Abstract
Innate immunity in the CNS depends primarily on the functions of glial cells, astrocytes and microglia, which are important for the early control of pathogen replication and direct the recruitment and activation of cells of the adaptive immune system required for pathogen clearance. Efficient immune responses are required for clearance of an invading pathogen, but dysregulation of a pro-inflammatory response in the CNS could lead to the development of autoimmunity. This review summarizes the activation of Toll-like receptors (TLRs) expressed on glial cells and the functional outcome of these interactions for CNS health and disease which depends on a delicate balance of the protective and toxic effects of molecules induced in the CNS following TLR ligation.
Introduction
Toll like receptors (TLRs) are a large family of evolutionarily conserved pattern recognition receptors in the vertebrate immune system. At least 13 TLR genes exist in mammals, and functional ligands have been identified for 10 [for review see (Uematsu and Akira, 2006)]. TLRs recognize conserved pathogen-associated molecular patterns (PAMPs) from diverse organisms, including bacteria, viruses, yeast, fungi and parasites. Many well known PAMPs signal through TLRs, including lipopolysaccharide (LPS; TLR4), double stranded RNA (dsRNA; TLR3), peptidoglycans (PGN; TLR2 with TLR1/6), and CpG DNA (TLR9) (Fig. 1A). All known TLRs with the exception of TLR3 signal through the adaptor protein MyD88 and lead to the activation of the transcription factor nuclear factor κB (NFκB). TLR3 and TLR4 signal through an MyD88-independent pathway dependent on the adaptor TRIF and activate both NFκB and Interferon response factor 3 (IRF3).
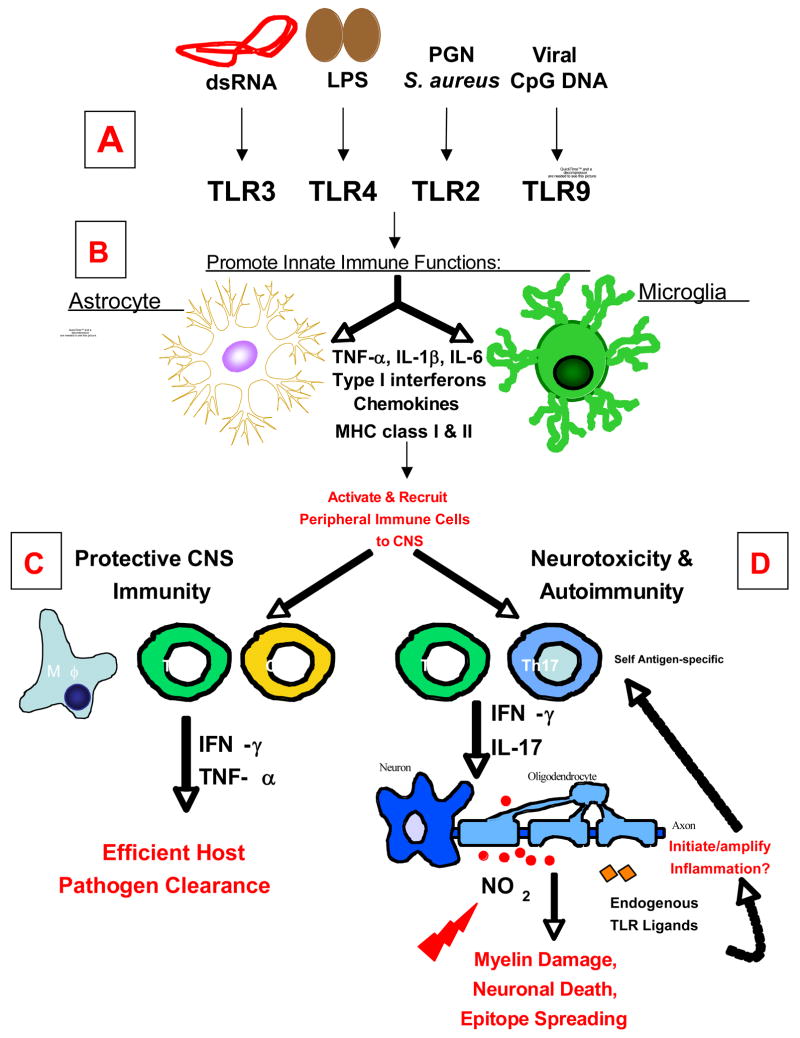
Figure 1. Schematic Representation of the Roles of TLR Signaling on Glial Cells in CNS Immunity and Disease.
