Abstract
OBJECTIVE
To explore the extent to which biochemical testing is used to diagnose androgen deficiency before initiating treatment and to learn whether recommendations for clinical monitoring of men taking androgen therapy are being followed.
DESIGN
Population-based retrospective cohort study.
SETTING
Winnipeg, Man.
PARTICIPANTS
A total of 902 men who filled at least 2 prescriptions for androgen therapy.
MAIN OUTCOME MEASURES
Whether men had had baseline prostate-specific antigen (PSA) and testosterone testing before initiation of therapy and whether men had beenmonitored during the first year of treatment.
RESULTS
Of the 902 men who filled first-time prescriptions during the study period, only 475 (52.7%) had ever had PSA or testosterone tests. Before starting therapy, 315 men (34.9%) had had PSA tests, and 152 men (16.9%) had had testosterone tests. Less than 1% of the entire sample had had 3 or more tests during the year following initiation of therapy.
CONCLUSION
Indications for androgen therapy in this population appear to be based on clinical symptoms rather than on demonstrated biochemical androgen deficiency. Recommendations for clinical monitoring of men taking androgen therapy are not followed consistently.
RÉSUMÉ
OBJECTIF
Déterminer à quel point des examens de laboratoire sont effectués pour établir un diagnostic de déficience androgénique avant d’instituer un traitement et vérifier si les recommandations de surveillance durant l’androgénothérapie sont suivies.
TYPE D’ÉTUDE
Étude de cohorte rétrospective de nature démographique
CONTEXTE
Winnipeg, Manitoba.
PARTICIPANTS
Un total de 902 hommes ayant utilisé au moins 2 prescriptions pour androgénothérapie.
PRINCIPALES QUESTIONS À L’ÉTUDE
Y a-t-il eu une mesure des niveaux de base de l’antigène prostatique spécifique (PSA) et de la testostérone avant le début du traitement ainsi qu’une surveillance durant la première année du traitement?
RÉSULTATS
Sur les 902 hommes qui ont utilisé une première prescriptiondurant la période couverte par l’étude, seulement 475 (52,7%) avaient eu un dosage de la PSA ou de la testostérone. Avant l’instauration du traitement, 315 hommes (34,9%) avaient eu un dosage de PSA et 152 (16,9%) un dosage de testostérone. Moins de 1% des sujets avaient eu 3 dosages ou plus durant la première année du traitement.
CONCLUSION
Dans la population étudiée, les raisons pour débuter une androgénothérapie semblent reposer sur des symptômes cliniques plutôt que sur la démonstration d’une déficience androgénique. Les recommandations de surveillance clinique des patients qui reçoivent des androgènes ne sont pas suivies de façon régulière.
Since 1993, there has been a 500% overall increase in the number of prescriptions for all testosterone products in North America. After the release of topical testosterone preparations in 2000, there was a further 67% increase in prescriptions.1 Although testosterone replacement (androgen) therapy is suggested to counteract the symptoms of andropause, such as fatigue, perception of lack of well-being, loss of energy, muscle wasting, sexual dysfunction, cognitive decline, and bone density loss, the very existence of the andropause syndrome has been questioned.2
Testosterone levels decline by 1% to 2% per year from the peak levels achieved in young adulthood, and 20% of men older than 60 have levels below the normal range for young men.3 Potential risks of treatment include local effects on the prostate, promotion of growth of occult prostate cancer cells, and prostatic hypertrophy. Other systemic effects include fluid retention, sleep apnea, gynecomastia, polycythemia, and increased risk of cardiovascular disease.4
Generally accepted recommendations for clinical monitoring of men taking androgen therapy include ascertaining pretreatment testosterone levels to establish a diagnosis of hypogonadism.5 These levels should be measured at 2 separate times at least 2 weeks apart.6 It is recommended that men receiving androgen therapy have a digital rectal examination (DRE) and baseline prostate-specific antigen (PSA) testing before beginning therapy and be monitored every 3 to 6 months for the first year of treatment and then yearly after that.5–7 These recommendations were published in widely available journals before the period during which data were collected for this study. Results of PSA tests are used as a marker of prostate cancer,8 and any rise in PSA levels or abnormal findings on DRE should be followed by more invasive testing for the presence of prostate disease.
