Abstract
OBJECTIVE
To summarize the evidence linking dietary habits to the incidence of several types of cancer with special emphasis on the chemoprotective properties of foods that originate from plants.
QUALITY OF EVIDENCE
A large body of epidemiologic, animal, and laboratory literature indicates that as many as 30% of all cancer cases are linked to poor dietary habits. The proportion reaches 70% for cancers of the gastrointestinal tract.
MAIN MESSAGE
Studies have consistently linked abundant consumption of plant-based food to a substantial reduction in risk of developing various cancers. Laboratory studies show that this chemopreventive effect is related to the high levels of numerous phytochemicals in this food. These phytochemicals interfere with several cellular processes involved in the progression of cancer and also with inflammatory processes that foster development of cancer.
CONCLUSION
Dietary factors play an important role in the high incidence of several types of cancer in Canada. Modification of dietary habits to include daily intake of plant-based food containing anticancer and anti-inflammatory phytochemicals thus represents a promising approach to preventing the development of cancer.
RÉSUMÉ
OBJECTIF
Faire le point sur les données qui indiquent que l’incidence de plusieurs types de cancers est reliée aux habitudes alimentaires, en insistant particulièrement sur les propriétés chimioprotectrices de certains aliments d’origine végétale.
QUALITÉ DES PREUVES
Les résultats d’un grand nombre d’études épidémiologiques et d’expériences chez l’animal ou en laboratoire indiquent que jusqu’à 30% de tous les cas de cancer sont liés à de mauvaises habitudes alimentaires. Pour les cancers du tube digestif, cetteproportion atteint 70%.
PRINCIPAL MESSAGE
Les études ont régulièrement montré que la consommation d’une bonne quantité d’aliments d’origine végétale est associée à un risque considérablement moindre de développer certains types de cancers. Les études en laboratoire révèlent que cet effet chimioprotecteur est dû aux niveaux élevés de plusieurs phytochimiques contenus dans ces aliments. Cesphytochimiques interfèrent avec plusieurs des processus cellulaires nécessaires à la progression du cancer et ralentissent les processus inflammatoires qui favorisent son développement.
CONCLUSION
Divers facteurs alimentaires jouent un rôle important dans l’incidence élevée de plusieurs types de cancer au Canada. Un changement dans les habitudes alimentaires favorisant un apport quotidien d’aliments d’origine végétale riches en phytochimiques anticancéreux et anti-inflammatoires représente donc une stratégie prometteuse pour prévenir le cancer.
In their landmark study, Doll and Peto showed that 75% to 80% of all cancers diagnosed in the United States in 1970 might have been prevented by altering lifestyle factors, such as smoking and diet.1 Subsequently, their conclusions were confirmed by a large number of studies. Researchers currently estimate that diet could account for approximately 30% of cancer deaths, similar to the number accounted for by smoking.2
The close relationship between diet and cancer is suggested by the large variation in rates of specific cancers in different countries and by the spectacular changes observed in the incidence of cancer in migrating populations.1–3 For example, studies have shown that Asians have a 25-fold lower incidence of prostate cancer and a 10-fold lower incidence of breast cancer than inhabitants of Western countries do, but that rates of these cancers dramatically increase following migration to the West.2 The importance of lifestyle factors in the development of cancer was also shown in studies of mono-zygotic twins (who share all genes). Inherited genetic factors were shown to be responsible for only about 15% of all cancer cases.4 These observations indicate that most cancers are not of hereditary origin and that lifestyle factors, such as dietary habits, have a profound influence on their development.
Quality of evidence
Relevant papers were identified by searching PubMed from January 1980 to December 2006 using the key words cancer, diet, prevention, angiogenesis, inflammation, and phytochemicals. Articles on the chemopreventive potential of specific foods or food groups were sought using the key words for these groups (eg, cruciferous, allium, soy, citrus, tomato, turmeric, berries, and green tea) or the key words for the anticancer molecules found in these foods (eg, isothiocyanates, sulforaphane, sulfides, resveratrol, curcumin, and epigallocatechin-3-gallate). Evidence of the chemopreventive effects of various components of food comes from several hundred case-control and prospective epidemiologic studies, and this relationship is strengthened by a large body of evidence derived from animal and laboratory studies.