Glial cells, astrocytes and microglia, express a wide variety of TLRs. (A) Stimulation with TLR ligands: dsRNA (TLR3), LPS (TLR4), peptidoglycans (PGN; TLR2 with TLR1/6), and viral CpG DNA (TLR9) promotes an array of immune functions in glial cells, including, the secretion of pro-inflammatory cytokines, chemokines, type I interferons (IFN-α/β), and an increase in MHC class I and II expression. (B) Secretion of these molecules affects the adaptive immune responses in the CNS by promoting a pro-inflammatory response thereby recruiting leukocytes (macrophages, dendritic cells, and CD4+ and CD8+ T cells) to the CNS. (C) Together these functions are essential for initiating protective immunity and efficient clearance of invading pathogens from the CNS. (D) However recent evidence suggests that depending on the type of pathogen and/or route of infection, chronic activation of TLRs on glial cells may be detrimental to the host, causing neurotoxicity and oligodendrocyte or neuronal cell death. In addition to the role of TLR signaling on glial cells in protective immunity, it may also be important for the development of CNS autoimmune diseases in the absence of pathogens. The CNS inflammatory response triggered by a virus or injury leads to further CNS tissue damage. The release of sequestered myelin epitopes further propagates inflammation and self-antigen specific immune responses (Th1 and Th17 responses), thus initiating autoimmunity. TLRs on glial cells can also be activated by endogenous ligands released during tissue damage that may initiate and/or further amplify inflammation in CNS autoimmune disease.
The limited immune surveillance of the central nervous system (CNS) makes it crucial that resident cells be able to rapidly recognize and respond to infection. Innate immunity in the CNS depends primarily on the functions of glial cells, astrocytes and microglia, which are important for the early control of pathogen replication and direct the recruitment and activation of cells of the adaptive immune system required for pathogen clearance (Bailey et al., 2006). In this review, we will focus on the activation of TLRs expressed on glial cells and the functional outcomes for the CNS. We will discuss the role of TLRs in innate immune responses of glia in vitro as well as their roles during CNS infection in vivo. The ability to mount an effective innate immune response in the CNS is likely critical to eliminate pathogens and often vital to host survival. However, it is also evident that chronic or dysregulated inflammation in the CNS can cause tissue damage and neurodegeneration and the role of TLRs in this process will be discussed. Finally, we will discuss how TLRs might be involved during the development of CNS autoimmunity.
Expression of TLRs in CNS Glial Cells
Microglia are CNS tissue resident macrophages and act as immune sentinels of the brain. In accordance with this view, primary microglia in vitro constitutively express a wide complement of TLRs (TLRs1-9) at varying levels (Bsibsi et al., 2002; Olson and Miller, 2004). In comparison, primary astrocytes also express a wide variety of TLRs, but at lower levels. Murine astrocytes express TLRs1-9, with particularly high levels of TLR3 (Carpentier et al., 2005; McKimmie and Fazakerley, 2005), suggesting that astrocytes may be particularly important for anti-viral responses in the CNS. To date, human astrocytes have been reported to express TLRs1-5 and TLR9, also with particularly high expression of TLR3 (Bsibsi et al., 2006; Bsibsi et al., 2002; Jack et al., 2005). The lack of TLR6-8 may be a difference between species or the result of varying isolation and culture conditions. There is also evidence that both oligodendrocytes and neurons can express TLRs, but their role in innate immune responses during CNS infection and autoimmunity are less well defined and are therefore not a focus of this review (Bsibsi et al., 2002; Lehnardt et al., 2006; Prehaud et al., 2005).
