequence identities of the PXR LBD across species are the lowest in the NR superfamily, whose other members tend to have comparable identities between species at least 10–15% higher [1,10].
Figure 2. Sequence alignment of PXR LBDs along with CAR and VDR.
The location of the α-helices above the amino acid sequence are based on the structures of human PXR [43] and human VDR [160]. Amino acid residues in bold type are residues show to directly interact with ligand in high-resolution x-ray crystallographic structures of human PXR [40,41,43,44], human VDR [160–163], human CAR [164], and mouse CAR [165,166]. Note that the orthologous positions of many of the amino acid residue positions involved in binding of ligand in PXR are also involved in ligand binding in VDRs and CARs. The extreme sequence divergence between PXRs is especially seen in the region between helices 1 and 3, where some PXRs (e.g., chicken, frog) lack sequence relative to other PXRs and where the zebrafish PXR has a stretch of sequence similar in length to that of mammalian PXRs but very dissimilar in sequence similarity.
PXR even shows extensive sequence variation at amino acid positions corresponding to residues that interact directly with ligands in X-ray crystallographic structures of human PXR. Even within mammals, there is substantial divergence of PXR ligand-binding residues [40,41,43,44], an especially unusual finding in the NR superfamily [67]. This is likely the most extreme divergence of ligand-binding residues of any of the ligand-activated NRs in vertebrates [31]. In contrast, ligand-binding residues are strongly conserved in the VDRs (a ‘classic’ endocrine NR closely related to PXR), with only 4 residues showing any difference across vertebrate species ranging from human to sea lamprey (a jawless fish). Only one ligand-binding residue varies at all between mammalian VDRs [31].
4.2 Phylogenetic analyses of vertebrate PXRs
The section above describes variation of amino acid sequence. The cross-species variation in the LBD of PXR is even more striking when DNA sequences are compared, in particular by analyzing the rate of nonsynonymous (changes amino acid sequence of a codon) and synonymous (does not change amino acid sequence) nucleotide substitution rates. The ratio of the rate of non-synonymous versus the rate of synonymous nucleotide variation (i.e., how many non-synonymous or synonymous changes have occurred in comparison to the total number of non-synonymous or synonymous changes possible; dN/dS or ω ratio) provides some indication into evolutionary selective forces acting on a given gene [70]. Synonymous substitutions are considered to be ‘neutral’ with respect to functional consequences, an assumption that is probably true most of the time, although there are documented exceptions [71]. For most gene comparisons, the ω ratio is less than one, often less than 0.1, reflective of ‘negative’ or ‘purifying’ selection to maintain a conserved amino acid sequence (i.e., changes in amino acid sequence are deleterious to function) [70]. An ω ratio of 1 reflects neutral selection (a finding that would be expected for a non-functional pseudogene), while an ω ratio greater than 1 suggests ‘positive’ selection (in this case non-synonymous substitutions are actually favored over synonymous, ‘neutral’ substitutions). Large-scale comparisons of genes between species show that very few genes, or even gene domains, have ω ratios equal to or exceeding one. Most of the genes with these properties were either viral proteins or proteins with immune or reproductive properties [72]. In two-species comparisons between human genes and either mouse or rat genes, PXR genes have ω ratios severalfold higher than the average for all other NR genes [1,68].
More sophisticated phylogenetic analysis with the ability to analyze individual codons within genes, using techniques such as the maximum likelihood method [73–75], reveals that the PXR LBD has a sub-population of codons with the highest ω ratios of any gene in the vertebrate NR superfamily [66,67,69]. These results suggest that natural selection has favored sequence diversity in the LBD of PXR, possibly to adapt to cross-species differences in important ligands. PXR may therefore represent an unusual example of an NR gene that has changed ligand specificities across vertebrate species to adapt to cross-species differences in exogenous and/or endogenous toxic compounds [8,10,66,67,76,77].
The Xenopus laevis (frog) PXRs (benzoate receptors α and β or BXRα and BXRβ) show the strongest evidence for positive selection. Relative to other PXRs such as those from zebrafish, chicken, or mammals, the BXRs have lost broad specificity for ligands, gained high efficacy activation by endogenous benzoates, and altered tissue expression pattern to play a developmental role in the frog [20,63,64]. Phylogenetic analysis by maximum likelihood shows that 23 codons in BXRα and/or BXRβ show strong evidence for positive selection, with nearly half of these codons having predicted influence on ligand specificity [66,67]. Overall, phylogenetic analyses of PXR genes across species strongly suggest that the LBD of PXRs has changed across species to evolutionary advantage.
4.3 Genetic variation of PXR within humans
The marked diversity of the PXR LBD across vertebrates contrasts with detailed ‘re-sequencing’ studies of the human PXR gene showing that mutations in the PXR coding region are quite rare, although variation in non-coding regions or due to splice variants may have clinical importance [78]. Re-sequencing of 100 individuals from multiple ethnic groups for the PXR gene showed nucleotide diversity lower than the genome-wide average for human genes and no aminoacid changing mutations in the LBD [79]. Sequencing of 205 Japanese subjects found non-synonymous substitutions in two individuals that caused modest reductions in a transactivation assay using a mammalian cell line [80,81]. Two separate substitutions in the PXR LBD were discovered in a re-sequencing study of 74 Africans and 418 Caucasians [17]. Sequence differences in the coding region of PXR do not account for well-described inter-individual differences in metabolism, such as variation in baseline activity or inducibility of CYP3A4 in liver or intestine [78].
In addition, sequence divergence is also low between human, chimpanzee, and rhesus monkey PXRs [10,67,68]. Specifically, the nucleotide divergence between the human and chimpanzee PXR genes is lower than the average for other genes in the human and chimpanzee genomes [68,69,82]. This suggests that important ligands for PXR, at least in terms of influencing reproductive fitness, do not vary between humans, or perhaps not even between humans and other primates, but do vary between primates and other animals.
5. Evolutionary significance of PXR activators
5.1 Ligand specificity and PXR evolution
The broad ligand specificity of PXR, coupled with its transcriptional targets in the liver and intestine, suggests that a major function of this receptor is to protect animals against toxic compounds [32,34,36,76,77,83]. From an evolutionary standpoint, these toxic compounds may be endogenous (hormones, bile salts) or exogenous (likely of dietary origin). An important goal of comparative evolutionary studies of PXR is to explain why PXR shows little sequence and functional variation within humans, or even between humans and other primates, but such striking sequence variation between primates and other species in the LBD. This implies that key ligands for PXR vary across species due to differences in physiology, environmental exposure, and/or diet.
5.2 Bile salts as PXR ligands
Bile salts such as cholic acid (3α, 7α, 12α-trihydoxy-5β-cholan-24-oic acid) are the end-products of cholesterol metabolism and also solubilize lipophilic compounds in the gut [84]. Bile salts are synthesized in the liver and stored in the gallbladder (in those animals that have this organ), and are generally not toxic even when micromolar concentrations accrue in the circulating plasma. An exception is lithocholic acid (3α-hydroxy-5β-cholan-24-oic acid), a mono-hydroxylated ‘secondary’ bile acid formed by the action of bacterial 7-dehydroxlases on primary bile acids such as chenodeoxycholic acid (3α,7α-dihydroxy-5β-cholan-24-oic acid). High levels of lithocholic acid are cytotoxic and implicated as a factor in colon cancer [85].
Mouse models have illustrated the importance of PXR-mediated pathways in lithocholic acid toxicity. PXR ‘knockout’ mice are more susceptible to exogenously administered lithocholic acid [35,86–89]. Conversely, administration of PXR activators, or genetically engineering mice to express high levels of PXR, reduces the toxic sequelae of high doses of exogenous lithocolic acid [86,87,89]. Lithocholic acid is a low potency PXR activator, with maximal effects occurring only at concentrations around 100 μM. PXR activation by lithocholic acid leads to upregulation of several genes that can detoxify lithocholic acid, including the CYP3A enzymes [88]. VDR is also activated by micromolar concentrations of lithocholic acid; this has physiologic importance particularly in the gut where high levels of lithocholic acid can accumulate [90].
In severe cholestatic liver injury, serum concentrations of bile acids other than lithocholic acid reach concentrations high enough to activate PXR [91]. This was demonstrated clearly in a mouse bile duct ligation model, in which hepatic CYP3A11 (ortholog of human CYP3A4) expression was upregulated even though serum levels of lithocholic acid were not increased, whereas serum levels of 6β-hydroxylated bile acids were markedly increased [92]. Given that 6β-hydroxylated bile acids such as β-muricholic (3α,6β,7β-trihydroxy-5β-cholan-24-oic acid) and murideoxycholic acid (3α,6β-dihydroxy-5β-cholan-24-oic acid; also known as murocholic acid) are efficacious activators of mouse PXR, but not other NR1I subfamily members [66,90], hepatic CYP3A11 upregulation in the bile duct ligation model is likely PXR-mediated.
The importance of PXR in bile salt metabolism and elimination is also illustrated by the rare disease cerebrotendinous xanthomatosis (CTX), an inborn error of metabolism caused by deficiency of CYP27A1. The enzyme defect of CTX results in pathological accumulation of 27-carbon (C27) bile alcohols (which retain the entire carbon skeleton of cholesterol), leading to gallstones, xanthomas, and neurologic dysfunction [93]. Interestingly, knockout of the Cyp27a gene in mice did not reproduce the symptoms seen in human CTX due to a dramatic increase of CYP3A expression that allowed the Cyp27a−/− mice to bypass the enzyme deficiency and detoxify the bile acid precursor 5β-cholestan-3α,7α,12α-triol (‘5β-cholestantriol’) [94,95]. Two research groups later showed that 5β-cholestantriol activates mouse, but not human, PXR (more precisely 5β-cholestantriol is probably a very weak partial agonist of human PXR) [57,96]. Because 5β-cholestantriol does not effectively activate human PXR, individuals with CTX are unable to prevent pathological accumulation of 5β-cholestantriol and other bile acid precursors.
