Abstract
Myogenic tone (MT), a fundamental stretch-sensitive vasoconstrictor property of resistance arteries and veins, is a key determinant of local blood flow regulation. We evaluated the pathways involved in MT development. The role of the RhoA/Rho kinase, p38 MAP kinase and HSP27 in MT was investigated in the rabbit facial vein, previously shown to possess MT at a pressure level equivalent to 20mmHg. Indeed, venous MT is poorly understood, although venous diseases affect a large proportion of the population. Stretched facial veins are characterized by a temperature-sensitive MT, which is normal at 39°C but fails to develop at 33°C. This allows for the discrimination of the pathways involved in MT from the multiple pathways activated by stretch. Isolated vein segments were mounted in organ baths and stretched. Temperature was then set at 33°C or 39°C. MT was associated to the translocation of RhoA to the plasma membrane and the Rho kinase inhibitor Y27632 decreased stretch-induced MT by 93.1 ±4.9%. MT was also associated to an increase in p38 (131,0±12,5% at 39°C versus 100% at 33°C) and HSP27 phosphorylation (196.1±13,3% versus 100%) and the p38 MAP kinase inhibitor SB203580 decreased MT by 36.5±8.1%. (39°C, compared to RFV stretched at 33°C). Finally, phosphorylation of p38 was blocked by Y27632 and Hsp27 phosphorylation was inhibited by SB203580 and Y27632.
Thus, myogenic tone and the associated p38 and Hsp27 phosphorylation depend on RhoA/Rho kinase activation in the rabbit facial vein.
Keywords: Amides, pharmacology, Animals, Biological Transport, Cell Membrane, metabolism, Enzyme Inhibitors, pharmacology, Face, blood supply, Heat-Shock Proteins, metabolism, Imidazoles, pharmacology, Intracellular Signaling Peptides and Proteins, Male, Muscle, Smooth, Vascular, physiology, Phosphorylation, drug effects, Physical Stimulation, Protein-Serine-Threonine Kinases, antagonists & inhibitors, physiology, Pyridines, pharmacology, Rabbits, Temperature, Vasoconstriction, physiology, Veins, physiology, p38 Mitogen-Activated Protein Kinases, metabolism, rho-Associated Kinases, rhoA GTP-Binding Protein, metabolism, physiology
Keywords: RhoA, Myogenic tone, resistance arteries, MAP kinase, p38, ERK1/2, pressure, stretch, vascular, Y27632, HSP27, veins, facial vein
Introduction
The process of matching blood flow to metabolic demand through changes in perfusion pressure is determined to a large extent by myogenic tone (MT). Myogenic properties of vessels include two processes: basal MT and myogenic response. Basal MT is a constant vasocontraction due to the transmural pressure or stretch applied to the arterial wall. Myogenic response is characterized by a smooth muscle cells contraction in response to increase in pressure or stretch (1). The myogenic response participates in the local regulation of blood flow and protects downstream capillary beds from large increases in hydrostatic pressure, such as that induced by postural changes, and a rise in the amplitude of MT is associated with hypertension (2) and diabetes mellitus (3). Signaling mechanisms that contribute to MT require both calcium entry, protein kinase C and phospholipase C activation (4) as well as calcium-sensitization of the contractile apparatus (5–7). There is also growing evidence that actin polymerization and the dynamic remodeling of the actin cytoskeleton play an important role in MT (8). In addition, Ca2+ and myosin light chain (MLC) phosphorylation are key regulators of the dynamic reorganization of actin filaments. In recent years, evidence has accumulated that the ras-related small GTP binding protein Rho is another important signaling element that mediates various actin-dependent cytoskeletal functions, including smooth muscle contraction.
The role of mitogen-activated protein (MAP) kinases, which may also affect smooth muscle contractility (9), in pressure myogenic contraction have not been fully explored (6). Using the rabbit facial vein (RFV), we have previously shown that ERK1/2 activation, although stimulated by stretch, is not linked to MT (10). In another study, in resistance arteries, we found that activation of p38 MAP kinase contributes to vascular smooth muscle contraction induced by thromboxane A2 (11). Furthermore, it has been shown that endothelin-1 activates p38 MAP kinase pathways and heat shock protein (HSP) 27, and that p38 could regulate phosphorylation of HSP27 (12). A specific role has been found for HSP27 in the regulation of actin cytoskeletal dynamics, based on the ability of HSP27 to modulate phosphorylation dependent actin polymerization (13). Thus we hypothesized that RhoA/Rho kinase and p38-Hsp27 might play a role in myogenic contraction.