Under resting conditions in vivo, TLRs1-9 have been detected in the CNS by quantitative real time PCR, with particularly strong expression of TLR3 (McKimmie et al., 2005). Constitutive expression of TLRs is primarily in microglia and largely restricted to the circumventricular organs (CVOs) and meninges, areas with direct access to the circulation, although they may be expressed at lower levels in other regions as well (Chakravarty and Herkenham, 2005; Laflamme and Rivest, 2001; Laflamme et al., 2001). This unique localization allows the CNS to recognize pathogens which are present in the periphery as well as those which have directly invaded the CNS. The levels of TLRs in the CNS can be upregulated by viral and bacterial infection, treatment with TLR stimuli, or CNS autoimmunity (Bsibsi et al., 2002; Laflamme et al., 2001; McKimmie et al., 2005; Zekki et al., 2002), providing a mechanism for amplification of inflammatory responses to pathogens infecting the CNS. These stimuli upregulate multiple TLRs in a coordinated fashion, not only the TLR involved in recognition of a particular pathogen or class of pathogens. For example, treatment of astrocytes with the dsRNA synthetic mimic polyinosinic-polycytidylic acid (poly I:C), a viral stimulus, upregulates its own receptor TLR3, and also upregulates TLR2 and TLR4, which are normally used to recognize bacterial products (Carpentier et al., 2005). Similarly, infection of mice with rabies broadly increases CNS expression of TLRs1-4 and 6–9 (McKimmie et al., 2005). The pattern of TLR upregulation is not fixed and varies with the particular pathogen encountered, even with pathogens of a similar class. For example, in contrast to rabies virus, Semliki forest virus infection in the CNS fails to upregulate TLR4 and TLR6, but does increase expression of TLR13 (McKimmie et al., 2005). The upregulation of TLRs in the CNS is likely in part due to the infiltration of TLR-expressing inflammatory cells, and in part due to the upregulation of receptor expression on astrocytes and microglia, which occurs in response to a variety of inflammatory stimuli (Carpentier et al., 2005; Olson and Miller, 2004). The amplification of receptor expression may particularly important in astrocytes and in regions outside the CVOs, where constitutive expression of TLRs is quite low.
TLRs in the Induction of Innate Immune Functions of Glia in Vitro
It is well known that both astrocytes and microglia have the potential to contribute to innate immunity in the CNS following exposure to pathogens or PAMPs (Fig. 1B). The immune signals to which glia are able to respond and their functions during both innate and adaptive immune responses have been reviewed extensively elsewhere (Bailey et al., 2006), and we will only summarize them briefly here. Innate immune functions of astrocytes and microglia include anti-microbial properties via the production of type I IFNs, important for early control of infection through the inhibition of translation, degradation of dsRNA and upregulation of major histocompatibility complex (MHC) class I and class II antigens, and the induction of inducible nitric oxide synthase (iNOS), leading to high levels of NO and peroxynitrite production with microbicidal activity. Both astrocytes and microglia also produce a variety of chemokines in response to stimulation with TLRs or pathogens, including CCL2, CCL3, CCL4, CCL5 and CXCL10, which are important for recruiting leukocytes into the inflamed CNS. Glial cells are also sources of cytokines that may impact developing adaptive immune responses in the CNS, most often interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and IL-β, which have the potential to activate a pro-inflammatory response in the both the CNS and the periphery and have also been implicated in mediating blood-brain barrier (BBB) damage and promoting efficient leukocyte entry into the CNS. It is interesting to note here that microbial products or direct viral infection induces the production of cytokines which promote Th1 and Th17 CD4+ T cell differentiation and survival as well antigen-presenting cell functions of microglia but not astrocytes (Bailey et al., 2006; Carpentier et al., 2005; Olson and Miller, 2004). It is unclear whether this is due to differences in TLR signaling between the two cells or if there are additional pathways acting in microglia. For the purposes of this review, we will focus on the innate immune functions of astrocytes and microglia which have been directly attributed to TLR signaling.