In contrast to the data in mammals described above, the first major study to systematically compare multiple non-mammalian and mammalian PXRs found that the zebrafish PXR was not activated by a variety of bile acids and synthetic bile acid derivatives [10]. However, biliary bile salts vary significantly across vertebrate species and the bile acids found in humans, mice, and most other mammals are not found in zebrafish and some other fish [97–99]. Most mammals and birds, and even the majority of present-day bony fish, convert 27-carbon cholesterol predominantly to C24 bile acids such as cholic acid and chenodeoxycholic acid, conjugated to either glycine or taurine. In contrast, the evolutionarily ‘earliest’ fish (i.e., the fish most distantly related to humans), represented now by jawless fish (lampreys, hagfish), cartilaginous fish (e.g., sharks, skates, rays) and some bony fish (like zebrafish), synthesize C27 bile alcohols conjugated with sulfate (see Figure 1) [99,100]. In these ‘early’ fish, C27 bile alcohol sulfates account for nearly all biliary lipids [100]. The zebrafish does not produce any C24 bile acids and instead synthesizes 5α-cyprinol (5α-cholestan-3α,7α,12α,26,27-pentol) sulfate [101,102], a bile alcohol sulfate very similar to the bile salts found in the earliest vertebrates to evolve, the jawless fish [103,104]. The bile salt synthetic pathway leading to C27 bile alcohol sulfates is a simpler pathway than that needed to produce C24 bile acids such as cholic acid (avoiding for example the need to cleave the cholesterol side-chain) and likely represents the first bile salt synthetic pathway to evolve in vertebrates [100,102].
In a functional assay, zebrafish PXR was activated efficaciously by cyprinol sulfate, scymnol (5β-cholestan-3α,7α,12α,24,26,27-hexol) sulfate (from the Spotted eagle ray, a cartilaginous fish), and essentially by no other bile salts [66,67]. Further, human, mouse, rat, rabbit, and chicken PXRs were all activated by cyprinol sulfate and scymnol sulfate [66]. Activation by C27 bile alcohol sulfates thus appears to be a ‘basal’ property of PXRs and has been retained as a vestigial function in mammalian and chicken PXRs, even though these animals, including humans, produce only minute quantities of C27 bile alcohols except in rare inborn errors of bile salt metabolism. The ability to be activated by C24 bile acids is likely a more recent evolutionary innovation for PXRs.
Overall, the variation of bile salts across species parallels the sequence variation of the PXR LBD. Bile salts vary little between primates [98], but do show differences between humans and other mammals (e.g., α- and β-muricholic acids are the main primary bile acids in rodents while cholic and chenodeoxycholic acids are dominant in humans and other primates) [97,98]. Even within mammals, there is evidence that PXRs have adapted to variations in biliary bile salts [66]. As described above, there is even more divergence between human and early fish bile salts, and PXRs again appear to have adapted to these differences. Thus, biliary bile salts are plausible endogenous ligands whose variation across species has influenced the ligand specificity of PXRs. This hypothesis can be strengthened by more extensive testing in additional vertebrates, particularly cartilaginous and jawless fish, and in reptiles.
PXR activation by high circulating levels of bile acids could be a protective response to cholestasis of various etiologies. Even if high circulating levels of some bile salts do not directly result in toxicity, the presence of elevated bile acids signifies likely impairment of hepatobiliary excretion of xenobiotics and some endogenous compounds. PXR activation would thus be an attempted adaptive response to increase metabolism and elimination of toxic compounds by alternative pathways. The ability of PXR to mediate detoxification of bile acids suggests that activators of this receptor may find therapeutic effect in treating cholestasis and diseases such as primary biliary cirrhosis where abnormally high levels of bile acids accumulate [105]. Indeed, the PXR activator rifampin has shown therapeutic benefit in the treatment of primary biliary cirrhosis [106]. It remains to be seen if selective PXR agonists can be developed to detoxify bile acids while avoiding adverse effects.
5.3 Steroid hormones as PXR ligands
PXRs generally share the property of being activated by micromolar concentrations of androstane and pregnane steroids. This property is conserved across all PXRs, except for the divergent Xenopus laevis BXRs. For example, both 5β-pregnan-3,20-dione and 5α-androstan-3α-ol activate human, rhesus monkey, dog, pig, mouse, rat, rabbit, chicken, and zebrafish PXRs [10]. Though it is tempting to view such conservation as significant, physiologic relevance of steroid effects on PXR have been difficult to prove.
The concentrations of individual steroid hormones that affect PXR are much higher than concentrations typically found in human serum or plasma, even during pregnancy or fetal development. What has not been examined in detail is the ability of combinations of steroid hormones, for instance at levels found in pregnancy, to activate PXRs. A recently published clinical study clearly shows that CYP3A activity, as measured by N-demethylation of dextromethorphan, is increased approximately 35% throughout all trimesters of pregnancy [107], confirming previous more limited investigations of drug metabolism during pregnancy, suggesting that hormonal changes may influence CYP3A expression. However, hormonal changes during the menstrual cycle have generally not been shown to affect CYP3A expression [108–110]. The increase in CYP3A during pregnancy may thus be mainly due to fetal or placental contribution [107], although this may not explain the significant rise in CYP3A activity in the first trimester of pregnancy.
Whether hormonal factors during pregnancy increase CYP3A activity, possibly via PXR-mediated pathways, warrants more careful investigation. One possibility would be to expose human hepatocytes or cells that recombinantly express PXR to maternal serum or to combinations of hormones found in pregnancy. The elevation of CYP3A activity in pregnancy serves the possible function of protecting the developing fetus from harmful compounds and is a potential evolutionarily important adaptation. It will be interesting to see if this finding is seen in other animals and, if so, whether PXR mediates the effect.
The physiologic roles of the pregnane and androstane steroids most active at PXRs are not well understood. A recent report provides evidence that PXR activation by 5β-dihydroprogesterone mediates chronic uterine relaxation during pregnancy via regulation of inducible nitric oxide synthase expression [111]. Several of the androstane steroids that activate PXRs (and are inverse agonists at mouse CAR) [112] have documented pheromone activities in some mammals. These include 5α-androst-16-en-3α-ol and 5α-androst-16-en-3-one, musk-scented compounds with pheromone activities in boars but unclear effects in humans [113,114]. The role of androstane and pregnane steroids such as 5β-pregnan-3,20-dione, 5α-androstan-3α-ol, or 5α-androst-16-en-3α-ol is basically unknown in non-mammalian species. Overall, the physiologic and evolutionary relevance of pregnane and androstane steroids as PXR activators remains an open question.
5.4 Xenobiotics as PXR ligands
The impressive ability of PXR to be activated by xenobiotics in humans suggests that a possible evolutionary function of PXR is to detect toxic exogenous compounds, acquired through diet or environmental exposure. A similar role may also be performed by the aryl hydrocarbon receptor, a key regulator of the CYP1A genes, which has recently been shown to be activated by dietary compounds in cow’s milk [115]. Somewhat surprisingly, evidence that dietary ligands activate PXR has been slow to accumulate, although vitamin E [116–118] and carotenoids [119] are now documented PXR activators. The identification of dietary or environmental ligands for PXR and CAR will be aided by cloning and functional expression of these genes from more species, particularly focusing on species that show diversity of evolutionary history and diet.
6. PXR functions in non-mammalian species
6.1 Function and evolution of non-mammalian PXRs
In contrast to the wealth of data on mammalian PXRs, there has been much less functional characterization of PXRs and of the regulation of liver and intestine metabolism in non-mammalian species. This differs considerably from the aryl hydrocarbon receptors, key regulators of CYP1A expression, for which fish models have provided considerable insight into toxicology, pharmacology, and receptor function [120]. Similar to mammals, some drugs and endogenous compounds can induce CYP3A gene expression in reptiles, amphibians, and fish. Chemically-induced upregulation of CYP3A expression has been demonstrated in microsomes from a Xenopus laevis kidney cell line by dexamethasone and corticosterone [121], in microsomes from alligator liver by phenobarbital and 3-methylcholanthrene [122], in Atlantic cod by alkylphenols [123], in rainbow trout and killifish by ketoconazole [124], in adult zebrafish liver by pregnenolone 16α-carbonitrile (but not clotrimazole or nifedipine) [125], and in larval zebrafish foregut by rifampin and dexamethasone [126]. The molecular mechanism of CYP3A induction in the species mentioned above has not been precisely determined.
Curiously, the compounds shown to increase CYP3A expression in zebrafish adult liver (pregnenolone 16α-carbonitrile) [125] or larval foregut (rifampin, dexamethasone) [126] did not activate zebrafish PXR in an in vitro reporter assay [10], whereas clotrimazole and nifedipine, which did activate zebrafish PXR in vitro [10], did not induce CYP3A in adult zebrafish liver [125]. This raises the possibility that other NRs (e.g., glucocorticoid receptors) or other receptors regulate hepatic and intestinal metabolism and elimination in fish. Overall, much work remains to be done with non-mammalian models of PXR function.
The origins and evolution of the NR1I subfamily, which currently includes PXR, VDR, and CAR, is an area of active inquiry. Based on current genetic data, multiple NR1I subfamily members have been found only in vertebrates. The orthologs (if any) of the NR1I subfamily members in the fruit fly Drosphila melanogaster or the nematode Caenorhabditis elegans are unknown. A single NR1I-like gene equally similar to VDR/PXR/CAR and a Drosophila NR is found in the draft genome of the urochordate Ciona intestinalis, an invertebrate much more closely related to vertebrates than flies or nematodes [127]. The functional properties of this invertebrate NR remain uncharacterized.