Nevertheless, a main difficulty in studying MT, in addition to its location to small blood vessels, is that stretch per se activates multiple pathways not necessarily involved in MT. Indeed, stretch and MT cannot easily be dissociated; classically, arteries submitted to pressure (thus developing MT due to stretch) are compared to unstretched arteries (absence of pressure). To bypass this difficulty, we used the RFV that develops MT to a degree similar to that observed in resistance arteries and which is highly temperature sensitive (5). At equal stretch, MT that is observed in the RFV at 39°C is absent at 33°C (5). Using the RFV is also opening an important perspective in the understanding of vein pathophysiology. Indeed, the control of venous tone is poorly understood and sparsely studied. Venous tone changes with aging (14), hypertension (15) or diabetes (16). MT occurs in the RFV with a stretch level (5mN in a 3–4 mm long segment) corresponding to a blood pressure approximately equal to 20 mmHg, which is within the normal range in veins. Thus, MT may play a role in venous tone regulation even though the system is operating at low pressures.
Therefore, we used the RFV model allowing the comparison of stretched vessels with (39°C) or without (33°C) MT, to test the hypothesis that p38, HSP27 or RhoA activation could be involved in or affected by the development of MT in RFV.
Materiel and Methods
Rabbit facial vein
Buccal segments of the facial vein were isolated as previously described (5) from male New Zealand white rabbits (2.5–2.7 kg). The procedure used was in accordance with the European Community standards on the care and use of laboratory animals (authorization # 00577). Three mm-long ring segments of RFV were mounted between parallel stainless steel wires in 5ml organ baths. One wire was attached to a fixed support, while the other was connected to a moveable holder supporting a tension transducer, so that isometric force could be recorded and data was collected (Biopac MP 100, La Jolla, CA, USA). Vein segments were maintained at 33°C in a physiological salt solution (PSS) equilibrated at pH 7.4, pO2 160 mmHg, pCO2 37 mmHg (5).
Each segment was submitted to one of the following protocols:
RFV segments were stretched to 5.0 mN and allow to stabilize for 30 min, at 33°C.
RFV segments, maintained at 33°C or 39°C were exposed or not to one of the following agents (10 nmol/L to 10 μmol/L): the Rho kinase inhibitor Y27632 or the p38 MAP kinase inhibitor SB203580.
RFV were just bathed in PSS, without stretch, at 33°C or 39°C.
Four or five segments were obtained from each rabbit, so that vein segments from the same rabbit could be submitted to different experimental conditions. In each segment force was measured in one of the condition described above and immediately frozen for biochemical analysis, so that wall force and activation of p38, HSP27 or RhoA could be correlated.
P38 MAP kinase and HSP27 activation
Phosphorylation of p38 (phospho-p38) and HSP27 (phospho-HSP27) was quantified in RFV segments using Western-blot analysis. Tissue extraction was done as previously described (10): frozen vessel segments were pulverized in liquid nitrogen. The powders were resuspended in lysis buffer [500 mmol/L Tris-HCl pH 7.4, 20% sodium dodecyl sulfate, 100mmol/L sodium orthovanadate, and protease inhibitors (Boehringer Mannheim)]. For Western-blot analysis, lysates containing 15 μg of protein were electrophoresed on polyacrylamide gels and transferred to nitrocellulose membranes (Amersham ECL). Membranes were incubated overnight at 4°C with primary antibody against phospho-p38 or phospho HSP27 (New England Biolabs) and incubated with HRP-conjugated secondary antibodies (Amersham). Phospho-p38 and phospho-HSP27 bands were visualized using the ECL-Plus Chemiluminescence kit (Amersham) and expressed as a percentage of the activity measured in parallel-processed control vessels. Staining membranes with Ponceau red was used to normalize for loading variations.
Rho A activation
Activation of Rho A is characterized by its translocation to plasma membrane. Frozen vascular segments were pulverized in liquid nitrogen. The powders were resuspended in ice-cold homogenization buffer of the following composition [300 mmol/l sucrose, 1 mmol/L NaN3, 20 mmol/L HEPES pH 7,4 and protease inhibitors (Boehringer Mannheim)] and centrifuged at 31 000 × g for 30 minutes (ultracentrifuge; Beckman). The supernatant was collected as the cytosolic fraction. Pellets were resuspended, and the membrane proteins were extracted by incubation in homogenization buffer [0,1 mol/L NaCl, 30 mmol/L imidazole, 8 % sucrose, 1 mmol/L NaN3, pH 6.8 and protease inhibitors (Boehringer Mannheim)]. The extract was centrifuged at 10 000 × g at 4°C. The supernatant was collected as the membrane fraction. Immunoreactive bands for Rho A in cytosolic and membrane fractions were processed as above for western blots, with anti-Rho A (Santa Cruz) as primary antibody.