Several TLRs have been shown to be important for anti-bacterial responses of microglia and astrocytes. For example, TLR4 is important for the induction of TNF-α in microglia exposed to LPS (Qin et al., 2005). Similarly, TLR2 is obligatory for microglial responses to group B Streptococcus (GBS) bacteria, at least as measured by nitric oxide production (Lehnardt et al., 2006). TLR2 is also crucial for the production of cytokines and chemokines by microglia and astrocytes following exposure to PGN (Esen et al., 2004; Kielian et al., 2005a). Interestingly, TLR2 also mediates astrocyte responses to intact Staphylococcus aureus, but it is dispensable for microglial activation by the same bacteria (Esen et al., 2004; Kielian et al., 2005a), indicating that microglia, but not astrocytes, have multiple mechanisms by which recognition of this bacteria occurs. It therefore appears that TLR signaling is important for mediating pro-inflammatory responses of glia to both bacterial products and whole bacteria, although microglia in particular may have some degree of overlapping, redundant signaling. Such redundancy is necessary for protective immunity, since many pathogens have evolved mechanisms to block innate immune signaling pathways as a mechanism of immune evasion. Non-TLR innate immune signaling pathways may also contribute to glial responses to bacteria. Alternate receptors which are also activated by bacterial products include scavenger receptors, mannose receptors, and nucleotide-binding oligomerization domain (NOD) proteins and NOD-like receptors. For more information regarding these pathways we refer readers to several recent reviews (Delbridge and O’Riordan, 2007; Taylor et al., 2005).
TLR3, which recognizes dsRNA, is likely to be important for anti-viral responses, since many viruses have dsRNA genomes or produce dsRNA during their replication cycle. TLR3 is critical for production of cytokines, chemokines, and type I IFNs in response to poly I:C treatment in astrocytes and microglia (Carpentier et al., 2007; Scumpia et al., 2005; Town et al., 2006). Interestingly, we have observed that TLR3 is dispensable for most pro-inflammatory responses of astrocytes after infection with Theiler’s murine encephalomyelitis virus (TMEV) (Carpentier et al., 2007). Instead, TMEV-infected astrocytes appear to rely more heavily on the intracellular double-stranded RNA activated protein kinase R (PKR) for the induction of IFNs, cytokines, and chemokines (Carpentier et al., 2007). Other intracellular dsRNA receptors, such as the DExD/H box-containing RNA helicases retinoic acid inducible gene 1 (RIG-1) and melanoma differentiation associated gene 5 (Mda5), may also activated by virus replicating directly in the cytoplasm (Yoneyama et al., 2004). Given that TLR3 is reported to be expressed extracellularly on astrocytes (Bsibsi et al., 2002), it is more likely to be important for responses to highly lytic infections, in which large amounts of dsRNA may be released into the extracellular milieu.