Many epidemiologic studies have consistently linked abundant consumption of foods of plant origin, such as fruit, vegetables, whole grains, legumes, nuts, seeds, and tea, with decreased risk of developing various of cancers.2,5 When results of studies on all types of cancer are taken together, as many as 80% show a substantial decrease in risk with higher intake of at least 1 vegetable or fruit category examined2 (Table 12). This is particularly true for cancers of the upper gastrointestinal tract (Table 22); for example, studies indicate that as many as 75% of colorectal cancers, the second leading cause of death due to cancer in Canada, could be prevented by increasing the amount of plant-based food in the diet.6
Table 1.
Summary of results of case-control and cohort studies of all types of cancer showing an inverse association between consumption of various categories of fruit and vegetables and risk of cancer: Overall level of evidence is I.
| FRUIT OR VEGETABLE CATEGORIES | NO. OF STUDIES SHOWING DECREASED RISK | TOTAL NUMBER OF STUDIES | % OF TOTAL STUDIES SHOWING DECREASED RISK |
|---|---|---|---|
| All vegetables | 59 | 74 | 80 |
| All fruit | 36 | 56 | 64 |
| Raw vegetables | 40 | 46 | 87 |
| Cruciferous vegetables | 38 | 55 | 69 |
| Allium vegetables | 27 | 35 | 77 |
| Green vegetables | 68 | 88 | 77 |
| Tomatoes | 36 | 51 | 71 |
| Citrus fruit | 27 | 41 | 66 |
Adapted from American Institute for Cancer Research.2
Table 2.
Levels of evidence for decreased risk of various types of cancer with consumption of fruit and vegetables
| TYPE OF CANCER | LEVEL OF EVIDENCE |
|---|---|
| Stomach, esophagus, mouth and pharynx, colon, rectum, lung | I |
| Larynx, pancreas, breast, bladder | II |
| Ovary, endometrium, cervix, thyroid, prostate, kidney, liver | III |
Adapted from American Institute for Cancer Research.2
These observations suggest that food of vegetable origin is an essential source of molecules with chemopreventive properties. This hypothesis is strengthened by many experimental data obtained from studies using cellular and animal models in which molecules isolated from various food sources were found to interfere with development of several cancers.7,8
Interfering with the development of precancerous tumours
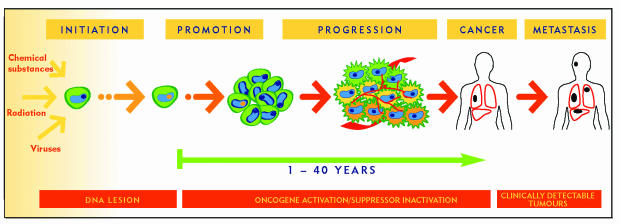
Carcinogenesis is generally a slow process during which precancerous cells must accumulate several mutations in the genes involved in growth control, resistance to apoptosis, and induction of angiogenesis in order to grow and invade the host tissues9 (Figure 110). This process, which might take place over several years (even several decades), offers a large therapeutic window for blocking the development of cancer. The low genetic diversity of precancerous cells and the absence of an appropriate blood supply render the cells much more vulnerable to anticancer molecules than mature tumour cells would be.
Figure 1. Steps in carcinogenesis: For many years, or even decades, cancerous cells remain very vulnerable; only a few will succeed in reaching the malignant stage.
This vulnerability makes it possible to intervene and interfere at several stages of tumour development and thus prevent onset of disease.