Drugs
SB203580 and Y27632 were purchased from Calbiochem (La Jolla, USA); all other reagents were purchased from Sigma (St Louis, USA). SB203580 and Y27632 were dissolved in DMSO (10 mmol/L solution) and then in PSS. In control experiments an equivalent concentration of DMSO was added to the organ baths.
Statistical Analysis
Results are expressed as mean ± SEM. The significance of the different treatments was determined by 2-factor ANOVA for repeated measures (comparison of concentration-response curves) or two-tailed Student’s t-test. P values <0.05 were considered to be significant.
Results
In RFV segments MT developed at 39°C, whereas it was absent at 33°C (figure 1). As previously shown (5), after reducing temperature to 33°C, the addition of papaverine (10 μmol/L) does not further relax the vein (not shown).
Figure 1.
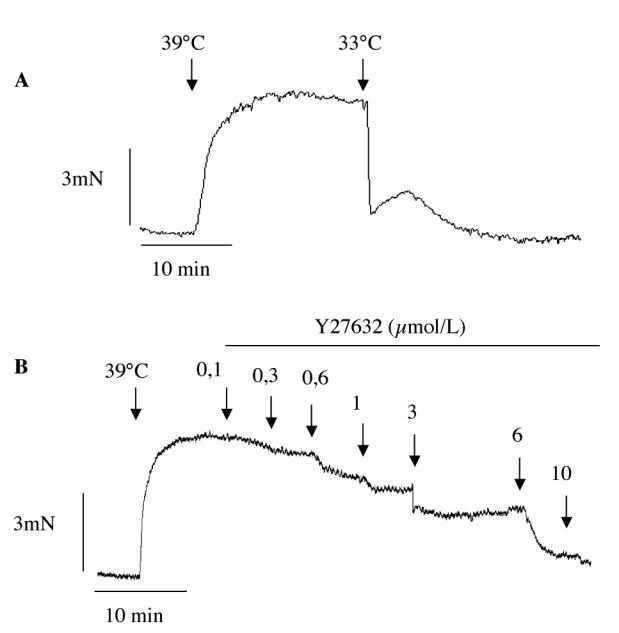
Typical recordings showing wall tension in a rabbit facial vein segment mounted in a myograph. Top panel shows that myogenic tone fully developed at 39°C and is absent when bath temperature is 33°C. Bottom panel: inhibitory effect of cumulative concentration (0,1 to 10 μmol/L) of the Rho kinase inhibitor Y27632.
In RFV at 39°C exposed to stretch, Y27632 induced a concentration-dependent reduction of MT (figure 1). SB203580 attenuated MT in a dose-dependent way but to lesser extent than Y27632 (figure 2): Y27632 (10 μmol/L) reduced MT to 6.9±5.0 % of control (stretched RFV at 39°C) and SB230580 (10 μmol/L) to 63.5±8.1 % of control.
Figure 2.
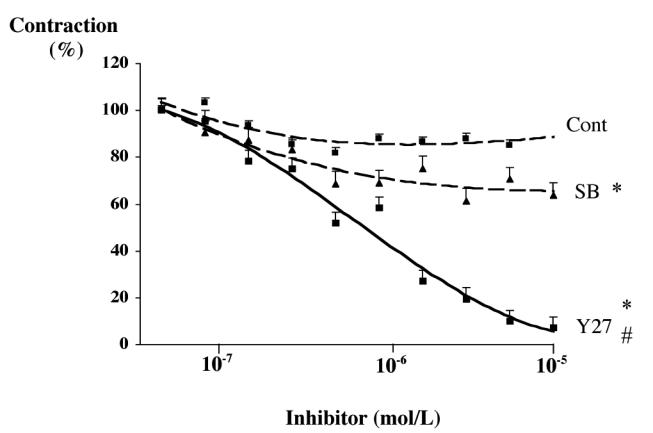
Effect of the Rho kinase inhibitor Y27632 (Y27) and of the p38 MAP kinase inhibitor SB203580 (SB) on myogenic tone in rabbit facial vein segments mounted in a myograph. Data is expressed as % of control. Myogenic tone in the presence of the Y27632 or SB203580 was compared to a time-control experiment (Cont). Mean ± sem is presented (n= 6 per group).
*P<0.05, 2-factor ANOVA, versus control.
#P<0.05, 2-factor ANOVA, Y27632 versus SB203580.
The involvement of RhoA/Rho kinase in stretch-induced MT was confirmed by the blot (figure 3B) showing that in absence of MT (33°C) RhoA was more concentrated in the cytosolic fraction whereas in the presence of MT (39°C) RhoA was mainly located to the plasma membrane fraction. In unstretched segments no translocation was observed at 33 or 39°C (figure 3B).
Figure 3.