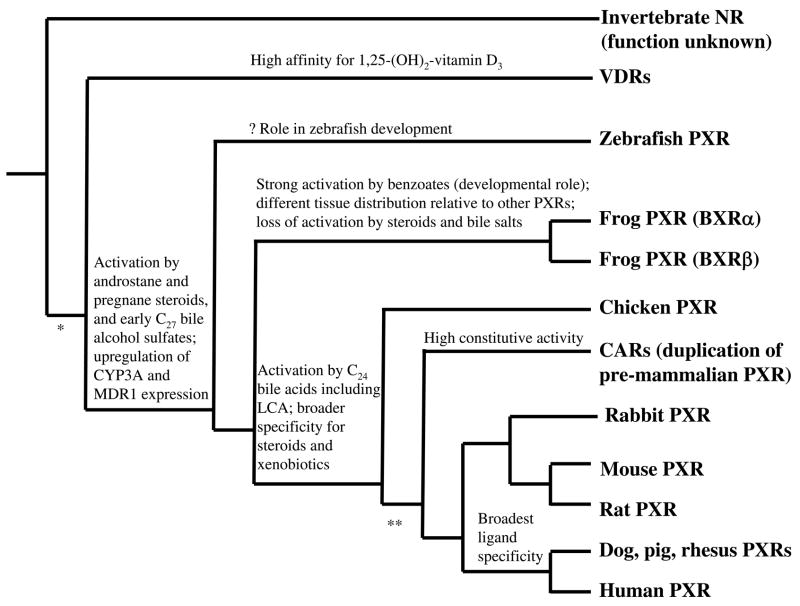
The evolutionary origins of VDR and PXR in vertebrates also remain unclear [31], although it appears probable that a single NR gene duplicated early in vertebrate evolution. These two genes then diverged from each other to become the separate PXR and VDR genes found in modern-day species [31]. Additional duplications have resulted in multiple PXR and VDR genes in some non-mammalian species. For example, the pufferfish has two VDR genes [6] and the Xenopus laevis frog has two PXR genes (BXRα and BXRβ) [20,63]. Distinct PXR and CAR genes appear to be solely found in mammals. An elegant series of studies demonstrated that the chicken only has a single ‘xenobiotic-responsive’ NR1I gene (currently classified as a PXR although some researchers debate this), the product of which has properties similar to both CAR and PXR [128]. Genome sequencing studies in two pufferfishes [3,6,129], chicken [130], and the frog Xenopus tropicalis have so far failed to find evidence for a CAR gene. A likely explanation is that an ancestral gene similar to the chicken PXR duplicated just prior to or early in mammalian evolution [31]. The two genes then diverged from other another to become the modern-day PXR and CAR genes found in all mammalian genomes sequenced so far (including opossum, two species of seals, dog, pig, mouse, rat, and rhesus monkey). The evolutionary benefit to mammals for having both PXR and CAR genes is not clear. Figure 3 shows a proposed phylogeny of the NR1I subfamily, focusing on PXR, and taking into account functional data and tissue expression patterns of the subfamily members.
Figure 3. Proposed phylogeny of PXRs showing functional characteristics.
The phylogenetic tree is derived from known phylogenetic relationships of the animal species integrated with tissue expression patterns and pharmacological properties. Characteristics included are tissue expression pattern; activation by pregnane steroids, androstane steroids, C27 bile alcohol sulfates (representative of the earliest bile salts to evolve in vertebrates), C24 bile acids (such as cholic acid and lithocholic acid, LCA); ability to upregulate expression of CYP3A and MDR1; and high constitutive activity. * and ** indicate proposed times when gene duplication occurred leading to expansion of the NR1I subfamily (that currently includes PXR, VDR, and CAR) when one of the duplicated genes diverged and took on different functions. An ancestral gene is thought to have duplicated in early vertebrate evolution, ultimately resulting in separate VDR and PXR genes (*). Just prior to or early in mammalian evolution, the PXR gene is thought to have duplicated (**); subsequent divergence of one of these genes resulted in the CAR gene.
6.2 PXR role in development
The dominant role of the Xenopus laevis BXRs in regulating early frog development [20,63,64] and the high levels of PXR expression in larval zebrafish [125], suggests that PXR may generally be a developmental regulator in non-mammalian vertebrates. Developmental roles of PXR in mammals have not been detected. PXR knockout mice are phenotypically normal unless challenged with potentially toxic compounds such as lithocholic acid or xenobiotics [88,131]. It remains to be seen if PXR mediates subtle functions during mammalian development.
7. Challenges in modeling and predicting human PXR function
7.1 Human hepatocytes
Xenobiotic-mediated induction of enzymes and transporters involved in drug metabolism and elimination are a significant cause of drug-drug and drug-herbal product interactions that can lead to morbidity and mortality in patients [132]. CYP3A4 alone has a role in metabolizing approximately 50% of prescription drugs in the United States [133] explaining why economical and accurate determination of CYP3A4 induction potential is a high priority in drug development [50,134–137]. Given the prominent role of PXR in regulating drug metabolism and elimination, assessing PXR activation is also important in drug development [138]. The most widely used in vitro methods for assessing PXR activation are cell culture-based reporter assays and human hepatocytes [24,135–137,139–141]. Aside from in vivo experiments, short-term hepatocyte cultures are the ‘gold standard’ for in vitro assessment of drug-mediated induction of enzymes and transporters, a topic that has been reviewed in detail elsewhere [24,137,139,140]. The main limitations of hepatocyte cultures are scarcity of supply, cost, and relatively high degrees of inter-individual variability [137,139].
7.2 In vitro reporter assays
Cell culture-based reporter assays are much cheaper than human hepatocyte culture and can be adapted for high throughput [136,138,141]. The reporter assays use either full-length PXR, in which the reporter gene is typically the CYP3A4 promoter driving expression of luciferase, or ‘two-hybrid’ reporter systems using the PXR LBD [136,138,141]. The advantages of reporter assays are the ability to specifically assess PXR activation without the contribution of other receptors, although careful controls must be used with full-length PXR assays to insure that the cell line used does not express endogenous PXR or other receptors (e.g., VDR or CAR) capable of activating the reporter gene [138].
The limitations of cell culture-based reporter assays are that the cell lines currently available cannot fully reproduce the hepatocyte. This can lead to discrepancies between experiments using reporter assays versus human hepatocytes. For example, St. John’s wort is more potent than rifampicin as a human PXR activator using reporter assays [60]; the situation is reversed when assessing CYP3A4 induction in human hepatocytes [12]. This type of discrepancy can occur when hepatocytes or intact livers metabolize the compound studied or when efflux transporters remove the compound from the hepatocyte [12]. Therefore, while reporter assays can be used for high-throughput screening for PXR activation, positive results should be confirmed in vivo or in human hepatocytes, if indicated. It is also critical that cytotoxicity for compounds be assessed as toxicity may lead to underestimates of PXR activation [142].
7.3 Animal models
The most widely used animal model for PXR function is the mouse [143]. PXR ‘knockout’ (PXR−/−) mice have been widely studied and have demonstrated the importance of PXR in detoxifying bile acids and xenobiotics [26,35,87–89,131,144–147] and in maintaining vitamin K homeostasis [148]. The first research group to generate PXR−/− mice showed clearly that PXR mediated the ability of pregnenolone 16α-carbonitrile to induce drug-metabolizing enzymes in mice [131]. Following the generation of PXR−/− mice, this group then created ‘humanized’ mice that lacked the mouse PXR but overexpressed an activated form of human PXR in the liver. Unlike wild-type mice, the humanized PXR mice responded to rifampin administration by upregulating CYP3A11 expression but, as expected, were unresponsive to pregnenolone 16α-carbonitrile [131]. These humanized mice remain the best animal system for modeling human PXR function in vivo [143,149].
The functional divergence of PXR across mammalian species complicates the use of other animal models. All the mammalian PXRs studied so far show significant differences so far in terms of PXR activation [10,18,22,66]. Human, dog, pig, and rabbit PXRs have very broad specificity for xenobiotics and endogenous compounds, whereas mouse PXR has more narrow ligand specificity [10]. There is an additional complication that CAR, a NR that has overlapping ligand specificity and functions with PXR, also shows significant divergence across species. Therefore, pre-clinical animal studies of drugs can show substantial differences between humans and other animals in the upregulation of enzymes and transporters involved in metabolism and elimination of xenobiotics and endogenous compounds. This reinforces the value of the humanized mouse strain described above and of the continuing development of mouse strains that express human genes in place of the endogenous genes.
The situation becomes even more difficult in non-mammalian species. PXRs in these animals are particularly divergent in sequence and function from human PXR [10,31,66,67]; furthermore, chicken, frog, and bony fish lack the CAR gene and cannot reproduce the mammalian situation of having both PXR and CAR genes [10,20,58,128]. Consequently, animals amenable to high-throughput small molecule or developmental toxicology screening, such as zebrafish or Xenopus laevis frogs, are poor models for human PXR function. These animals may, however, provide insight into subtle developmental effects or alternative pathways mediated by PXR not yet discovered using mouse models [20,64,125].
7.4 In silico models
Perhaps not surprisingly given the broad specificity that human PXR has for binding ligands, development of predictive in silico models of human PXR-ligand interactions has proven to be difficult. Similar to efforts to model enzymes and transporters with broad specificity, such as CYP3A4 [48,150] or the P-glycoprotein transporter [53,151–153], in silico modeling of PXR activation has important applications in drug development, particularly if such models can reliably eliminate drug candidates that would activate PXR and cause potentially harmful drug-drug interactions [154,155].
Three studies have utilized molecular modeling analysis for a series of PXR ligands [156–158]. The first study looked at barbiturate, hydantoin, and macrolide antibiotic activation of human PXR but only performed limited computational analysis involving intra-atomic distances between atoms in the ligands [156]. The second study utilized data on 12 diverse ligands (rifampicin, pregnenolone 16α-carbonitrile, dexamethasone, RU-486, clotrimazole, SR12813, TCPOBOP, androstanol, 5β-pregnane-3,20-dione, hyperforin, lithocholic acid, and 3-keto-lithocholic acid) from three different studies [11,60,88] to develop a three-dimensional pharmacophore using Catalyst® (Accelrys, Inc.) [157]. This study was completed prior to the first report of a human PXR LBD crystal structure [43], and the reported pharmacophore was found to be in agreement with the predominantly hydrophobic nature of the PXR LBD as revealed by the crystal structure. The developed pharmacophore was validated using an external test set of 28 known PXR ligands.