Function of TLRs during CNS Infection In Vivo
The use of mice genetically deficient for various TLRs has allowed the study of their in vivo role during CNS infection, although potential unique contributions of TLR signaling on glia in the CNS have not yet been thoroughly assessed. Recently, two groups have used a bone marrow (BM) transplant system to assess the role of TLR4 on peripheral and CNS-resident cells in an LPS-induced injury model (Chakravarty and Herkenham, 2005; Zhou et al., 2006). In these studies, wild type (WT) or TLR4-deficient mice were lethally irradiated, destroying their peripheral immune system but leaving CNS resident cells, including slowly dividing microglia and astrocytes, intact. The peripheral immune system is then reconstituted with bone marrow from wild type or TLR4−/− mice, allowing the generation of mice with TLR4 expression only in the CNS (TLR4−/− BM→ WT mice) or only in the periphery (WT BM→ TLR4−/− mice). Mice specifically lacking peripheral TLR4 had defective cytokine production in the serum after peripheral LPS injection, but cytokine production in the CNS was relatively intact. In contrast, mice specifically lacking CNS expression of TLR4 were able to mount normal pro-inflammatory responses in the periphery, but the production of pro-inflammatory cytokines in the CNS and the infiltration of leukocytes into the CNS were inhibited (Chakravarty and Herkenham, 2005; Zhou et al., 2006). These studies for the first time implicate that specific expression of TLRs in the CNS have distinct consequences for this tissue.
We are still awaiting the results of more studies using this bone marrow transplant approach or cell-type specific deletions to more fully define the role of CNS-specific TLRs, work that is ongoing in our lab and others. Even so, there are several groups which have used varying routes of infection to define roles for individual TLRs that are specific to the CNS. For example, TLR2−/− mice are highly susceptible to intravenous S. aureus infection, demonstrating increased mortality and increased bacterial titers in the blood (Takeuchi et al., 2000). In contrast, there is no difference between TLR2−/− and wild type mice in the survival, clinical symptoms or bacterial load associated with S. aureus-induced brain abscess, although they did display somewhat decreased inflammation in the brain, as measured by TNF-α, CXCL2 and iNOS production (Kielian et al., 2005b). Consistent with these results, it has been observed that TLR2 is important for the induction of cytokines from macrophages, but not microglia, exposed to intact S. aureus (Kielian et al., 2005a; Takeuchi et al., 2000). The additional mechanisms by which microglia recognize S. aureus have not yet been determined. The observation that MyD88−/− mice develop significantly larger S. aureus-induced brain abscesses than wild type mice (Kielian et al., 2007) indicates that these additional pathways could also be TLR-mediated. However, we urge caution in the interpretation of these and other studies using MyD88−/− mice, as these mice also have defects in IL-1 signaling which could contribute to the observed phenotypes.
TLR2−/− mice are more susceptible to fatal meningitis induced by Streptococcus pneumoniae (Echchannaoui et al., 2002; Koedel et al., 2003), developing more severe clinical symptoms, increased S. pneumoniae load in the CNS, massive although delayed leukocyte infiltration, and increased BBB permeability. One group has reported no differences in bacterial titer or cytokine production in the periphery of TLR2−/− and wild type mice (Echchannaoui et al., 2002), while another has noted an increase in blood, but not splenic bacterial load in TLR2−/− mice (Koedel et al., 2003). The differences between these two studies could potentially be explained by the route of infection: the former study used an intracerebral route of infection with lower levels of bacteria, while the latter infected intracisternally with higher numbers of bacteria, which led to higher peripheral bacterial load and faster onset of clinical symptoms. In both cases, however, the increase in mortality appears to be primarily due to the increased bacterial load specifically in the CNS.
However, TLR activation in response to a pathogen is not always protective in the CNS. TLR2 is important for mediating peripheral responses to herpes simplex virus 1 (HSV-1), as TLR2−/− mice show decreased peripheral inflammatory responses and serum cytokine levels after HSV-1 infection (Kurt-Jones et al., 2004). However, these mice are protected from lethal encephalitis, with decreased CNS cytokine and chemokine expression. Similarly, TLR3−/− mice are protected from lethal West Nile virus encephalitis (Wang et al., 2004). In the periphery, these mice have increased viral loads and decreased inflammatory responses, as would be expected if TLR3 mediates responses to this virus. The decreased inflammatory response in the periphery failed to damage the BBB, and the virus was unable to access the CNS and cause fatal disease. In contrast, intracerebral infection of West Nile virus had identical outcomes in wild type and TLR3−/− mice, indicating that TLR3 in the CNS is not important for responses to this virus (Wang et al., 2004). These studies demonstrate that host inflammatory responses in the CNS during infection may be more responsible for self tissue damage than direct effects of the pathogen itself. They also suggest that treatment strategies for CNS infections which promote inflammation in the CNS need to be carefully considered and studied before use, as they could result in significant neurologic side effects.