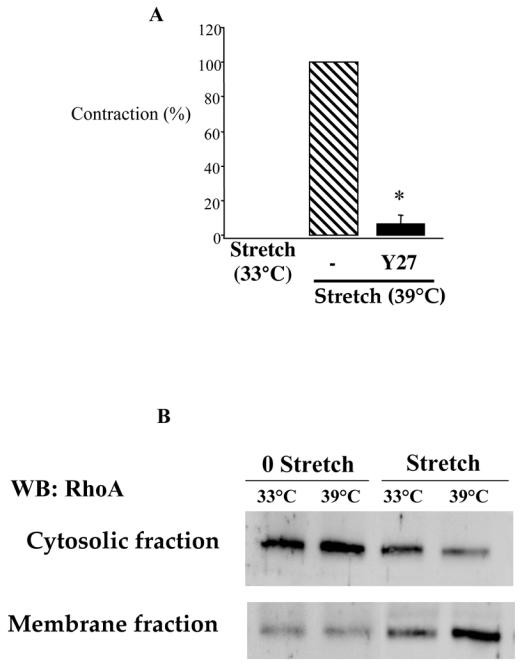
Inhibition of myogenic tone by Y27632 (l0 μmpl/L), in rabbit facial vein segments mounted in a myograph (A). Data is presented as percentage (%) of myogenic tone. 100% is corresponding to myogenic tone in the absence of inhibitor and control is corresponding to the vein segments stretched at 33°C, thus not developing myogenic tone. Mean ± sem is presented (n= 7 per group). *P<0.05, Student’s t-test, versus control.
The low panel (B) represents a Western-blot analysis of Rho in control conditions (vein segments stretched at 33°C) or in the presence of myogenic tone (stretched segments at 39°C). Cytosolic (C) and membrane (M) fractions were separated before migration so that the translocation of Rho to the membrane could be visualized as a change in the ratio of the cytosolic to the membrane fraction. Temperature per se had no effect on RhA translocation (no stretch at 33 versus 39°C). Each blot is representative of 5 different experiments.
The phosphorylation of p38-MAP kinase was determined in RFV maintained at 33°C or 39°C, with or without Y27632. The effect of temperature was also evaluated and basal p38 phosphorylation was considered to be 100% in unstretched RFV at 33°C. p38 phosphorylation was not affected by temperature in unstretched veins (figure 4A). At 33°C, stretch induced a significant increase of p38 phosphorylation (141.0±11.0%) compared with unstretched veins. This activation of p38 was not significantly prevented by Y27632 (figure 4A).
Figure 4.
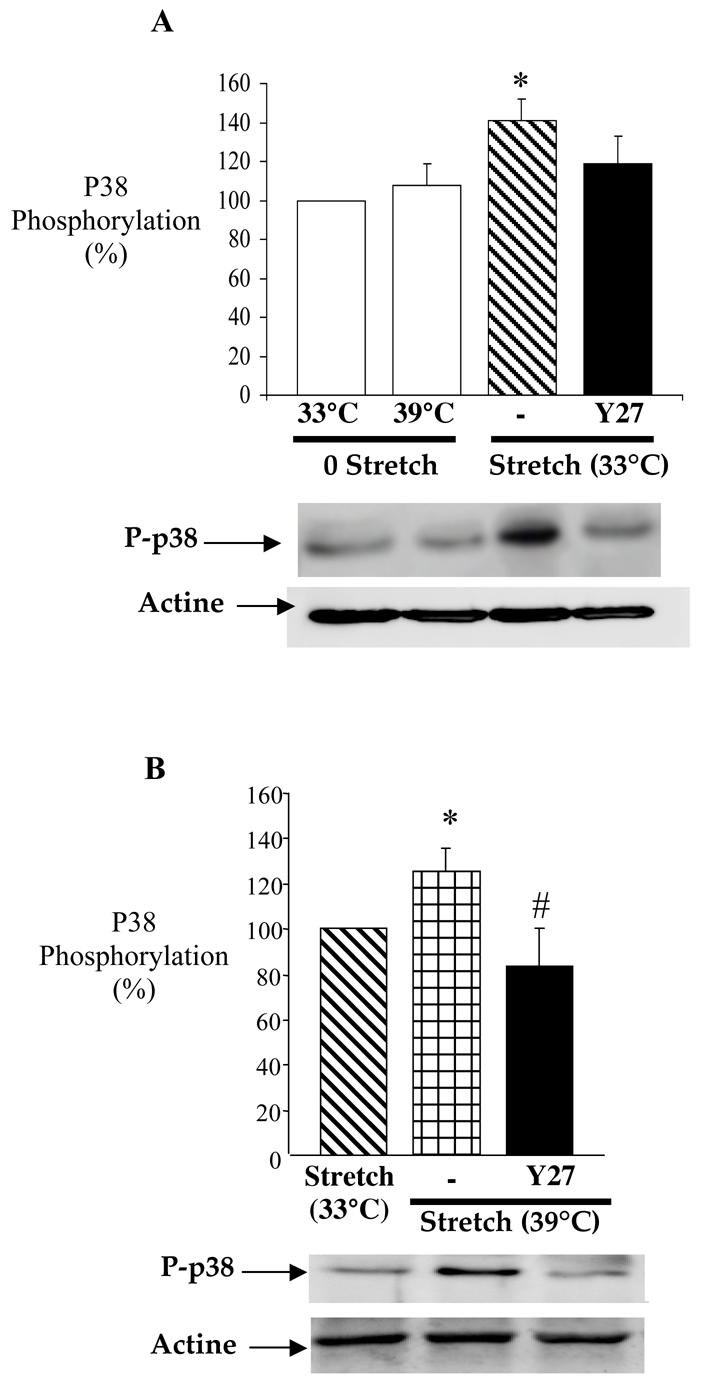
Panel A: Control for the effect of temperature on the relation between stretch and p38 phosphorylation. Phosphorylation of p38 MAP kinase was measured in rabbit facial vein segments unstretched at 33°C and 39°C and stretched at 33°C (stretch) with or without Y27632 (l0 μmol/L). Data is presented as percentage (%) of control (33°C without stretch). Mean ± sem is presented (n= 6 per group). *P<0.05, Student’s t-test, versus control.