The third and more recent study examined a dataset of 54 compounds and developed both ligand-based and structure-based models, also using Catalyst® [158]. This study conclusively found only one structural feature that was common to all PXR ligands. However, highly active PXR ligands were found to share certain unique features that were postulated to enhance receptor activation by occupying the large binding pocket. Some of the models developed in this third study may be amenable to screening large databases of compounds, but this was not extensively validated. This study also investigated two pairs of structurally similar ligands (docetaxel/paclitaxel and cortisol/cortisone) to explain their differential activity at human PXR. Both of the studies using Catalyst® [157,158] were technically sound but do have the caveat of having used data from multiple previous studies rather than a dataset collected by a single protocol. Predictive modeling of PXR would be aided by the collection of a large dataset of ligands using a consistent experimental method.
So far, it has not been possible to utilize the published crystal structures of human PXR for predictive high-throughput docking studies. The flexible nature of the human PXR LBD, discussed above in Section 3.2, makes structure-based modeling very challenging [157,158]. The determinants that mediate ligand selectivity of human PXR are still not fully understood, partly due to the flexibility of the human PXR LBD. Comparisons between human and mouse PXR [43] or rat PXR [159] have helped in determining some of the structural factors that differentiate human PXR from the rodent PXRs, aided by a homology model of the rat PXR [159]. Overall, however, much remains to be learned about the structural factors mediating cross-species differences in PXR ligand specificity.
8. Expert opinion and conclusion
PXR is a key regulator of the metabolism and excretion of xenobiotics and endogenous compounds. PXR underlies a number of clinically important adverse drug interactions. The broad ligand specificity of PXR and the high degree of sequence divergence across animal species complicates the translation of in silico and animal models to human physiology.
Similar to efforts with enzymes and transporters with broad specificity, such as CYP3A4 or P-glycoprotein, predictive animal and in silico modeling of PXR-ligand interactions has important clinical and drug development applications but is challenging. For in silico models, the field would be aided by the collection of a large dataset of PXR activation by a consistent validated protocol, looking at subsets of both structurally diverse and structurally related compounds. Prior efforts reporting in silico models have had the limitation of using data from multiple previous studies using different experimental designs.
Lastly, the unusually high variability of the PXR LBD offers a unique opportunity to understand the evolution of a protein that putatively protects animals against toxic endogenous and exogenous compounds. There still remain open questions into what ligands have shaped the evolution of the PXR LBD and what consequences there are to mammals in having the CAR gene in addition to the PXR gene. Cross-species differences in biliary bile salt composition is one possible evolutionary driving force for diversification of the PXR LBD, but this hypothesis has not yet been solidified by structural comparisons of PXRs from different species, whether by experimentally determined or predicted structures. A potentially important finding would be the identification of hitherto unidentified dietary activators of PXR. Such activators may explain inter-individual variation in parameters such as CYP3A4 or P-glycoprotein expression. It may be that foods consumed by humans in hunter-gatherer types of societies, or by wild animals, have PXR activators not found or rarely encountered by people consuming processed foods.
The extreme divergence of the zebrafish and frog PXRs from their mammalian counterparts means that these model organisms respond very differently from humans in terms of compounds that alter liver and intestinal metabolism. In addition, the small size and rapid development of zebrafish, an advantage for high-throughput studies, has limited the ability to perform detailed studies equivalent to the dissociated human hepatocyte models. This needs to be taken into account when using these animals for toxicology and drug discovery studies.
Overall, much progress has been made in understanding the biology of PXR. Future studies should continue to aid our understanding of the regulation of liver and intestinal metabolism and elimination and how to apply this knowledge to clinical benefit.
Acknowledgments
MD Krasowski is supported by a Clinical-Scientist Development Award from the National Institutes of Health (K08-GM074238-01A1).
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1•.ZHANG Z, BURCH PE, COONEY AJ, et al. Genomic analysis of the nuclear receptor family: new insights into structure, regulation, and evolution from the rat genome. Genome Res. 2004;14(4):580–590. doi: 10.1101/gr.2160004. Comparison of nuclear hormone receptor genes in the human, rat, and mouse genomes demonstrating the unusual evolution of the PXR LBD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ROBINSON-RECHAVI M, CARPENTIER A-S, DUFFRAISSE M, LAUDET V. How many nuclear hormone receptors are there in the human genome? Trends Genet. 2001;17(10):554–556. doi: 10.1016/s0168-9525(01)02417-9. [DOI] [PubMed] [Google Scholar]
- 3.JAILLON O, AURY J-M, BRUNET F, et al. Genome duplication in the telost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature. 2004;431(7011):946–956. doi: 10.1038/nature03025. [DOI] [PubMed] [Google Scholar]
- 4.ROBINSON-RECHAVI M, BOUSSAU B, LAUDET V. Phylogenetic dating and characterization of gene duplications in vertebrates: the cartilaginous fish reference. Mol Biol Evol. 2004;21(3):580–586. doi: 10.1093/molbev/msh046. [DOI] [PubMed] [Google Scholar]
- 5.ESCRIVA H, MANZON L, YOUSON J, LAUDET V. Analysis of lamprey and hagfish genes reveals a complex history of gene duplications during early vertebrate evolution. Mol Biol Evol. 2002;19(9):1440–1450. doi: 10.1093/oxfordjournals.molbev.a004207. [DOI] [PubMed] [Google Scholar]
- 6•.MAGLICH JM, CARAVELLA JA, LAMBERT MH, WILLSON TM, MOORE JT, RAMAMURTHY L. The first completed genome sequence from a teleost fish (Fugu rubripes) adds significant diversity to the nuclear receptor superfamily. Nucleic Acids Res. 2003;31(14):4051–4058. doi: 10.1093/nar/gkg444. Excellent study of the nuclear hormone receptor complement in the pufferfish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.BLUMBERG B, SABBAGH W, JUGUILON H, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12(20):3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KLIEWER SA, MOORE JT, WADE L, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92(1):73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 9.LEHMANN JM, MCKEE DD, WATSON MA, WILLSON TM, MOORE JT, KLIEWER SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102(5):1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.MOORE LB, MAGLICH JM, MCKEE DD, et al. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol. 2002;16(5):977–986. doi: 10.1210/mend.16.5.0828. Most complete comparative pharmacology study of PXR and CAR across species. [DOI] [PubMed] [Google Scholar]
- 11.MOORE LB, PARKS DJ, JONES SA, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275(20):15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 12.KOMOROSKI BJ, ZHANG S, CAI H, et al. Induction and inhibition of cytochromes P450 by the St. John’s wort constituent hyperforin in human hepatocyte cultures. Drug Metab Dispos. 2004;32(5):512–518. doi: 10.1124/dmd.32.5.512. [DOI] [PubMed] [Google Scholar]
- 13.BERTILSSON G, HEIDRICH J, SVENSSON K, et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Nat Acad Sci USA. 1998;95(21):12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SYNOLD TW, DUSSAULT I, FORMAN BM. The orphan nuclear receptor SXR coordinately regulated drug metabolism and efflux. Nature Med. 2001;7(5):584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 15.RIPP SL, FITZPATRICK JL, PETERS JM, PROUGH RA. Induction of CYP3A expression by dehydroepiandrosterone: involvement of the pregnane X receptor. Drug Metab Dispos. 2002;30(5):570–575. doi: 10.1124/dmd.30.5.570. [DOI] [PubMed] [Google Scholar]
- 16.JONES SA, MOORE LB, SHENK JL, et al. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol. 2000;14(1):27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 17.HUSTERT E, ZIBAT A, PRESECAN-SIEDEL E, et al. Natural protein variants of pregnane X receptor with altered transactivation activity toward CYP3A4. Drug Metab Dispos. 2001;29(11):1454–1459. [PubMed] [Google Scholar]