To explore the extent to which these recommendations are being followed, we examined use of PSA and testosterone testing among a cohort of patients who filled prescriptions for androgen therapy.
METHODS
Approvals from the University of Manitoba’s Research Ethics Board and from Manitoba Health’s Health Information Privacy Committee were obtained before initiation of the study. Patients who filled prescriptions for androgen therapy (testosterone undecanoate and testosterone cypionate were the only available preparations in Canada at the time) were identified from the Manitoba Drug Information Network. This database reflects prescriptions dispensed by Manitoba retail pharmacists and has been validated in other studies.9 The database is maintained by Manitoba Health and is updated in the Manitoba Centre for Health Policy repository annually.
Records in the database can be linked to other Manitoba databases in the repository through the uniquely scrambled Personal Health Information Number attached to each record. It is thus possible to link the records in the Manitoba Drug Information Network database with records in other components of the repository.10 Linkage with the physician registry can determine whether prescribing physicians are generalists or specialists without identifying the physicians; linkage with the patient registry can provide information on patients’ age and other demographics.
Cohort
A file was created of a cohort of men who had filled at least 2 prescriptions for androgens between April 1, 2000, and March 31, 2003, identifying the date of their initial prescriptions for androgens. This file was merged with laboratory files for PSA and testosterone tests from the 8 laboratories that perform all these tests in Manitoba for the period January 1, 2000, to December 31, 2003. We excluded patients who had prescriptions for androgens before January 2000 as we were unable to explore use of pretreatment testing before that date. We followed the cohort for 9 months after their prescriptions were filled from March 31 to December 31, 2003.
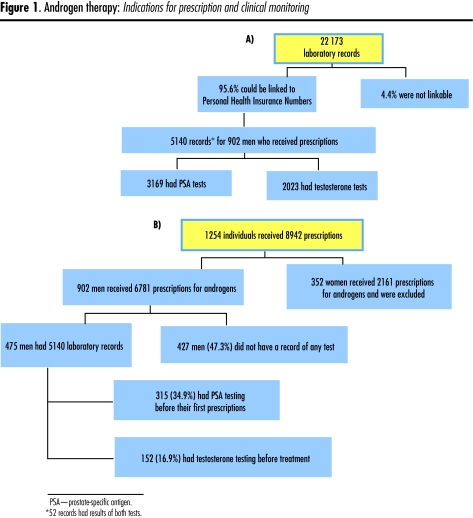
Only total testosterone testing is available routinely. We were thus able to determine which of the androgen users had undergone PSA and testosterone testing during the 3 months before initiating androgen therapy and which of them had undergone the recommended monitoring with PSA testing during treatment (Figure 1).
Figure 1. Androgen therapy.
Indications for prescription and clinical monitoring
Statistical analysis
We calculated rates of baseline PSA and testosterone testing and determined the rate of ongoing PSA monitoring during the first year of androgen use. Rates were compared for various demographic groups using χ2 analysis.
We then explored the relationship between patient and physician characteristics and the likelihood of having baseline PSA and testosterone tests with logistic regression analysis. We included patients’ age, socioeconomic status (as reflected by the socioeconomic status index developed at the Manitoba Centre for Health Policy),11 region (urban vs rural), and physician type (family physician vs specialist) in the analysis. All analyses were performed using SAS software.
RESULTS
From the administrative data, we identified all men who filled prescriptions for the first time for androgens during the years 2001, 2002, and 2003. Most of the men were of an age where testosterone levels are expected to decrease (35.8% were 50 to 59 years old, and 27.7% were 60 years or older), but 22.3% were between 40 and 49 years, and their physiologic levels of testosterone should still have been in the normal range12 (Table 1). Figure 1 shows the distribution of available laboratory records and patients who filled prescriptions. A total of 1254 people received 8942 prescriptions; 352 of these people (28.1%) were women who received 2161 prescriptions and were excluded from the study. We were able to link 95.6% (22 173) of the laboratory tests to patients in the repository. After linking laboratory and prescription data, we had 5140 records of which 2023 were results of testosterone tests and 3169 were results of PSA tests. Fifty-two records contained results of both testosterone and PSA tests.