Panel B: In another series of experiments, phosphorylation of p38 MAP kinase was measured in vein segments stretched at 39°C in the presence of Y27632 (l0 μmol/L). Data is presented as percentage (%) of control vein segments not developing myogenic tone (stretch 33°C). Mean ± sem is presented (n= 6 rabbit per group; the different experimental conditions shown in each panel were performed in vein segments obtained from the same animal). *P<0.05, Student’s t-test, Stretch at 39°C versus control (stretch 33°C); # P<0.05, Student’s t-test, vessels stretched at 39°C with Y27632 versus stretch at 39°C.
Then we measured p38 activation in the presence of stretch at 33°C (without MT) and at 39°C (with MT). Basal p38 phosphorylation was considered to be 100% in vein segments stretched at 33°C. Stretch at 39°C was also associated to an increased in p38 phosphorylation (131.0±12.5%) and Y27632 completely blocked p38 phosphorylation associated with MT (figure 4B).
In another series of experiments, HSP27 phosphorylation was determined in RFV segments submitted or not to stretch at 33°C or 39°C and in the presence or absence of Y27632 (10 μmol/L) or SB203480 (10 μmol/L). In unstretched RFV segments the temperature had no significant effect on HSP27 phosphorylation (fig. 5A). In stretched RFV segments maintained at 33°C, HSP27 phosphorylation was not different from that measured in unstretched segments at 33°C, and neither Y27632 nor SB239580 had an effect on HSP27 phosphorylation. Compared with stretched vein segments at 33°C, HSP27 phosphorylation was higher in stretched vein segments at 39°C and thus developing MT (196.1±13.3% of control, figure 5B). Both SB203580 and Y27632 significantly inhibited HSP27 phosphorylation associated with MT.
Figure 5.
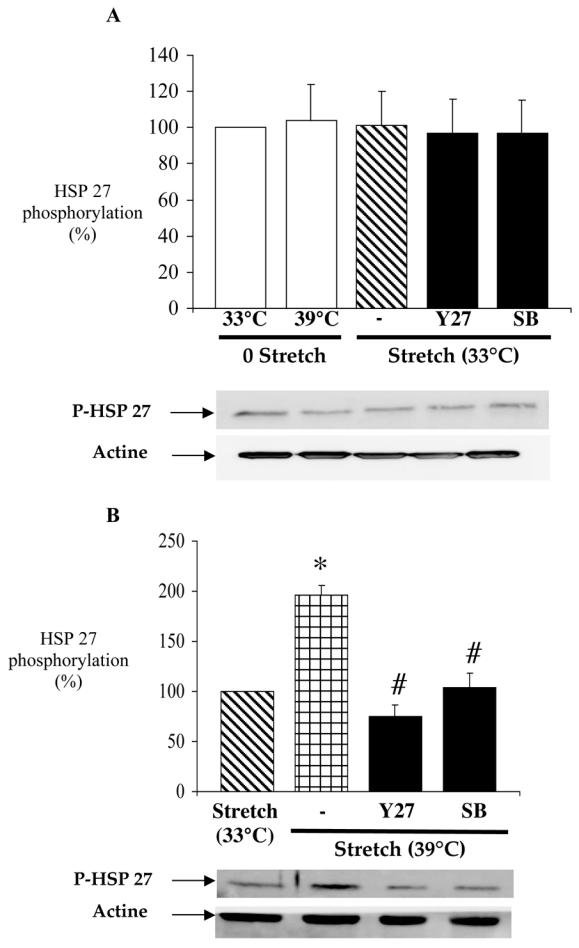
Panel A: Control for the effect of temperature on the relation between stretch and HSP27 phosphorylation. Phosphorylation of HSP27 was measured in rabbit facial vein segments unstretched at 33°C and 39°C and stretched at 33°C (stretch) with or without Y27632 (l0 μmol/L) or SB203580 (l0 μmol/L). Data is presented as percentage (%) of control (33°C without stretch). Mean ± sem is presented (n= 6 rabbit per group; the different experimental conditions shown in each panel were performed in vein segments obtained from the same animal). No difference between groups (Student’s t-test).