- 18.SAVAS U, WESTER MR, GRIFFIN KJ, JOHNSON EF. Rabbit pregnane X receptor is activated by rifampicin. Drug Metab Dispos. 2000;28(5):529–537. [PubMed] [Google Scholar]
- 19.WENTWORTH JM, AGOSTINI M, LOVE J, SCHWABE JW, CHATTERJEE VKK. St John’s wort, a herbal antidepressant, activates the steroid X receptor. J Endocrinol. 2000;166(3):R11–R16. doi: 10.1677/joe.0.166r011. [DOI] [PubMed] [Google Scholar]
- 20•.BLUMBERG B, KANG H, BOLADO J, et al. BXR, an embryonic orphan nuclear receptor activated by a novel class of endogenous benzoate metabolites. Genes Dev. 1998;12(9):1269–1277. doi: 10.1101/gad.12.9.1269. Original study demonstrating the unusual properties of the Xenopus laevis PXRs – BXRα and BXRβ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HARTLEY DP, DAI X, HE YD, et al. Activators of the rat pregnane X receptor differentially modulate hepatic and intestinal gene expression. Mol Pharmacol. 2004;65(5):1159–1171. doi: 10.1124/mol.65.5.1159. [DOI] [PubMed] [Google Scholar]
- 22.ZHANG H, LECULYSE E, LIU L, et al. Rat pregnane X receptor: molecular cloning, tissue distribution, and xenobiotic regulation. Arch Biochem Biophys. 1999;368(1):14–22. doi: 10.1006/abbi.1999.1307. [DOI] [PubMed] [Google Scholar]
- 23.DROCOURT L, PASCUSSI J-M, ASSENAT E, FABRE J-M, MAUREL P, VILAREM M-J. Calcium channel modulators of the dihydropyridine family are human pregnane X receptor activators and inducers of CYP3A, CYP2B, and CYP2C in human hepatocytes. Drug Metab Dispos. 2001;29(10):1325–1331. [PubMed] [Google Scholar]
- 24.GERBAL-CHALOIN S, PASCUSSI J-M, PICHARD-GARCIA L, et al. Induction of CYP2C genes in human hepatocytes in primary culture. Drug Metab Dispos. 2001;29(3):242–251. [PubMed] [Google Scholar]
- 25•.MAGLICH JM, STOLTZ CM, GOODWIN B, HAWKINS-BROWN D, MOORE JT, KLIEWER SA. Nuclear pregnane X receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic distribution. Mol Pharmacol. 2002;62(3):638–646. doi: 10.1124/mol.62.3.638. Demonstrates the overlapping transcriptional targets of PXR and CAR. [DOI] [PubMed] [Google Scholar]
- 26.XIE W, YEUH M-F, RADOMINSKA-PANDYA A, et al. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Nat Acad Sci USA. 2003;100(7):4150–4155. doi: 10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.HO RH, KIM RB. Transporters and drug therapy: implications for drug disposition and disease. Clin Pharmacol Ther. 2005;78(3):260–277. doi: 10.1016/j.clpt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 28.BURK O, ARNOLD KA, NUSSLER AK, et al. Antimalarial artemisin drugs induce cytochrome P450 and MDR1 expression by activation of xenosensors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol. 2005;67(6):1954–1965. doi: 10.1124/mol.104.009019. [DOI] [PubMed] [Google Scholar]
- 29.GEICK A, EICHELBAUM M, BURK O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276(18):14581–14587. doi: 10.1074/jbc.M010173200. [DOI] [PubMed] [Google Scholar]
- 30.PASCUSSI JM, GERBAL-CHALOIN S, DROCOURT L, MAUREL P, VILAREM MJ. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta. 2003;1619(3):243–253. doi: 10.1016/s0304-4165(02)00483-x. [DOI] [PubMed] [Google Scholar]
- 31••.RESCHLY EJ, KRASOWSKI MD. Evolution and function of the NR1I nuclear hormone receptor subfamily (VDR, PXR, and CAR) with respect to metabolism of xenobiotics and endogenous compounds. Curr Drug Metab. 2006 doi: 10.2174/138920006776873526. in press. Recent review of the evolution and function of VDR, PXR, and CAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.KLIEWER SA, GOODWIN B, WILLSON TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocrine Rev. 2002;23(5):687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 33.KLIEWER SA. The nuclear pregnane X receptor regulates xenobiotic detoxification. J Nutrit. 2003;133(7 Suppl):2444S–2447S. doi: 10.1093/jn/133.7.2444S. [DOI] [PubMed] [Google Scholar]
- 34.SONODA J, ROSENFELD JM, XU L, EVANS RM, XIE W. A nuclear receptor-mediated xenobiotic response and its implication in drug metabolism and host protection. Curr Drug Metab. 2003;4(1):59–72. doi: 10.2174/1389200033336739. [DOI] [PubMed] [Google Scholar]
- 35.SONODA J, XIE W, ROSENFELD JM, BARWICK JL, GUZELIAN PS, EVANS RM. Regulation of a xenobiotics sulfonation cascade by nuclear pregnane X receptor (PXR) Proc Nat Acad Sci USA. 2002;99(21):13801–13806. doi: 10.1073/pnas.212494599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DUSSAULT I, FORMAN BM. The nuclear receptor PXR: a master regulator of “homeland” defense. Crit Rev Eukaryotic Gene Express. 2002;12(1):53–64. doi: 10.1615/critreveukaryotgeneexpr.v12.i1.30. [DOI] [PubMed] [Google Scholar]
- 37.VAN BUREN D, WIDEMAN CA, RIED M, et al. The antagonistic effect of rifampin upon cyclosporine bioavailability. Transplant Proc. 1984;16(6):1642–1645. [PubMed] [Google Scholar]
- 38.SCHWARZ UI, BÜSCHEL B, KIRCH W. Unwanted pregnancy on self-medication with St John’s wort despite hormonal contraception. Br J Clin Pharmacol. 2003;55(1):112–113. doi: 10.1046/j.1365-2125.2003.t01-1-01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SKOLNICK JL, STOLER BS, KATZ DB, ANDERSTON WH. Rifampin, oral contraceptives, and pregnancy. JAMA. 1976;236(12):1382. [PubMed] [Google Scholar]
- 40•.WATKINS RE, DAVIS-SEARLES PR, LAMBERT MH, REDINBO MR. Coactivator binding promotes the specific interaction between ligand and the pregnane X receptor. J Mol Biol. 2003;331(4):815–828. doi: 10.1016/s0022-2836(03)00795-2. X-ray crystallographic study showing how the coactivator SRC-1 modulates the ligand specificity of human PXR. [DOI] [PubMed] [Google Scholar]
- 41•.WATKINS RE, MAGLICH JM, MOORE LB, et al. 2.1 Å crystal structure of human PXR in complex with the St. John’s wort compound hyperperforin. Biochemistry. 2003;42(6):1430–1438. doi: 10.1021/bi0268753. X-ray crystal structure of human PXR showing flexibility of the receptor in binding the ligand hyperforin. [DOI] [PubMed] [Google Scholar]
- 42.WATKINS RE, NOBLE SM, REDINBO MR. Structural insights in the promiscuity and function of the human pregnane X receptor. Curr Opin Drug Discov Devel. 2002;5(1):150–158. [PubMed] [Google Scholar]
- 43••.WATKINS RE, WISELY GB, MOORE LB, et al. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science. 2001;292(5525):2329–2333. doi: 10.1126/science.1060762. First published X-ray crystal structure of the human PXR showing unusual features relative to other nuclear hormone receptors. [DOI] [PubMed] [Google Scholar]
- 44.CHRENCIK JE, ORANS J, MOORE LB, et al. Structural disorder in the complex of human PXR and the macrolide antibiotic rifampicin. Mol Endocrinol. 2005;19(5):1125–1134. doi: 10.1210/me.2004-0346. [DOI] [PubMed] [Google Scholar]
- 45.ORANS J, TEOTICO DG, REDINBO MR. The nuclear xenobiotic receptor PXR: recent insights and new challenges. Mol Endocrinol. 2005;19(12):2891–2900. doi: 10.1210/me.2005-0156. [DOI] [PubMed] [Google Scholar]
- 46•.INGRAHAM HA, REDINBO MR. Orphan nuclear receptors adopted by crystallography. Curr Opin Struct Biol. 2005;15(6):708–715. doi: 10.1016/j.sbi.2005.10.009. Reviews the known crystal structures of the ‘orphan’ nuclear hormone receptors, including PXR. [DOI] [PubMed] [Google Scholar]
- 47••.CARNAHAN VE, REDINBO MR. Structure and function of the human nuclear xenobiotic receptor PXR. Curr Drug Metab. 2005;6(4):357–367. doi: 10.2174/1389200054633844. Excellent review of the structural properties of PXR. [DOI] [PubMed] [Google Scholar]
- 48.EKINS S, BRAVI G, WIKEL JH, WRIGHTON SA. Three-dimensional-quantitative structure activity relationship analysis of cytochrome P-450 3A4 substrates. J Pharmacol Exp Ther. 1999;291(1):424–433. [PubMed] [Google Scholar]
- 49.LI AP, KAMINSKI DL, RASMUSSEN A. Substrates of human hepatic cytochrome P450 3A4. Toxicology. 1995;104(1–3):1–8. doi: 10.1016/0300-483x(95)03155-9. [DOI] [PubMed] [Google Scholar]
- 50.LUO G, GUENTHNER T, GAN L-S, HUMPHREYS WG. CYP3A4 induction by xenobiotics: biochemistry, experimental methods and impact on drug discovery and development. Curr Drug Metab. 2004;5(6):483–505. doi: 10.2174/1389200043335397. [DOI] [PubMed] [Google Scholar]
- 51.QUATTROCHI LC, GUZELIAN PS. CYP3A regulation: from pharmacology to nuclear receptors. Drug Metab Dispos. 2001;29(5):615–622. [PubMed] [Google Scholar]
- 52.WRIGHTON SA, SCHUETZ EG, THUMMEL KE, SHEN DD, KORZEKWA KR, WATKINS PB. The human CYP3A subfamily: practical considerations. Drug Metab Rev. 2000;32(3–4):339–361. doi: 10.1081/dmr-100102338. [DOI] [PubMed] [Google Scholar]
- 53.EKINS S, KIM RB, LEAKE BF, et al. Three-dimensional quantitative structure-activity relationships of inhibitors of P-glycoprotein. Mol Pharmacol. 2002;61(5):964–973. doi: 10.1124/mol.61.5.964. [DOI] [PubMed] [Google Scholar]
- 54.HIGGINS CF. The multidrug resistance P-glycoprotein. Curr Opin Cell Biol. 1993;5(4):684–687. doi: 10.1016/0955-0674(93)90140-l. [DOI] [PubMed] [Google Scholar]