Preventing cancer by blocking development of these precancerous cells is extremely important because even healthy people have a certain number of latent tumours in their tissues. Studies of people who died from causes unrelated to cancer have shown that 30% to 50% of women aged 40 to 50 years had premalignant microscopic breast tumours, and 40% of similarly aged men had precancerous cells in the prostate.11 Even more striking, virtually every person (98%) had small latent tumours in the thyroid, although these tumours are only very rarely observed in the clinic (Table 311). It thus seems that the spontaneous formation of small tumours happens frequently over a lifetime, but that in most cases the growth of these small tumours is tightly controlled by our natural defence mechanisms, and they remain in a microscopic and harmless state.12 The appearance of clinically detectable cancer thus implies that precancerous cells have overwhelmed these defences and acquired the ability to grow and invade the host tissues.
Table 3.
Proportion of tumours detected at autopsy and clinically: Tumours had formed spontaneously.
| ORGAN | % OF PATIENTS WITH TUMOURS DETECTED AT AUTOPSY | % OF PATIENTS WITH TUMOURS DETECTED CLINICALLY |
|---|---|---|
| Breast (40- to 50-year-old women) | 33 | 1 |
| Prostate (40- to 50-year-old men) | 40 | 2 |
| Thyroid | 98 | 0.1 |
Adapted from Black and Welch.11
Anticancer properties of plant-based food: the importance of phytochemicals
There is now considerable evidence that the chemopreventive properties of plant-based food are related to their ability to block the progression of latent microtumours. These properties arise from the high content of phytochemicals, molecules that target several key events in the development of cancer. Intensive research conducted over the last few years has shown that phytochemicals derived from the diet interfere with tumour progression by acting directly on tumour cells as well as by modifying the tumour’s microenvironment (stroma) and creating physiologic conditions that are hostile to tumour growth.
Direct inhibitory actions on tumour cells
Reduction of damage to DNA
Free radicals, environmental or diet-associated chemicals, and some metabolites all have the capacity to severely damage cell DNA, which might ultimately lead to cancer.13 Several chemopreventive phytochemicals elicit their anticancer effects by modulating the enzymatic systems responsible for neutralizing these carcinogens, either by reducing their carcinogenic potential or by increasing their excretion.14–16 For example, isothiocyanates, compounds found in abundance in cruciferous vegetables, inhibit tumourigenesis by reducing genetic damage induced by a wide variety of chemical carcinogens.17,18 Phytochemicals, which modulate the host’s defence mechanism against DNA-damaging molecules, are also found in several other types of fruit and vegetables, including members of the genus Allium (such as garlic)19 and citrus fruit.20 The ability of these molecules to reduce the oncogenic potential of carcinogens thus makes them an efficient first-line defence against cancer.16–19
Cytotoxicity against tumour cells
Several phytochemicals also inhibit tumour growth by directly inducing cancer cell death by apoptosis. For example, phenethyl isothiocyanate from cruciferous vegetables, curcumin from turmeric, and resveratrol from grapes have all been shown to possess strong pro-apoptotic activity against cells isolated from a variety of tumours.21–23 This activity correlates with inhibition of tumour growth in animals. Recent work has shown that some phytochemicals also sensitize tumour cells to apoptotic cues derived from natural pro-apoptotic stimuli, such as tumour necrosis factor–related apoptosis-inducing ligand.24,25 Overall, the cytotoxic properties of diet-derived phytochemicals contribute to the chemopreventive effects associated with intake of plant-derived foods and could play an essential role in preventing growth of cells that have already acquired an initiated phenotype (precancerous cells).