Panel B: In another series of experiments, phosphorylation of HSP27 was measured in vein segments stretched at 39°C in the presence of SB203580 (l0 μmpl/L) or Y27632 (l0 μmpl/L). Data is presented as percentage (%) of control vein segments not developing myogenic (stretched at 33°C). Mean ± sem is presented (n= 6 rabbit per group; the different experimental conditions shown in each panel were performed in vein segments obtained from the same animal). *P<0.05, Student’s t-test, Stretch 39°C versus control (stretch 33°C); # P<0.05, Student’s t-test, vessels stretched at 39°C with Y27632 or SB203580 versus vessels stretched at 39°C.
Ponceau red staining, measured in each group was not significantly affected in the different experimental condition, suggesting that the blots were loading with similar amounts of proteins. In addition, the amount of actin was found to be similar in each group.
Discussion
This study shows that MT was associated to RhoA translocation from the cytosol to the plasma membrane and to Rho kinase activation. These results are consistent with a previous work showing that Y27632 caused a dose-dependent inhibition of basal tone in mesenteric resistance arteries (17). In rat tail arteries Y27632 only partly attenuated MT (1). This discrepancy may reflect a difference in the degree of participation of Rho kinase in MT in different vessels (1). It has been shown that a Ca2+ sensitization may contribute to MT (18), and recent evidence for the involvement of the small GTPase RhoA in Ca2+ sensitization in smooth muscle contraction has been reported by several laboratories (18,19). Rho kinase, the RhoA effector, can directly modulate smooth muscle contraction through MLC phosphorylation, independently of the Ca2+-calmodulin-dependent myosin light chain kinase pathway (20). Rho kinase is able to regulate MLC phosphorylation by the direct phosphorylation of MLC and by the inactivation of myosin phosphatase through phosphorylation of the myosin binding subunit (20). Therefore, Rho kinase and myosin phosphatase coordinately regulate the phosphorylation state of MLC, and as a result induce smooth muscle contraction.
Our results also indicate that p38 MAP kinase inhibition attenuated in part MT (30% maximal inhibition). In addition, p38 phosphorylation was higher in RFV segments developing MT. Furthermore, our findings show that Y27632 inhibited contraction-induced p38 phosphorylation, suggesting that RhoA/Rho kinase activation was upstream of p38 and induced its activation. Our findings are consistent with a previous study suggesting that p38 MAP kinase activation could be involved in the mechanotransduction of wall tension in gracilis muscle arterioles (6). Studies in large vessels and isolated smooth muscle cells suggested that MAP kinases activation contributes to vascular smooth muscle contraction induced by endothelin-1 (ET1) (21) and angiotensin II (22). Some of the downstream events coupled with p38 MAP kinase activation include phosphorylation of MAPK-activated protein (MAPKAP) kinases 2 and 3, which in turn phosphorylate HSP27 (23). Phosphorylation of HSP27 is another MAP kinase-mediated mechanism possibly modulating the contractile response of vascular smooth muscle (12). We found that HSP27 was activated in the presence of MT and that RhoA and p38 were required for this activation. Nevertheless, that HSP27 is phosphorylated does not necessarily imply that it is directly involved in MT. Several reports suggest that HSP27 might modulate actin filament dynamics and be involved in contraction of smooth muscle cells. HSP27, in its non-phosphorylated state, behaves as a phosphorylation-regulated-F-actin capping protein capable of inhibiting actin polymerization (24). Phosphorylation of HSP27, via activation of p38, might markedly modify the equilibrium in favor of polymerized actin, thereby contributing to the maintenance of the microfilament network. Studies in cells transiently transfected with dominant negative RhoA suggest that i) RhoA might exert its effects on cytoskeleton reorganization through HSP 27 and ii) cytoskeletal proteins might interact to induce sustained smooth muscle contraction (25). It is still unclear whether RhoA interacts with HSP27, but our work suggests that RhoA via the activation of Rho kinase might stimulate p38 leading to HSP27 phosphorylation.