- 55.WACHER VJ, WU CY, BENET LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implication for drug delivery and activity in cancer chemotherapy. Mol Carcinogenesis. 1995;13(3):129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 56.YU DK. The contribution of P-glycoprotein to pharmacokinetic drug-drug interactions. J Clin Pharmacol. 1999;39(12):1203–1211. doi: 10.1177/00912709922012006. [DOI] [PubMed] [Google Scholar]
- 57.GOODWIN B, GAUTHIER KC, UMETANI M, et al. Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc Nat Acad Sci USA. 2003;100(1):223–228. doi: 10.1073/pnas.0237082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.HANDSCHIN C, PODVINEC M, MEYER UA. CXR, a chicken xenobiotic-sensing orphan nuclear receptor, is related to both mammalian pregnane X receptor (PXR) and constitutive androstane receptor (CAR) Proc Nat Acad Sci USA. 2000;97(20):10769–10774. doi: 10.1073/pnas.97.20.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.HANDSCHIN C, PODVINEC M, AMHERD R, LOOSER R, OURLIN J-C, MEYER UA. Cholesterol and bile acids regulate xenosensor signaling in drug-mediated induction of cytochromes P450. J Biol Chem. 2002;277(33):29561–29567. doi: 10.1074/jbc.M202739200. [DOI] [PubMed] [Google Scholar]
- 60.MOORE LB, GOODWIN B, JONES SA, et al. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Nat Acad Sci USA. 2000;97(13):7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.ZHANG W, PURCHIO A, CHEN K, BURNS SM, CONTAG CH, CONTAG PR. In vivo activation of the human CYP3A4 promoter in mouse liver and regulation by pregnane X receptors. Biochem Pharmacol. 2003;65(11):1889–1896. doi: 10.1016/s0006-2952(03)00188-6. [DOI] [PubMed] [Google Scholar]
- 62.MATHENY CJ, ALI RY, YANG X, POLLACK GM. Effect of prototypical inducting agents on P-glycoprotein and CYP3A expression in mouse tissues. Drug Metab Dispos. 2004;32(9):1008–1014. [PubMed] [Google Scholar]
- 63.GRÜN F, VENKATESAN RN, TABB MM, et al. Benzoate X receptors α and β are pharmacologically distinct and do not function as xenobiotic receptors. J Biol Chem. 2002;277(46):43691–43697. doi: 10.1074/jbc.M206553200. [DOI] [PubMed] [Google Scholar]
- 64.HEATH LA, JONES EA, OLD RW. Expression pattern of BXR suggests a role for benzoate ligand-mediated signalling in hatching gland function. Int J Dev Biol. 2000;44(1):141–144. [PubMed] [Google Scholar]
- 65.NISHIKAWA J, SAITO K, SASAKI M, TOMIGAHARA Y, NISHIHARA T. Molecular cloning and functional characterization of a novel nuclear receptor similar to an embryonic benzoate receptor BXR. Biochem Biophys Res Comm. 2000;277(1):209–215. doi: 10.1006/bbrc.2000.3649. [DOI] [PubMed] [Google Scholar]
- 66••.KRASOWSKI MD, YASUDA K, HAGEY LR, SCHUETZ EG. Evolution of the pregnane X receptor: adaptation to cross-species differences in biliary bile salts. Mol Endocrinol. 2005;19(7):1720–1739. doi: 10.1210/me.2004-0427. Provides evidence for the hypothesis that cross-species variation in endogenous ligands, specifically biliary bile salts, have shaped evolution of the PXR LBD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.KRASOWSKI MD, YASUDA K, HAGEY LR, SCHUETZ EG. Evolutionary selection across the nuclear hormone receptor superfamily with a focus on the NR1I subfamily (vitamin D, pregnane X, and constitutive androstane receptors) Nucl Rec. 2005;3(1):2. doi: 10.1186/1478-1336-3-2. Most detailed phylogenetic analysis of the entire vertebrate nuclear hormone receptor superfamily, showing evidence for positive selection of the human PXR and CAR LBDs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.CLARK AG, GLANOWSKI S, NIELSEN R, et al. Inferring nonneutral evolution from human-chimp-mouse orthologous gene trios. Science. 2003;302(5652):1960–1963. doi: 10.1126/science.1088821. [DOI] [PubMed] [Google Scholar]
- 69.THOMPSON EE, KUTTAB-BOULOS H, KRASOWSKI MD, DI RIENZO A. Functional constraints in the constitutive androstane receptor inferred from human sequence variation and cross-species comparisons. Hum Genomics. 2005;2(3):168–178. doi: 10.1186/1479-7364-2-3-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.YANG Z, BIELAWSKI JP. Statistical methods for detecting molecular adaptation. Trends Ecol Evol. 2000;15(12):496–503. doi: 10.1016/S0169-5347(00)01994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.PAGANI F, RAPONI M, BARALLE FE. Synonymous mutations in CFTR exon 12 affect splicing and are not neutral in evolution. Proc Nat Acad Sci USA. 2005;102(18):6368–6372. doi: 10.1073/pnas.0502288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.ENDO T, IKEO K, GOJOBORI T. Large-scale search for genes on which positive selection may operate. Mol Biol Evol. 1996;13(5):685–690. doi: 10.1093/oxfordjournals.molbev.a025629. [DOI] [PubMed] [Google Scholar]
- 73.YANG Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol Biol Evol. 1998;15(5):568–573. doi: 10.1093/oxfordjournals.molbev.a025957. [DOI] [PubMed] [Google Scholar]
- 74.YANG Z. Maximum likelihood estimation of large phylogenies and analysis of adaptive evolution in human influenza virus A. J Molec Evol. 2000;51(5):423–432. doi: 10.1007/s002390010105. [DOI] [PubMed] [Google Scholar]
- 75.NIELSEN R, YANG Z. Likelihood models for detecting positively selected amino acid sites and applications to the HIV-1 envelope gene. Genetics. 1998;148(3):929–936. doi: 10.1093/genetics/148.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.SCHUETZ EG, STROM S, YASUDA K, et al. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001;276(42):39411–39418. doi: 10.1074/jbc.M106340200. [DOI] [PubMed] [Google Scholar]
- 77.XIE W, BARWICK JL, SIMON CM, et al. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev. 2000;14(23):3014–3023. doi: 10.1101/gad.846800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78•.LAMBA J, LAMBA V, SCHUETZ E. Genetic variants of PXR (NR1I2) and CAR (NR1I3) and their implications in drug metabolism and pharmacogenetics. Curr Drug Metab. 2005;6(4):369–383. doi: 10.2174/1389200054633880. Excellent review of the known genetic variants of PXR and CAR. [DOI] [PubMed] [Google Scholar]
- 79.ZHANG J, KUEHL P, GREEN ED, et al. The human pregnane X receptor: genomic structure and identification and functional characterization of natural allelic variants. Pharmacogenetics. 2001;11(7):555–572. doi: 10.1097/00008571-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 80.KOYANO S, KUROSE K, OZAWA S, et al. Eleven novel single nucleotide polymorphisms in the NR1I2 (PXR) gene, four of which induce non-synonymous amino acid alterations. Drug Metab Pharmacokin. 2002;17(6):561–565. doi: 10.2133/dmpk.17.561. [DOI] [PubMed] [Google Scholar]
- 81.KOYANO S, KUROSE K, SAITO Y, et al. Functional characterization of four naturally occurring variants of human pregnane X receptor (PXR): one variant causes dramatic loss of both DNA binding activity and the transactivation of the CYP3A4 promoter/enhancer region. Drug Metab Dispos. 2004;32(1):149–154. doi: 10.1124/dmd.32.1.149. [DOI] [PubMed] [Google Scholar]
- 82.EBERSBERGER I, METZLER D, SCHWARZ C, PÄÄBO S. Genomewide comparison of DNA sequences between humans and chimpanzees. Am J Hum Genet. 2002;70(6):1490–1497. doi: 10.1086/340787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.WEI P, ZHANG J, DOWHAN DH, HAN Y, MOORE DD. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenomics J. 2002;2(2):117–126. doi: 10.1038/sj.tpj.6500087. [DOI] [PubMed] [Google Scholar]
- 84.HOFMANN AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159(22):2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 85.HOFMANN AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab Rev. 2004;36(3–4):703–722. doi: 10.1081/dmr-200033475. [DOI] [PubMed] [Google Scholar]
- 86.UPPAL H, TOMA D, SAINI SPS, REN S, JONES TJ, XIE W. Combined loss of orphan receptors PXR and CAR heightens sensitivity to toxic bile acids in mice. Hepatology. 2005;41(1):168–176. doi: 10.1002/hep.20512. [DOI] [PubMed] [Google Scholar]
- 87.ZHANG J, HUANG W, QATANANI M, EVANS RM, MOORE DD. The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity. J Biol Chem. 2004;279(47):49517–49522. doi: 10.1074/jbc.M409041200. [DOI] [PubMed] [Google Scholar]
- 88.STAUDINGER JL, GOODWIN B, JONES SA, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Nat Acad Sci USA. 2001;98(6):3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.XIE W, RADOMINSKA-PANDYA A, SHI Y, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Nat Acad Sci USA. 2001;98(6):3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.MAKISHIMA M, LU TT, XIE W, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296(5571):1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 91.FISCHER S, BEUERS U, SPENGLER U, ZWIEBEL FM, KOEBE H-G. Hepatic levels of bile acids in end-stage chronic cholestatic liver disease. Clin Chim Acta. 1996;251(2):173–186. doi: 10.1016/0009-8981(96)06305-x. [DOI] [PubMed] [Google Scholar]