Effects on tumour microenvironment
Antiangiogenic properties
Angiogenesis is the process by which tumour cells stimulate formation of new blood vessel networks that sustain the development of cancer by providing oxygen and nutrients to tumour cells.26 Work from our laboratory has shown that several phytochemicals possess strong antiangiogenic activity and that this effect likely plays an important role in their chemopreventive properties.27,28 For example, epigallocatechin-3-gallate (EGCG), an abundant polyphenol found in green tea, potently inhibits vascular endothelial growth factor receptor-2, a key receptor involved in tumour angiogenesis.29 The inhibitory effect occurs with low concentrations of EGCG (concentrations achievable through diet), suggesting that this antiangiogenic effect is relevant in vivo.28 Accordingly, oral infusion of a polyphenolic fraction isolated from green tea at a human achievable dose (equivalent to 6 cups of green tea per day) strongly inhibits development of prostate cancer and increases the survival of transgenic mice that spontaneously develop this cancer.30 Interestingly, moderate consumption of green tea by humans was also recently associated with clear clinical benefits for some patients with chronic lymphocytic leukemia.31
In a similar manner, ellagic acid,32 a phenolic acid found in high quantities in some fruit, such as raspberries and strawberries, and delphinidin,33 an anthocyanidin abundant in blueberries, also block vascular endothelial growth factor receptor-2 activity and also strongly inhibit the activity of another receptor found in perivascular cells, platelet-derived growth factor receptor.32,33 This combined inhibitory effect of 2 receptor tyrosine kinases that are both essential for angiogenesis leads to inhibition of this process in both in vitro and in vivo assays29,33 in a manner similar to the synergistic anticancer effects observed with pharmacologic inhibition of these receptors.34 There is growing evidence that the antiangiogenic effects of these phytochemicals play a crucial role in their chemopreventive activity by preventing latent tumours from acquiring the blood vessel network necessary to sustain their growth and permit them to invade host tissues.
Anti-inflammatory effects
It is becoming increasingly clear that inflammatory stimuli participate in the progression of several cancers, including those of the colorectum, breast, and lung.35 The close relationship between inflammation and cancer is suggested by the identification of a number of inflammatory conditions that predispose patients to cancer (Table 436) as well as by the chemopreventive effects that anti-inflammatory cyclooxygenase-2 (COX-2) inhibitors, such as celecoxib, have on risk of colorectal cancer.37 Although the severe cardiovascular side effects associated with these drugs preclude their use as prophylactic agents,38 these effects nevertheless indicate that reducing inflammation represents a promising approach to preventing cancer.
Table 4.
Some inflammatory conditions that predispose patients to cancer
| INFLAMMATORY STIMULUS | MALIGNANCY |
|---|---|
| Inflammatory bowel disease | Colorectal cancer |
| Helicobacter pylori-induced gastritis | Gastric cancer |
| Prostatitis | Prostate cancer |
| Salpingitis, endometriosis | Ovarian cancer |
| Barrett syndrome | Esophageal cancer |
| Asbestos inhalation | Bronchial carcinoma, mesothelioma |
Adapted from Balkwill et al.36
There is now considerable evidence that Western diets rich in refined starches, sugar, and saturated and trans fatty acids and poor in fruit, vegetables, fibre, ω-3 fatty acids, and whole grains promote inflammation.39 Inflammatory and immune cells from those consuming typical Western diets contain a high proportion of the pro-inflammatory ω-6 polyunsaturated fatty acid (PUFA) arachidonic acid and a low proportion of anti-inflammatory ω-3 PUFAs eicosapentenoic acid and docosahexenoic acid.40 As arachidonic acid is the precursor of 2-series prostaglandins and 4-series leukotrienes, which are highly active mediators of inflammation, a high dietary ω-6–to–ω-3 ratio results in generation of a pro-inflammatory state that could sustain the onset of several diseases, including cardiovascular disease, diabetes, and certain types of cancer.39,41
In addition to the important role of dietary PUFAs, there is also growing evidence that several phytochemicals from dietary sources reduce inflammatory processes, and that this anti-inflammatory effect contributes to their anticancer properties.42 For example, curcumin (the yellow pigment of turmeric), the green tea polyphenol EGCG, and resveratrol from grapes have all been shown to markedly reduce the expression of COX-2, an effect related to blockading the activity of the nuclear factor-κB transcription factor.7,42,43 This effect is likely to occur in humans, as reflected by the decrease in serum levels of the inflammatory prostaglandin PGE2 following oral administration of curcumin.44
Conclusion
A large number of epidemiologic, animal, and laboratory studies indicate that abundant consumption of food of plant origin reduces the risk of several types of cancer. The chemopreventive effect is related to the high content in these foods of phytochemicals with potent anticancer and anti-inflammatory properties. These properties block precancerous cells from developing into malignant cells by interfering directly with tumour cells and by preventing generation of an inflammatory microenvironment that would sustain the progression of the tumours. In many cases, these anticancer phytochemicals interfere with tumour promotion and progression by mechanisms identical to those through which synthetic molecularly targeted chemotherapeutic agents exert their activity (Table 5).7,42,45
Table 5.