It should be noted that we used a vein which may not be representative of resistance arteries, most usually used in the study of MT. Nevertheless, the RFV shares several common features with arteries, including histological features and a strong capacity to contract (5). Nevertheless, each specific blood vessel (and this applies to arteries) has specific features and extrapolation must always be considered carefully. The RFV has this interesting feature allowing comparing stretched vessels with or without MT. This is a key issue of the present study as stretch per se activates many pathways not necessarily involved in MT, as we have previously shown with ERK1/2 (10). Of course, we cannot totally exclude that MT in the RFV does not rely on a highly temperature sensitive step in the process involved in MT. Nevertheless, only myogenic tone can be turned off and on between 33 and 37°C whereas the other forms of tone are unaffected (angiotensin II, 5HT, KCl…).
Our findings in the RFV open an important perspective in the study of venous tone. It is estimated that 75% of the total blood volume in the resting state is contained within venules and veins. Therefore, constriction or dilation of veins would have a greater effect on changing blood volume distribution than any other part of the vasculature (26). The factors controlling venoconstriction are complex and include both neural and humoral mechanisms. The increased sympathetic nerve activity results in augmented venoconstriction, which predominates in the development of spontaneous hypertension (15). The present study suggests that pharmacological modulation of venous myogenic tone may represent another way to change venous tone, besides sympathetic tone.
Finally, the experimental protocol used did not allow removing the endothelium from RFV segments. Thus we cannot distinguish the effect of stretch on the smooth muscle cells from that on endothelial cells. Nevertheless, the ratio of endothelial cells to smooth muscle cells is relatively small.
In conclusion, this study brings new insights in the understanding of venous myogenic contraction showing a complete involvement of RhoA/Rho kinase and a partial involvement of p38 MAP kinase; and might provide a novel mechanism insight on increased venoconstriction in cardiovascular diseases. Indeed, in conditions of MT development, pressure might activate RhoA which plays a major role in the sensitization of the contractile apparatus to calcium. In parallel, RhoA/Rho kinase would also activate p38 MAP kinase, which would then activate HSP27 phosphorylation, possibly inducing cytoskeletal rearrangement by facilitating thin-filament regulation of smooth muscle contraction.
Acknowledgments
Laurent Loufrani and Caroline Dubroca were fellows of the French Foundation for Medical research (Fondation pour la recherche médicale: FRM, Paris, France).
References
- 1.Schubert R, Kalentchuk VU, Krien U. Rho kinase inhibition partly weakens myogenic reactivity in rat small arteries by changing calcium sensitivity. Am J Physiol Heart Circ Physiol. 2002;283:H2288–95. doi: 10.1152/ajpheart.00549.2002. [DOI] [PubMed] [Google Scholar]
- 2.Hughes JM, Bund SJ. Arterial myogenic properties of the spontaneously hypertensive rat. Exp Physiol. 2002;87:527–34. doi: 10.1113/eph8702399. [DOI] [PubMed] [Google Scholar]
- 3.Ungvari Z, Pacher P, Kecskemeti V, Papp G, Szollar L, Koller A. Increased myogenic tone in skeletal muscle arterioles of diabetic rats. Possible role of increased activity of smooth muscle Ca2+ channels and protein kinase C. Cardiovasc Res. 1999;43:1018–28. doi: 10.1016/s0008-6363(99)00106-6. [DOI] [PubMed] [Google Scholar]
- 4.Osol G, Laher I, Kelley M. Myogenic tone is coupled to phospholipase C and G protein activation in small cerebral arteries. Am J Physiol. 1993;265:H415–20. doi: 10.1152/ajpheart.1993.265.1.H415. [DOI] [PubMed] [Google Scholar]
- 5.Henrion D, Laher I, Bevan JA. Intraluminal flow increases vascular tone and 45Ca2+ influx in the rabbit facial vein. Circ Res. 1992;71:339–45. doi: 10.1161/01.res.71.2.339. [DOI] [PubMed] [Google Scholar]
- 6.Massett MP, Ungvari Z, Csiszar A, Kaley G, Koller A. Different roles of PKC and MAP kinases in arteriolar constrictions to pressure and agonists. Am J Physiol Heart Circ Physiol. 2002;283:H2282–7. doi: 10.1152/ajpheart.00544.2002. [DOI] [PubMed] [Google Scholar]