Table 1.
Characteristics of patients who filled prescriptions for androgen therapy: N = 902.
| CHARACTERISTICS | N (%) |
|---|---|
| Rural | 268 (29.7) |
| Urban | 634 (70.3) |
| Age (y) | |
| • 0–39 | 128 (14.2) |
| • 40–49 | 201 (22.3) |
| • 50–59 | 323 (35.8) |
| • >60 | 250 (27.7) |
Of the 902 patients who had first-time prescriptions during the study period, only 475 (52.7%) had had PSA or testosterone tests at all. Among these patients, 152 (16.9%) had had testosterone tests and 315 (34.9%) had had PSA tests before starting therapy (Table 2). Family physicians wrote 78.4% of all prescriptions, urologists wrote 3%, and a variety of other specialists wrote the remaining prescriptions. Less than 1% of the entire cohort had had 3 or more PSA tests during the year following initiation of therapy. We tested the relationships between physician and patient characteristics and baseline PSA and testosterone testing using logistic regression. The models identified age as a predictor of testing before receiving a prescription for an androgen. Older users (mean age 56 years) were more likely to have had PSA tests, and younger users were more likely to have had their testosterone measured (Table 2). Rural androgen users were likely to have had their testosterone levels measured, but were less likely than those living in urban areas to have had their PSA measured before initiation of therapy. Neither socioeconomic status nor type of physician had a statistically significant effect.
Table 2.
Patient characteristics associated with baseline prostate-specific antigen and testosterone testing
| A)
| |||||
|---|---|---|---|---|---|
| CHARACTERISTICS | HAD BASELINE PSA TEST (% OF ALL MEN IN CATEGORY) N = 315 | DID NOT HAVE BASELINE PSA TEST (% OF ALL MEN IN CATEGORY) N = 587 | P VALUE | TOTAL (%) N = 902 | ODDS RATIO (95% CONFIDENCE INTERVAL) |
| Urban | 239 (37.7) | 395 (62.3) | 634 (70.3) | ||
| Rural | 76 (28.4) | 192 (71.6) | .0286 | 268 (29.7) | 0.67 (0.48–0.92) |
| Mean age (y) | 56.2 | 50.9 | <.0001 | 1.03 (1.02–1.05) | |
| B)
| |||||
| CHARACTERISTICS | HAD BASELINE TESTOSTERONE TEST (% OF ALL MEN IN CATEGORY) N = 152 | DID NOT HAVE BASELINE TESTOSTERONE TEST (% OF ALL MEN IN CATEGORY) N = 750 | P VALUE | TOTAL (%) N = 902 | ODDS RATIO (95% CONFIDENCE INTERVAL) |
| Urban | 77 (12.1) | 557 (87.9) | 634 (70.3) | ||
| Rural | 75 (28.0) | 193 (72.0) | <.0001 | 268 (29.7) | 2.76 (1.93–3.95) |
| Mean age (y) | 50.5 | 53.2 | .0138 | 0.99 (0.97–0.99) | |
PSA—prostate-specific antigen.
DISCUSSION
Our results show that recommendations for clinical monitoring of men taking androgen therapy are not being followed. While we do not know the indication for androgen therapy in this population, it appears to be based on clinical symptoms or patient request rather than demonstrated biochemical androgen deficiency, as recommended.5–7 If baseline testosterone levels were not measured, a definitive diagnosis of androgen insufficiency could not be made.
The recommendation for testing patients receiving androgen therapy includes establishing baseline PSA and testosterone levels and monitoring PSA levels every 3 to 6 months during the first year of treatment. While 34.9% of patients did have baseline PSA levels measured, less than 1% of patients received the recommended ongoing monitoring. Some of these patients, however, would have discontinued therapy and thus would not have required ongoing monitoring.