Pharmacologic targets of diet-derived phytochemicals
| TARGET | EXAMPLES | BEST FOOD SOURCES |
|---|---|---|
| Inhibition | ||
| Tumour invasion and metastasis | Epigallocatechin gallate | Green tea |
| Growth factor receptor–mediated signal transduction | Delphinidin, ellagic acid | Blueberries, raspberries, nuts |
| Inflammatory enzymes (eg, cyclooxygenase-2) | Curcumin, resveratrol | Turmeric, grapes |
| Transcription factor activity (eg, nuclear factor-κB, activator protein-1) | Curcumin, resveratrol | Turmeric, grapes |
| Multidrug resistance | Diallyl disulfide | Garlic |
| Angiogenesis | Epigallocatechin gallate | Green tea |
| Estrogenic actions | Genistein | Soy |
| Metabolic activation of carcinogens via phase I enzymes | Indole-3-carbinol | Cabbage |
| Activation | ||
| Tumour cell apoptosis | Phenethyl isothiocyanate | Cabbage, watercress |
| Immune system function | Lentinan | Shiitake mushrooms |
| Detoxification via phase II enzymes | Sulforaphane | Broccoli |
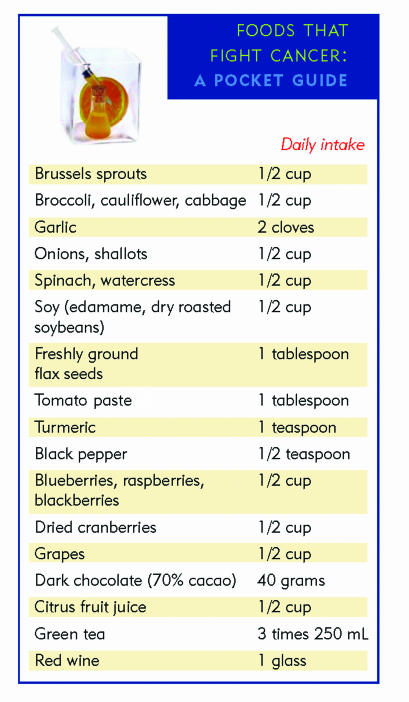
Unlike synthetic molecules, however, whose inherent toxicity limits their use for prevention (eg, COX-2 inhibitors), the anticancer molecules present naturally in food are selected by evolution to be beneficial for health and, therefore, do not have secondary harmful effects. Daily intake of food rich in anticancer molecules could thus be compared to a preventive, non-toxic version of chemotherapy that is harmless to the physiology of normal tissue and stops microtumours from attaining a stage that would allow them to have pathologic consequences. Based on these considerations, there is no doubt that all patients, particularly those who have family history of cancer, should be strongly encouraged to change their dietary habits to include at least 5 to 10 servings daily of plant-based food, especially food with the highest content of anticancer phytochemicals (Figure 210). Such a change, coupled with a reduction in consumption of red meat (which is associated with an increased risk of colorectal46 and some types of breast47 cancer), maintenance of physical activity and appropriate body mass, and not smoking could substantially reduce the burden of cancer in Canada.
Figure 2.
Including foods with chemopreventive properties in a daily diet
Levels of evidence
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
Acknowledgement
This work was supported by grants from the Fondation Charles-Bruneau and the Cancer Research Society of Canada Inc. Dr Béliveau holds the Chaire en prévention et en traitement du cancer at the University of Quebec at Montreal and the Chaire Claude-Bertrand en neurochirurgie at the University of Montreal.
EDITOR’S KEY POINTS
Even healthy people can have microtumours in their tissues. It appears that the growth of these small tumours is tightly controlled by our natural defence mechanisms, but these defences can be overwhelmed.