- 92.STEDMAN C, ROBERTSON G, COULTER S, LIDDLE C. Feed-forward regulation of bile acid detoxification by CYP3A4: studies in humanized transgenic mice. J Biol Chem. 2004;279(12):11336–11343. doi: 10.1074/jbc.M310258200. [DOI] [PubMed] [Google Scholar]
- 93.CALI JJ, HSIEH C-L, FRANCKE U, RUSSELL DW. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem. 1991;266(12):7779–7783. [PMC free article] [PubMed] [Google Scholar]
- 94.HONDA A, SALEN G, MATSUZAKI Y, et al. Side chain hydroxylations in bile acid biosynthesis catalyzed by CYP3A are markedly up-regulated in Cyp27−/−mice but not in cerebrotendinous xanthomatosis. J Biol Chem. 2001;276(37):34579–34585. doi: 10.1074/jbc.M103025200. [DOI] [PubMed] [Google Scholar]
- 95.HONDA A, SALEN G, MATSUZAKI Y, et al. Differences in hepatic levels of intermediates in bile acid biosynthesis between Cyp27(−/−) mice and CTX. J Lipid Res. 2001;42(2):291–300. [PubMed] [Google Scholar]
- 96.DUSSAULT I, YOO H-D, LIN M, et al. Identification of an endogenous ligand that activates pregnane X receptor-mediated sterol clearance. Proc Nat Acad Sci USA. 2003;100(3):833–838. doi: 10.1073/pnas.0336235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.HOFMANN AF, SCHTEINGART CD, HAGEY LR. Species differences in bile acid metabolism. In: PAUMGARTNER G, BEUERS U, editors. International Falk Symposium: Bile acids in liver diseases. Kluwer Academic Publishers; Dordrecht: 1995. pp. 3–30. [Google Scholar]
- 98.HAGEY LR. Bile acid biodiversity in vertebrates: chemistry and evolutionary implications. UMI; Ann Arbor, MI: 1992. [Google Scholar]
- 99.UNE M, HOSHITA T. Natural occurrence and chemical synthesis of bile alcohols, higher bile acids, and short side chain bile acids. Hiroshima J Med Sci. 1994;43(2):37–67. [PubMed] [Google Scholar]
- 100.MOSCHETTA A, XU F, HAGEY LR, et al. A phylogenetic survey of biliary lipids in vertebrates. J Lipid Res. 2005;46(10):2221–2232. doi: 10.1194/jlr.M500178-JLR200. [DOI] [PubMed] [Google Scholar]
- 101.ANDERSON IG, BRIGGS T, HASLEWOOD GAD. Comparative studies of ‘bile salts’. 18 The chemistry of cyprinol. Biochem J. 1964;90(2):303–308. doi: 10.1042/bj0900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.GOTO T, HOLZINGER F, HAGEY LR, et al. Physicochemical and physiological properties of 5α-cyprinol sulfate, the toxic bile salt of cyprinid fish. J Lipid Res. 2003;44(9):1643–1651. doi: 10.1194/jlr.M300155-JLR200. [DOI] [PubMed] [Google Scholar]
- 103.HASLEWOOD GAD, TÖKÉS L. Comparative studies of bile salts: bile salts of the lamprey Petromyzon marinus L. Biochem J. 1969;114(2):179–184. doi: 10.1042/bj1140179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.HASLEWOOD GAD. Comparative studies of bile salts. Myxinol disulphate, the principal bile salt of hagfish (Myxinidae) Biochem J. 1966;100(1):233–237. doi: 10.1042/bj1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.KARPEN SJ. Exercising the nuclear option to treat cholestasis: CAR and PXR ligands. Hepatology. 2005;42(2):266–269. doi: 10.1002/hep.20833. [DOI] [PubMed] [Google Scholar]
- 106.BACHS L, PARES A, ELENA M, PIERA C, RODES J. Comparison of rifampicin with phenobarbitone for treatment of pruritus in biliary cirrhosis. Lancet. 1989;1(8638):574–576. doi: 10.1016/s0140-6736(89)91608-5. [DOI] [PubMed] [Google Scholar]
- 107•.TRACY TS, VENKATARAMANAN R, GLOVER DD, CARITIS SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A4 activity) during pregnancy. Am J Obstet Gynecol. 2005;192(2):633–639. doi: 10.1016/j.ajog.2004.08.030. Well-designed clinical study definitively demonstrating induction of CYP3A activity during pregnancy. [DOI] [PubMed] [Google Scholar]
- 108.KASHUBA AD, BERTINO JS, ROCCI ML, KULAWY RW, BECK DJ, NAFZIGER AN. Quantification of 3-month intraindividual variability and the influence of sex and menstrual cycle phase on CYP3A activity as measured by phenotyping with intravenous midazolam. Clin Pharmacol Ther. 1998;64(3):269–277. doi: 10.1016/S0009-9236(98)90175-8. [DOI] [PubMed] [Google Scholar]
- 109.KHARASCH ED, MAUTZ D, SENN T, LEUTZ G, COX K. Menstrual cycle variability in midazolam pharmacokinetics. J Clin Pharmacol. 1999;39(3):275–280. [PubMed] [Google Scholar]
- 110.KHARASCH ED, RUSSELL M, GARTON K, LENTZ G, BOWDLE TA, COX K. Assessment of cytochrome P450 3A4 activity during the menstrual cycle using alfentanil as a noninvasive probe. Anesthesiology. 1997;87(1):26–35. doi: 10.1097/00000542-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 111.MITCHELL BF, MITCHELL JM, CHOWDHURY J, et al. Metabolites of progesterone and the pregnane X receptor: a novel pathway regulating uterine contractility in pregnancy? Am J Obstet Gynecol. 2005;192(4):1304–1312. doi: 10.1016/j.ajog.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 112.FORMAN BM, TZAMELI I, CHOI H-S, et al. Androstane metabolites bind to and deactivate the nuclear receptor CAR-β. Nature. 1998;395(6702):612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- 113.SINGER AG. A chemistry of mammalian pheromones. J Steroid Biochem Mol Biol. 1991;39(4B):627–632. doi: 10.1016/0960-0760(91)90261-3. [DOI] [PubMed] [Google Scholar]
- 114.CLAUS R, ALSING W. Occurence of 5α-androst-16-en-3-one, a boar pheromone, in man and its relationship to testosterone. J Endocrinol. 1976;68(3):483–484. doi: 10.1677/joe.0.0680483. [DOI] [PubMed] [Google Scholar]
- 115.XU H, RAJESAN R, HARPER P, et al. Induction of cytochrome P450 1A by cow milk-based formula: a comparative study between human milk and formula. Br J Pharmacol. 2005;146(2):296–305. doi: 10.1038/sj.bjp.0706319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.TRABER MG. Vitamin E, nuclear receptors and xenobiotic metabolism. Arch Biochem Biophys. 2004;423(1):6–11. doi: 10.1016/j.abb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 117.BRIGELIUS-FLOHÉ R. Induction of drug metabolizing enzymes by vitamin E. J Plant Physiol. 2005;162(7):797–802. doi: 10.1016/j.jplph.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 118.LANDES N, PFLUGER P, KLUTH D, et al. Vitamin E activates gene expression via the pregnane X receptor. Biochem Pharmacol. 2003;65(2):269–273. doi: 10.1016/s0006-2952(02)01520-4. [DOI] [PubMed] [Google Scholar]
- 119.RÜHL R, SCZECH R, LANDES N, PFLUGER P, KLUTH D, SCHWEIGERT FJ. Carotenoids and their metabolites are naturally occurring activators of gene expression via the pregnane X receptor. Eur J Nutrit. 2004;43(6):336–343. doi: 10.1007/s00394-004-0475-1. [DOI] [PubMed] [Google Scholar]
- 120.HAHN ME. Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact. 2002;141(1–2):131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 121.SCHUETZ EG, SCHUETZ JD, GROGAN WM, et al. Expression of cytochrome P450 3A in amphibian, rat, and human kidney. Arch Biochem Biophys. 1992;294(1):206–214. doi: 10.1016/0003-9861(92)90159-t. [DOI] [PubMed] [Google Scholar]
- 122.ERTL RP, STEGEMAN JJ, WINSTON GW. Induction time course of cytochromes P450 by phenobarbital and 3-methylcholanthrene by pretreatment in liver microsomes of Alligator mississippiensis. Biochem Pharmacol. 1998;55(9):1513–1521. doi: 10.1016/s0006-2952(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 123.HASSELBERG L, MEIER S, SVARDAL A, HEGELUND T, CELANDER MC. Effects of alkylphenols on CYP1A and CYP3A expression in first spawning Atlantic cod (Gadus morhua) Aquat Toxicol. 2004;67(4):303–313. doi: 10.1016/j.aquatox.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 124.HEGELUND T, OTTOSSON K, RÅDINGER M, TOMBERG P, CELANDER MC. Effects of the antifungal imidazole ketoconazole on CYP1A and CYP3A in rainbow trout and killifish. Environ Toxicol Chem. 2004;23(5):1326–1334. doi: 10.1897/03-155. [DOI] [PubMed] [Google Scholar]
- 125.BRESOLIN T, DE FREITAS REBELO M, BAINY ACD. Expression of PXR, CYP3A, and MDR1 genes in liver of zebrafish. Comp Biochem Physiol Part C. 2005;140(3–4):403–407. doi: 10.1016/j.cca.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 126.TSENG H-P, HSEU T-H, BUHLER DR, WANG W-D, HU C-H. Constitutive and xenobiotics-induced expression of a novel CYP3A gene from zebrafish larva. Toxicol Appl Pharmacol. 2005;205(3):247–258. doi: 10.1016/j.taap.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 127.DEHAL P, SATOU Y, CAMPBELL RK, et al. The draft genome of Ciona intestinalis: insights into vertebrate origins. Science. 2002;298(5601):2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- 128••.HANDSCHIN C, BLAETTLER S, ROTH A, et al. The evolution of drug-activated nuclear receptors: one ancestral gene diverged into two xenosensor genes in mammals. Nucl Rec. 2004;2(1):7. doi: 10.1186/1478-1336-2-7. Study that demonstrated clearly that the chicken has only a single xenobiotic nuclear hormone receptor with resemblance to both PXR and CAR, suggesting that a similar receptor gene duplicated to form separate PXR and CAR genes in mammals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.APARICIO S, CHAPMAN J, STUPK E, et al. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science. 2002;297(5585):1301–1310. doi: 10.1126/science.1072104. [DOI] [PubMed] [Google Scholar]