The role of testosterone in development or progression of prostate cancer is controversial. Androgens are known to play a role in benign prostatic hyperplasia.13 Some have suggested that occult prostate cancer might progress rapidly in the presence of exogenous androgens,7,14,15 but others refute this.16,17 Some studies have shown a rise in PSA levels in men taking androgen therapy, but this rise does not appear to be associated with prostate cancer.18–20 Other studies do not seem to support this.21 A case series of 20 men diagnosed with prostate cancer while receiving androgen therapy showed that 55% developed prostate cancer within the first 2 years of initiation of therapy.22 Urologists were more likely to monitor PSA and do DREs than non-urologists were in this series. In our study, however, no such difference was noted.
We have no information on the frequency of PSA monitoring among patients not diagnosed with prostate cancer. In addition, the total number of men taking androgen therapy was not reported. Among those who did get prostate cancer, PSA testing was carried out before androgen therapy commenced for 100% of those treated by urologists and 75% of men treated by non-urologists. In our study, family physicians wrote 78.4% of all new prescriptions for androgens, but ordered 88% of baseline PSA testing. While the evidence suggesting an increased risk of prostate cancer with testosterone therapy is weak, evidence supporting the safety of testosterone therapy is also weak. A prudent course of action would be to suggest ongoing monitoring until such time as the safety of testosterone therapy has been proven.
Strength
A strength of this study is the use of population-based data. The reliability of this data source has been shown in numerous previous studies.23 The only previous study that looked at clinical monitoring of men taking androgen therapy used a retrospective chart audit in 6 practices.22
Limitations
A limitation of the study is that we were able to link only 95.6% of the laboratory tests to patients in the repository; it is possible that those receiving prescriptions for androgen therapy might have been disproportionately represented in the 4.4% of unlinked records. There is also no indication of the reasons for the PSA tests that were ordered. It is likely that some of these were done as part of prostate cancer screening rather than for monitoring during androgen therapy. We might thus be overestimating compliance with these recommendations. It is also possible that Canadian family physicians were not aware of the recommendations for baseline testosterone and PSA testing.
While there are no specific guidelines in Canada for use of testosterone supplementation, a recent study of family physicians in British Columbia found that 76.3% of those surveyed recognized the need for biochemical testing to establish a diagnosis of androgen insufficiency before initiation of treatment.24 The time that passed between our study and this later one might account for the increased exposure to the concept of andropause and its treatments.
Of note is that 28.1% of the prescriptions filled during this period were for women, a non-approved use of androgens. Although women were not the focus of this study, the high percentage of female testosterone users is of interest and warrants further study. Because the relationship between androgen therapy and prostate cancer has not been satisfactorily proven, important future research would involve prospective follow-up of patients who receive this therapy to monitor the incidence of prostate cancer. Qualitative research might shed some light on the reasons for lack of compliance with recommended monitoring. Our results indicate a need for similar studies in other areas to determine the generalizability of our findings.
Conclusion
Recent lessons from the Women’s Health Initiative on the harmful effects of hormone replacement therapy for women emphasize the need for caution in prescribing hormone therapy in the absence of proven biochemical deficiency. The lack of baseline testosterone testing indicates that prescribing androgen therapy is being driven by symptomatic management of a controversial syndrome that might be heavily influenced by direct-to-physician advertising.25 We do not know whether use of androgens in this population will lead to an increase in the incidence of prostate cancer, but the lack of ongoing monitoring is of concern, given the current understanding that prostate cancer is androgen dependent. The fact that less than 1% of this population had the recommended ongoing clinical monitoring might reflect physicians’ ignorance of the recommendations or an overwhelming rejection of the need for repeated PSA testing.
In the province of Manitoba, both PSA and testosterone testing are covered under the provincial health plan, so the cost of testing is not a barrier to compliance with recommendations for monitoring. For policy makers, the level of disregard of recommendations should be alarming, as the consequences could have a substantial effect on the health of the population.
Acknowledgment
This research was funded by the Health Sciences Centre Foundation. Data were accessed from the Population Health Research Data Repository at the Manitoba Centre for Health Policy in Winnipeg. Results and conclusions are those of the authors and no official endorsement by Manitoba Health is intended or should be inferred. The authors thank the data analysts at the Manitoba Centre for Health Policy (Randy Walld, Shelley Derksen and Dan Chateau) for assistance with the analysis.