Case-control, epidemiologic, and laboratory studies have shown that certain foods of plant origin have chemoprotective properties related to their ability to block the progression of latent microtumours into mature tumours.
Canada’s Food Guide recommends intake of 7 to 10 servings of fruit and vegetables daily for adults, depending on age and sex. It might be prudent to choose foods that have high levels of anticancer phytochemicals.
POINTS DE RÉPÈRE DU RÉDACTEUR
Même une personne en santé peut héberger des microtumeurs dans ses tissus. On croit que la croissance de ces petites tumeurs est étroitement contrôlée par les mécanismes de défense naturels, mais ces défenses peuvent être débordées.
Des études épidémiologiques, de cas-témoins et en laboratoire ont montré que certains aliments d’origine végétale possèdent des propriétés chimioprotectrices reliées à leur capacité de bloquer la transformation des microtumeurs latentes en tumeurs matures.
Le Guide alimentaire canadien recommande pour les adultes un apport quotidien de 7 à 10 portions de fruits et légumes, selon l’âge et le sexe. Il semblerait judicieux de choisir les aliments possédant un niveau élevé de phytochimiques anticancéreux.
Footnotes
This article has been peer reviewed.
Competing interests
None declared
References
- 1.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–308. [PubMed] [Google Scholar]
- 2.American Institute for Cancer Research, World Cancer Research Fund. Food, nutrition, and the prevention of cancer: a global perspective. Washington, DC: American Institute for Cancer Research; 1997. [DOI] [PubMed] [Google Scholar]
- 3.Willett WC. Diet and cancer. Oncologist. 2002;5:393–404. doi: 10.1634/theoncologist.5-5-393. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 5.Gescher A, Pastorino U, Plummer SM, Manson MM. Suppression of tumour development by substances derived from the diet—mechanisms and clinical implications. Br J Clin Pharmacol. 1998;45:1–12. doi: 10.1046/j.1365-2125.1998.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson CA, LeWinn K, Newton TR, Alberts DS, Martinez ME. Nutrition and diet in the development of gastrointestinal cancer. Curr Oncol Rep. 2003;5:192–202. doi: 10.1007/s11912-003-0110-y. [DOI] [PubMed] [Google Scholar]
- 7.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nature Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 8.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–40. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 10.Béliveau R, Gingras D. Foods that fight cancer: preventing cancer through diet. Toronto, ON: McClelland & Stewart Ltd; 2006. [Google Scholar]
- 11.Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328:1237–43. doi: 10.1056/NEJM199304293281706. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J, Kalluri R. Cancer without disease [abstract] Nature. 2004;427:787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 13.Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc Natl Acad Sci USA. 1995;92:5258–65. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioannides C, Lewis DF. Cytochromes P450 in the bioactivation of chemicals. Curr Top Med Chem. 2004;4(16):1767–88. doi: 10.2174/1568026043387188. [DOI] [PubMed] [Google Scholar]
- 15.Conney AH. Enzyme induction and dietary chemicals as approaches to cancer chemoprevention: the seventh DeWitt S. Goodman lecture. Cancer Res. 2003;63:7005–31. [PubMed] [Google Scholar]
- 16.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 17.Hecht SS. Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism. J Nutr. 1999;129:768S–74S. doi: 10.1093/jn/129.3.768S. [DOI] [PubMed] [Google Scholar]
- 18.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131:3027S–33S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 19.Guyonnet D, Belloir C, Suschetet M, Siess MH, Le Bon AM. Mechanisms of protection against aflatoxin B(1) genotoxicity in rats treated by organosulfur compounds from garlic. Carcinogenesis. 2002;23:1335–41. doi: 10.1093/carcin/23.8.1335. [DOI] [PubMed] [Google Scholar]