Neurotoxicity Caused by TLR Stimulation
Although the stimulation of TLRs on glial cells activate functions that are important for the elimination of pathogens (Fig. 1C), these same functions can be toxic to cells of the CNS that have limited regenerative capacity (Fig. 1D). Microglia stimulated with LPS in vitro are toxic to neurons and oligodendrocytes in a TLR4-dependent manner (Lehnardt et al., 2002; Lehnardt et al., 2003). Similarly, microglia exposed to GSB or S. pneumoniae serotype 2 also display neurotoxic properties, dependent on TLR2 (Hoffmann et al., 2007; Lehnardt et al., 2006). Some authors have observed that LPS-stimulated astrocytes are also neurotoxic, although others report that only microglia are required for toxicity (Bal-Price and Brown, 2001; Lehnardt et al., 2002). Toxicity appears to be mediated primarily via nitric oxide, as pharmacologic blockade of iNOS is able to prevent neuronal death in the presence of activated glia (Bal-Price and Brown, 2001; Lehnardt et al., 2006). TLR-stimulated astrocytes and microglia both produce high levels of iNOS (Carpentier et al., 2005; Olson and Miller, 2004), however, TLR-stimulated astrocytes also produce a variety of neurotrophic factors, which may mitigate their toxicity (Bsibsi et al., 2006). Stimulation of astrocytes with TLR ligands also inhibits their ability to uptake excess glutamate (Bal-Price and Brown, 2001; Scumpia et al., 2005), and therefore the role of astrocytes in neurotoxicity may be most important in models in which glutamate excitotoxicity is a major mechanism of death.
TLR stimulation also has neurotoxic properties in vivo. Injection of poly I:C or Pam3CysSK4 into the CNS causes can cause neurodegeneration in a TLR3- or TLR2-dependent manner, respectively (Hoffmann et al., 2007; Melton et al., 2003). LPS exposure also causes profound microglial activation associated with oligodendrocyte death, demyelination and increased vulnerability of neurons to injury, dependent on TLR4 signaling (Lehnardt et al., 2002; Lehnardt et al., 2003). Local injections of LPS directly into the CNS cause severe loss of dopamine neurons in the substantia nigra (Castano et al., 1998) and neurons in the hippocampus (Hauss-Wegrzyniak et al., 1998). Newborn neurons in the hippocampus and dopaminergic neurons in the substantia nigra appear to be exquisitely sensitive to the effects of LPS, as peripheral injection of even low levels of LPS reduces the number of these cells (Monje et al., 2003; Qin et al., 2007). Blockade of NOS is able to rescue substantia nigra neurons from death, indicating that the mechanism in these in vivo models is likely similar to the in vitro system (Arimoto and Bing, 2003).
These results demonstrate that TLR activation likely contributes to neuronal and/or oligodendroglial loss, which occurs during CNS infection. It is important to note here that TLR activation does not always lead to neurodegeneration and can actually have beneficial effects in the CNS under some circumstances. For example, the injection of LPS following toxin-induced injury to the corpus callosum promotes the clearance of myelin debris and enhances remyelination (Glezer et al., 2006). However, these protective functions have not yet been described during CNS infection or autoimmunity and are therefore not a focus of this review. We refer interested readers to an interesting recent review on the topic of TLRs and CNS repair (van Noort, 2007).