- 130.HILLIER LW, MILLER W, BIRNEY E, et al. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432(7018):695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- 131••.XIE W, BARWICK JL, DOWNES M, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406(6794):435–439. doi: 10.1038/35019116. First study of PXR ‘knockout’ mice also provided elegant studies with ‘humanized’ PXR mice. [DOI] [PubMed] [Google Scholar]
- 132.SHAPIRO LE, SHEAR NH. Drug interactions: proteins, pumps, and P-450s. J Am Acad Dermatol. 2002;47(4):467–484. doi: 10.1067/mjd.2002.126823. [DOI] [PubMed] [Google Scholar]
- 133.MICHALETS EL. Update: clinical significant cytochrome P-450 drug interactions. Pharmacotherapy. 1998;18(1):84–112. [PubMed] [Google Scholar]
- 134.DICKINS M. Induction of cytochromes P450. Curr Top Med Chem. 2004;4(16):1745–1766. doi: 10.2174/1568026043387115. [DOI] [PubMed] [Google Scholar]
- 135.SCHUETZ EG. Induction of cytochromes P450. Curr Drug Metab. 2001;2(2):139–147. doi: 10.2174/1389200013338595. [DOI] [PubMed] [Google Scholar]
- 136.YUEH M-F, KAWAHARA M, RAUCY J. High volume bioassays to assess CYP3A4-mediated drug interactions: induction and inhibition in a single cell line. Drug Metab Dispos. 2005;33(1):38–48. doi: 10.1124/dmd.104.001594. [DOI] [PubMed] [Google Scholar]
- 137.VERMEIR M, ANNAERT P, MAMIDI RNVS, ROYMANS D, MEULDERMANS W, MANNENS G. Cell-based models to study hepatic drug metabolism and enzyme induction in humans. Expert Opin Drug Metab Toxicol. 2006;1(1):75–90. doi: 10.1517/17425255.1.1.75. [DOI] [PubMed] [Google Scholar]
- 138.MOORE JT, KLIEWER SA. Use of the nuclear receptor PXR to predict drug interactions. Toxicology. 2000;153(1–3):1–10. doi: 10.1016/s0300-483x(00)00300-0. [DOI] [PubMed] [Google Scholar]
- 139.GÓMEZ-LECHÓN MJ, DONATO MT, CASTELL JV, JOVER R. Human hepatocytes as a tool for studying toxicity and drug metabolism. Curr Drug Metab. 2003;5(5):292–312. doi: 10.2174/1389200033489424. [DOI] [PubMed] [Google Scholar]
- 140.LECLUYSE EL, ALEXANDRE E, HAMILTON GA, et al. Isolation and culture of primary human hepatocytes. Methods Mol Biol. 2005;290(1):207–229. doi: 10.1385/1-59259-838-2:207. [DOI] [PubMed] [Google Scholar]
- 141.ZHU Z, KIM S, CHEN TC, et al. Correlation of high-throughput pregnane X receptor (PXR) transactivation and binding assays. J Biomol Screening. 2004;9 (6):533–540. doi: 10.1177/1087057104264902. [DOI] [PubMed] [Google Scholar]
- 142.VIGNATI LA, BOGNI A, GROSSI P, MONSHOUWER M. A human and mouse pregnane X receptor reporter gene assay in combination with cytotoxicity measurements as a tool to evaluate species-specific CYP3A induction. Toxicology. 2004;199:23–33. doi: 10.1016/j.tox.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 143•.DAI G, WAN YJ. Animal models of xenobiotic receptors. Curr Drug Metab. 2005;6(4):341–355. doi: 10.2174/1389200054633862. Recent review of animal models of xenobiotic receptors. [DOI] [PubMed] [Google Scholar]
- 144.NALLANI SC, GOODWIN B, MAGLICH JM, BUCKLEY DJ, BUCKLEY AR, DESAI PB. Induction of cytochrome P450 3A by paclitaxel in mice: pivotal role of the nuclear xenobiotic receptor, pregnane X receptor. Drug Metab Dispos. 2003;31(5):681–684. doi: 10.1124/dmd.31.5.681. [DOI] [PubMed] [Google Scholar]
- 145.ANAKK S, KALSOTRA A, KIKUTA Y, et al. CAR/PXR provide directives for Cyp3a41 gene regulation differently from Cyp3a11. Pharmacogenomics J. 2004;4(2):91–101. doi: 10.1038/sj.tpj.6500222. [DOI] [PubMed] [Google Scholar]
- 146.STAUDINGER J, LIU Y, MADA A, HABEEBU S, KLAASSEN CD. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos. 2001;29(11):1467–1472. [PubMed] [Google Scholar]
- 147.SONODA J, CHONG LW, DOWNES M, et al. Pregnane X receptor prevents hepatorenal toxicity from cholesterol metabolites. Proc Nat Acad Sci USA. 2005;102(6):2198–2203. doi: 10.1073/pnas.0409481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.TABB MM, SUN A, ZHOU C, et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic SXR. J Biol Chem. 2003;278(45):43919–43927. doi: 10.1074/jbc.M303136200. [DOI] [PubMed] [Google Scholar]
- 149••.XIE W, EVANS RM. Pharmaceutical use of mouse models humanized for the xenobiotic receptor. Drug Discov Today. 2002;7(9):509–515. doi: 10.1016/s1359-6446(02)02251-1. Excellent review of the use of humanized mice in drug discovery and development. [DOI] [PubMed] [Google Scholar]
- 150.KEMP CA, MARÉCHAL J-D, SUTCLIFFE MJ. Progress in cytochrome P450 active site modeling. Arch Biochem Biophys. 2005;433(2):361–368. doi: 10.1016/j.abb.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 151.PAJEVA IK, WIESE M. Pharmacophore model of drugs involved in P-glycoprotein multidrug resistance: explanation of structural variety (hypothesis) J Med Chem. 2002;45(26):5671–5684. doi: 10.1021/jm020941h. [DOI] [PubMed] [Google Scholar]
- 152.GOMBAR VK, POLLI JW, HUMPHREYS JE, WRING SA, SERABJIT CS. Predicting P-glycoprotein substrates by a quantitative structure-activity relationship model. J Pharm Sci. 2004;93(4):957–968. doi: 10.1002/jps.20035. [DOI] [PubMed] [Google Scholar]
- 153.EKINS S, KIM RB, LEAKE BF, et al. Application of three-dimensional quantitative structure-activity relationships of P-glycoprotein inhibitors and substrates. Mol Pharmacol. 2002;61(5):974–981. doi: 10.1124/mol.61.5.974. [DOI] [PubMed] [Google Scholar]
- 154.EKINS S, MIRNY L, SCHUETZ EG. A ligand-based approach to understanding selectivity of nuclear hormone receptors PXR, CAR, FXR, LXRα, and LXRβ. Pharm Res. 2002;19(12):1788–1800. doi: 10.1023/a:1021429105173. [DOI] [PubMed] [Google Scholar]
- 155.EKINS S, WRIGHTON SA. Application of in silico approaches to predicting drug-drug metabolism. J Pharmacol Toxicol Methods. 2001;45(1):65–69. doi: 10.1016/s1056-8719(01)00119-8. [DOI] [PubMed] [Google Scholar]
- 156.KOBAYASHI K, YAMAGAMI Y, HIGUCHI T, HOSOKAWA M, CHIBA K. Key structural features of ligands for activation of human pregnane X receptor. Drug Metab Dispos. 2004;32(4):468–472. doi: 10.1124/dmd.32.4.468. [DOI] [PubMed] [Google Scholar]
- 157••.EKINS S, ERICKSON JA. A pharmacophore for human pregnane X receptor ligands. Drug Metab Dispos. 2002;30(1):96–99. doi: 10.1124/dmd.30.1.96. First detailed in silico model for the pharmacophore for human PXR activation. [DOI] [PubMed] [Google Scholar]
- 158••.SCHUSTER D, LANGER T. The identification of ligand features essential for PXR activation. J Med Chem. 2005;45(2):431–439. doi: 10.1021/ci049722q. Most complete ligand-based and structure-based in silico models for activation of human PXR activation, incorporating the published crystallographic structures of the human PXR LBD. [DOI] [PubMed] [Google Scholar]
- 159.TIRONA RG, LEAKE BF, PODUST LM, KIM RB. Identification of amino acids in rat pregnane X receptor that determine species-specific activation. Mol Pharmacol. 2004;65(1):36–44. doi: 10.1124/mol.65.1.36. [DOI] [PubMed] [Google Scholar]
- 160.ROCHEL N, WURTZ JM, MITSCHLER A, KLAHOLZ B, MORAS D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000;5(1):173–179. doi: 10.1016/s1097-2765(00)80413-x. [DOI] [PubMed] [Google Scholar]
- 161.TOCCHINI-VALENTINI G, ROCHEL N, WURTZ JM, MITSCHLER A, MORAS D. Crystal structures of the vitamin D receptor complexed to superagonist 20-epi ligands. Proc Nat Acad Sci USA. 2001;98(10):5491–5496. doi: 10.1073/pnas.091018698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.TOCCHINI-VALENTINI G, ROCHEL N, WURTZ J-M, MORAS D. Crystal structure of the vitamin D receptor liganded with the vitamin D side chain analogues calicotriol and seocalcitol, receptor agonists of clinical importance. Insights into a structural basis for the switching of calcipotriol to a receptor antagonist by a further side chain modification. J Med Chem. 2004;47(8):1956–1961. doi: 10.1021/jm0310582. [DOI] [PubMed] [Google Scholar]
- 163.MIZWICKI MT, KEIDEL D, BULA CM, et al. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1α,25(OH)2-vitamin D3 signaling. Proc Nat Acad Sci USA. 2004;101(35):12876–12881. doi: 10.1073/pnas.0403606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.XU RX, LAMBERT MH, WISELY BB, et al. A structural basis for constitutive activity in the human CAR/RXRα heterodimer. Mol Cell. 2004;16(6):919–928. doi: 10.1016/j.molcel.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 165.SUINO K, PENG L, REYNOLDS R, et al. The nuclear xenobiotic receptor CAR: structural determinants of constitutive activation and heterodimerization. Mol Cell. 2004;16(6):893–905. doi: 10.1016/j.molcel.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 166.SHAN L, VINCENT J, BRUNZELLE JS, et al. Structure of the murine constitutive androstane receptor complexed to androstenol: a molecular basis for inverse agonism. Mol Cell. 2004;16(6):907–917. doi: 10.1016/j.molcel.2004.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]