- 7.Matchkov VV, Tarasova OS, Mulvany MJ, Nilsson H. Myogenic response of rat femoral small arteries in relation to wall structure and [Ca(2+)](i) Am J Physiol Heart Circ Physiol. 2002;283:H118–25. doi: 10.1152/ajpheart.00690.2001. [DOI] [PubMed] [Google Scholar]
- 8.Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002;16:72–6. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- 9.Matrougui K, Eskildsen-Helmond YE, Fiebeler A, Henrion D, Levy BI, Tedgui A, Mulvany MJ. Angiotensin II stimulates extracellular signal-regulated kinase activity in intact pressurized rat mesenteric resistance arteries. Hypertension. 2000;36:617–21. doi: 10.1161/01.hyp.36.4.617. [DOI] [PubMed] [Google Scholar]
- 10.Loufrani L, Lehoux S, Tedgui A, Levy BI, Henrion D. Stretch induces mitogen-activated protein kinase activation and myogenic tone through 2 distinct pathways. Arterioscler Thromb Vasc Biol. 1999;19:2878–83. doi: 10.1161/01.atv.19.12.2878. [DOI] [PubMed] [Google Scholar]
- 11.Bolla M, Matrougui K, Loufrani L, Maclouf J, Levy B, Levy-Toledano S, Habib A, Henrion D. p38 mitogen-activated protein kinase activation is required for thromboxane-induced contraction in perfused and pressurized rat mesenteric resistance arteries. J Vase Res. 2002;39:353–60. doi: 10.1159/000065547. [DOI] [PubMed] [Google Scholar]
- 12.Yamboliev IA, Hedges JC, Mutnick JL, Adam LP, Gerthoffer WT. Evidence for modulation of smooth muscle force by the p38 MAP kinase/HSP27 pathway. Am J Physiol Heart Circ Physiol. 2000;278:H1899–907. doi: 10.1152/ajpheart.2000.278.6.H1899. [DOI] [PubMed] [Google Scholar]
- 13.Landry J, Huot J. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochem Cell Biol. 1995;73:703–7. doi: 10.1139/o95-078. [DOI] [PubMed] [Google Scholar]
- 14.Monos E, Berczi V, Nadasy G. Local control of veins: biomechanical, metabolic, and humoral aspects. Physiol Rev. 1995;75:611–66. doi: 10.1152/physrev.1995.75.3.611. [DOI] [PubMed] [Google Scholar]
- 15.Moore A, Mangoni AA, Lyons D, Jackson SH. The cardiovascular system. Br J Clin Pharmacol. 2003;56:254–60. doi: 10.1046/j.0306-5251.2003.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin DS, Rodrigo MC, Appelt CW. Venous tone in the developmental stages of spontaneous hypertension. Hypertension. 1998;31:139–44. doi: 10.1161/01.hyp.31.1.139. [DOI] [PubMed] [Google Scholar]
- 17.Cheng X, Leung SW, Lim SL, Pang CC. Attenuated arterial and venous constriction in conscious rats with streptozotocin-induced diabetes. Eur J Pharmacol. 2003;458:299–304. doi: 10.1016/s0014-2999(02)02762-0. [DOI] [PubMed] [Google Scholar]
- 18.VanBavel E, van der Meulen ET, Spaan JA. Role of Rho-associated protein kinase in tone and calcium sensitivity of cannulated rat mesenteric small arteries. Exp Physiol. 2001;86:585–92. doi: 10.1113/eph8602217. [DOI] [PubMed] [Google Scholar]
- 19.Yeon DS, Kim JS, Ahn DS, Kwon SC, Kang BS, Morgan KG, Lee YH. Role of protein kinase C- or RhoA-induced Ca(2+) sensitization in stretch-induced myogenic tone. Cardiovasc Res. 2002;53:431–8. doi: 10.1016/s0008-6363(01)00496-5. [DOI] [PubMed] [Google Scholar]
- 20.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–4. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 21.Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- 22.Cain AE, Tanner DM, Khalil RA. Endothelin-1 --induced enhancement of coronary smooth muscle contraction via MAPK-dependent and MAPK-independent [Ca(2+)](i) sensitization pathways. Hypertension. 2002;39:543–9. doi: 10.1161/hy0202.103129. [DOI] [PubMed] [Google Scholar]
- 23.Meloche S, Landry J, Huot J, Houle F, Marceau F, Giasson E. p38 MAP kinase pathway regulates angiotensin II-induced contraction of rat vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2000;279:H741–51. doi: 10.1152/ajpheart.2000.279.2.H741. [DOI] [PubMed] [Google Scholar]
- 24.Larsen JK, Yamboliev IA, Weber LA, Gerthoffer WT. Phosphorylation of the 27-kDa heat shock protein via p38 MAP kinase and MAPKAP kinase in smooth muscle. Am J Physiol. 1997;273:L930–40. doi: 10.1152/ajplung.1997.273.5.L930. [DOI] [PubMed] [Google Scholar]
- 25.Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–61. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P, Bitar KN. Rho A regulates sustained smooth muscle contraction through cytoskeletal reorganization of HSP27. Am J Physiol. 1998;275:G1454–62. doi: 10.1152/ajpgi.1998.275.6.G1454. [DOI] [PubMed] [Google Scholar]