- 20.Hahn-Obercyger M, Stark AH, Madar Z. Grapefruit and oroblanco enhance hepatic detoxification enzymes in rats: possible role in protection against chemical carcinogenesis. J Agric Food Chem. 2005;53:1828–32. doi: 10.1021/jf048547a. [DOI] [PubMed] [Google Scholar]
- 21.Thornalley PJ. Isothiocyanates: mechanism of cancer chemopreventive action. Anticancer Drugs. 2002;13:331–8. doi: 10.1097/00001813-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Karunagaran D, Rashmi R, Kumar TR. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets. 2005;5:117–29. doi: 10.2174/1568009053202081. [DOI] [PubMed] [Google Scholar]
- 23.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 24.Jeon KI, Rih JK, Kim HJ, Lee YJ, Cho CH, Goldberg ID, et al. Pretreatment of indole-3-carbinol augments TRAIL-induced apoptosis in a prostate cancer cell line, LNCaP. FEBS Lett. 2003;544:246–51. doi: 10.1016/s0014-5793(03)00473-3. [DOI] [PubMed] [Google Scholar]
- 25.Jung EM, Park JW, Choi KS, Park JW, Lee HI, Lee KS, et al. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through CHOP-independent DR5 upregulation. Carcinogenesis. 2006;27:2008–17. doi: 10.1093/carcin/bgl026. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 27.Tosetti F, Ferrari N, De Flora S, Albini A. “Angioprevention”: angiogenesis is a common and key target for cancer chemopreventive agents”. FASEB J. 2002;16(1):2–14. doi: 10.1096/fj.01-0300rev. [DOI] [PubMed] [Google Scholar]
- 28.Béliveau R, Gingras D. Green tea: prevention and treatment of cancer by nutraceuticals. Lancet. 2004;364:1021–2. doi: 10.1016/S0140-6736(04)17076-1. [DOI] [PubMed] [Google Scholar]
- 29.Lamy S, Gingras D, Béliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62:381–5. [PubMed] [Google Scholar]
- 30.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanafelt TD, Lee YK, Call TG, Nowakowski GS, Dingli D, Zent CS, et al. Clinical effects of oral green tea extracts in four patients with low grade B-cell malignancies. Leuk Res. 2006;30:707–12. doi: 10.1016/j.leukres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Labrecque L, Lamy S, Chapus A, Mihoubi S, Durocher Y, Cass B, et al. Combined inhibition of PDGF and VEGF receptors by ellagic acid, a dietary-derived phenolic compound. Carcinogenesis. 2005;26:821–6. doi: 10.1093/carcin/bgi024. [DOI] [PubMed] [Google Scholar]
- 33.Lamy S, Blanchette M, Michaud-Levesque J, Lafleur R, Durocher Y, Moghrabi A, et al. Delphinidin, a dietary anthocyanidin, inhibits vascular endothelial growth factor receptor-2 phosphorylation. Carcinogenesis. 2006;27:989–96. doi: 10.1093/carcin/bgi279. [DOI] [PubMed] [Google Scholar]
- 34.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumour vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 38.Bertagnolli MM, Eagle CJ, Zauber AG, Redston N, Soloman SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 39.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–85. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 40.Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61:345–58. doi: 10.1079/pns2002166. [DOI] [PubMed] [Google Scholar]
- 41.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–45. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 42.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480–1:243–68. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann NY Acad Sci. 2004;1030:434–41. doi: 10.1196/annals.1329.054. [DOI] [PubMed] [Google Scholar]
- 44.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 45.D’Incalci M, Steward WP, Gescher AJ. Use of cancer chemopreventive phytochemicals as antineoplastic agents. Lancet Oncol. 2005;6:899–904. doi: 10.1016/S1470-2045(05)70425-3. [DOI] [PubMed] [Google Scholar]
- 46.Chao A, Thun MJ, Connell CJ, McCullough ML, Jacobs EJ, Flanders WD, et al. Meat consumption and risk of colorectal cancer. JAMA. 2005;293:172–82. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]
- 47.Cho E, Chen WY, Hunter DJ, Stampfer MJ, Colditz GA, Hankinson SE, et al. Red meat intake and risk of breast cancer among premenopausal women. Arch Intern Med. 2006;166:253–9. doi: 10.1001/archinte.166.20.2253. [DOI] [PubMed] [Google Scholar]