EDITOR’S KEY POINTS
Particularly since the advent of topical testosterone preparations in 2000, millions of prescriptions for testosterone products have been written for the treatment of andropause, the existence of which is questioned. Testosterone levels in men naturally decline by 1% to 2% each year from the peak achieved in early adulthood.
As testosterone treatment can increase the risk of prostate cancer, it is recommended that a diagnosis of hypogonadism be established by testosterone levels and that an ongoing monitoring program including prostate-specific antigen measurement and digital rectal examination be instituted in those prescribed a testosterone product.
The authors of this retrospective study found that most men started on testosterone did not have a testosterone level drawn before treatment or ongoing monitoring for the risk of prostate cancer.
POINTS DE REPÈRE DU RÉDACTEUR
Particulièrement depuis l’apparition en 2000 des préparations topiques de testostérone, on a rédigé des millions de prescriptions pour des préparations de testostérone pour traiter l’andropause, une entité dont l’existence même est mise en doute. Chez l’homme, les niveaux de testostérone diminuent de 1 à 2% par année à partir du pic du début de l’âge adulte.
Comme l’administration de testostérone peut accroître le risque de cancer de la prostate, il est recommandé d’établir un diagnostic d’hypogonadisme en mesurant le niveau de testostérone et d’établir un programme de surveillance des patients traités, incluant la mesure de l’antigène prostatique spécifique et le toucher rectal.
Les résultats de cette étude rétrospective montrent que la plupart des sujets chez qui un traitement à la testostérone avait été instauré n’avaient eu aucun dosage de la testostérone avant le traitement, ou aucun monitorage du risque de cancer de la prostate durant le traitement.
Footnotes
This article has been peer reviewed.
Contributors
Dr Alan Katz, Dr Anne Katz, and Mr Burchill conceived and designed the study, gathered and analyzed the data, and prepared the article for submission.
Competing interests
None declared
References
- 1.IMS Health Inc. IMS sales data 2001. Westport, CT: IMS Health Inc; 2001. [Google Scholar]
- 2.Juul A, Skakkeback N. Androgens and the aging male. Hum Reprod Update. 2002;8(5):423–33. doi: 10.1093/humupd/8.5.423. [DOI] [PubMed] [Google Scholar]
- 3.Moncada I. Testosterone and men’s quality of life. Aging Male. 2006;9(4):189–93. doi: 10.1080/13685530601003180. [DOI] [PubMed] [Google Scholar]
- 4.Tenover J. Tetosterone replacement therapy in older men. Int J Androl. 1999;22:300–6. doi: 10.1046/j.1365-2605.1999.00184.x. [DOI] [PubMed] [Google Scholar]
- 5.Nolten W. Androgen deficiency in the aging male: when to evaluate and when to treat. Curr Urol Rep. 2000;1:313–9. doi: 10.1007/s11934-000-0013-5. [DOI] [PubMed] [Google Scholar]
- 6.Morales A, Bain J, Ruijs A, Chapdelaine A, Tremblay R. Clinical practice guidelines for screening and monitoring male patients receiving testosterone supplementation. Int J Impot Res. 1996;8:95–7. [PubMed] [Google Scholar]
- 7.Guay AT, Perez J, Fitaihi W, Vereb M. Testosterone treatment in hypogonadal men: prostate specific antigen level and risk of prostate cancer. Endocr Pract. 2000;6(2):132–8. doi: 10.4158/EP.6.2.132. [DOI] [PubMed] [Google Scholar]
- 8.Partin A, Hanks G, Klein E, Moul J, Nelson W, Scher H. Prostate-specific antigen as a marker of disease activity in prostate cancer. Oncology. 2002;16(8):1024–47. [PubMed] [Google Scholar]
- 9.Kozyrskyj A, Mustard C. Validation of an electronic population-based prescription database. Ann Pharmacother. 1998;32:1152–7. doi: 10.1345/aph.18117. [DOI] [PubMed] [Google Scholar]
- 10.Roos L, Walld R, Wajda A, Bond R, Hartford K. Record linkage strategies, outpatient procedures and administrative data. Med Care. 1996;34(6):570–82. doi: 10.1097/00005650-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Frolich N, Fransoo R, Roos N. Health service use in the Winnipeg Regional Health Authority: variations across areas in relation to health and socioeconomic status. Healthc Manage Forum. 2002 Winter;(Suppl):9–14. doi: 10.1016/s0840-4704(10)60176-7. [DOI] [PubMed] [Google Scholar]
- 12.Morley J, Perry H., III Androgen treatment of male hypogonadism in older males. J Steroid Biochem Mol Biol. 2003;85:367–73. doi: 10.1016/s0960-0760(03)00203-6. [DOI] [PubMed] [Google Scholar]
- 13.Rolf C, Nieschlag E. Potential adverse effects of long-term testosterone therapy. Bailliere’s Clin Endocrinol Metab. 1998;12(3):521–34. doi: 10.1016/s0950-351x(98)80305-4. [DOI] [PubMed] [Google Scholar]
- 14.Morales A. Androgen replacement therapy and prostate safety. Eur Urol. 2002;41:113–20. doi: 10.1016/s0302-2838(01)00039-2. [DOI] [PubMed] [Google Scholar]
- 15.Slater S, Oliver R. Testosterone: its role in development of prostate cancer and potential risk from use as a hormone replacement therapy. Drugs Aging. 2000;17(6):431–9. doi: 10.2165/00002512-200017060-00001. [DOI] [PubMed] [Google Scholar]
- 16.Hajjar R, Kaiser F, Morely J. Outcomes of long-term testosterone replacement in older hypogonadal males: a retrosepctive analysis. J Clin Endocrinol Metab. 1997;82(11):3793–6. doi: 10.1210/jcem.82.11.4387. [DOI] [PubMed] [Google Scholar]
- 17.Snyder P, Peachey H, Berlin J, Hannoush P, Hadda G, Dlewati A, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670–7. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 18.Gerstenbluth R, Maniam P, Corty E, Seftel A. Prostate specific antigen changes in hypogonadal men treated with testosterone replacement. J Androl. 2002;23(6):922–6. [PubMed] [Google Scholar]
- 19.Sih B, Morley J, Kaiser F, Perry H, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661–7. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- 20.Svetec DA, Canby ED, Thompson IM, Sabanegh ES., Jr The effect of parenteral testosterone replacement on prostate specific antigen in hypogonadal men with erectile dysfunction. J Urol. 1997;158(5):1775–7. doi: 10.1016/s0022-5347(01)64126-0. [DOI] [PubMed] [Google Scholar]
- 21.Kenny AM, Prestwood KM, Raisz LG. Short-term effects of intramuscular and transdermal testosterone on bone turnover, prostate symptoms, cholesterol, and hematocrit in men over age 70 with low testosterone levels. Endocr Res. 2000;26(2):153–68. doi: 10.3109/07435800009066159. [DOI] [PubMed] [Google Scholar]
- 22.Gaylis F, Lin D, Ignatoff J, Amling C, Tutrone R, Cosgrove D. Prostate cancer in men using testosterone supplementation. J Urol. 2005;174(2):534–8. doi: 10.1097/01.ju.0000165166.36280.60. [DOI] [PubMed] [Google Scholar]
- 23.Roos L, Mustard C, Nicol J, McLerran D, Majenka D, Young T. Registration and administrative data: organization and accuracy. Med Care. 1993;31(3):201–12. doi: 10.1097/00005650-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Pommerville PJ. Andropause: knowledge and awareness among primary care physicians in Victoria, BC, Canada. Aging Male. 2006;9(4):215–20. doi: 10.1080/13685530601040661. [DOI] [PubMed] [Google Scholar]
- 25.Gutknecht D. Evidence-based advertising? A survey of four major journals. J Am Board Fam Pract. 2001;14(3):197–200. [PubMed] [Google Scholar]