Functions of TLRs in Autoimmunity
Multiple sclerosis (MS) is a CD4+ T cell-mediated autoimmune demyelinating disease of the CNS (Sospedra and Martin, 2005). Although its exact etiology is unknown, epidemiologic evidence suggests a viral trigger (Sospedra and Martin, 2005). The principal murine model of MS is experimental autoimmune encephalomyelitis (EAE), in which an autoimmune response is primed in the periphery by the administration of myelin peptides or proteins emulsified in complete Freund’s adjuvant (CFA), or by adoptive transfer of activated myelin-specific CD4+ T cells. TLR and/or IL-1 signaling is crucial for the development of EAE because MyD88−/− mice do not develop EAE under the typical immunization protocol (Prinz et al., 2006). TLR9−/− and TLR4−/− mice also show decreased clinical disease after peripheral priming, although the effect is less dramatic than the loss of all MyD88-dependent pathways (Prinz et al., 2006). At least some of this effect is probably due to the inability to effectively prime pro-inflammatory Th1 and/or Th17 responses, however, MyD88−/− mice also developed decreased clinical disease after the transfer of activated wild-type myelin-specific CD4+ T cells, indicating that lack of T cell activation can not fully explain these results (Prinz et al., 2006). Furthermore, bone marrow transplants have demonstrated that mice specifically deficient in CNS expression of MyD88 or TLR9 develop delayed and reduced disease compared to wild type mice, with decreases in leukocyte infiltration as well as axonal and myelin damage (Prinz et al., 2006). These studies define important roles for both TLR9- and other MyD88-dependent pathways in CNS-resident cells during EAE-associated neurologic damage.
The exact nature of the signals activating TLRs during EAE and MS is still unclear, since there is no infectious agent in the CNS (Sospedra and Martin, 2005). It has been suggested that the innate immune system is activated not only by microbial products, but also by signals of host cell damage, known as “endogenous ligands” (Zhang and Schluesener, 2006). These endogenous ligands include substances which may be released into the extracellular milieu during cell lysis, such as endogenous mRNA and heat shock proteins, and extracellular matrix breakdown products which may signal tissue damage, such as hyaluronan fragments, heparan sulfate, fibrinogen, and fibronectin extra domain A (Zhang and Schluesener, 2006). However, controversy exists regarding whether these are true endogenous ligands or were contaminated with microbial products during isolation or synthesis (Zhang and Schluesener, 2006). Despite the uncertainty over their exact identity, it is becoming clear that such endogenous ligands must exist because TLRs have significant role in pathology during sterile CNS injury (Kim et al., 2007; Tanga et al., 2005). Both TLR2 and TLR4 deficient mice show decreased activation of pro-inflammatory gene expression and decreased pain hypersensitivity after spinal nerve transection, implicating a role for endogenous ligands in activating TLR-mediated inflammation in the pathogenesis of neuropathic pain (Kim et al., 2007; Tanga et al., 2005). Such a model also attractively explains how the TLR-dependent inflammatory cascade could be activated and progress in the CNS during autoimmunity without the presence of a specific pathogen.
We have a proposed a model of CNS autoimmunity which is precipitated by bystander damage in the CNS and perpetuated by epitope spreading (Vanderlugt and Miller, 2002). In this model, the inflammatory response in the CNS is initiated by a virus (or other CNS insult) and the ensuing immune response causes tissue damage in the CNS, which could be mediated by TLRs, as discussed in the previous section. The tissue damage and subsequent release of previously sequestered myelin antigens perpetuates the inflammation and allows the de novo induction of antigen-specific immune responses in the CNS milieu to spread to myelin proteins, thereby initiating autoimmunity (Bailey et al., 2007; McMahon et al., 2005). An important implication of this model is that once the tissue damage is initiated, it is self-sustaining and no longer requires the presence of the pathogen. The ability of TLRs in the CNS to be activated by endogenous antigens released during ongoing tissue damage is an additional way in which TLRs could play a critical and ongoing role in CNS autoimmune disease (Fig. 1D). The role of TLRs in the process of epitope spreading is still unknown, however, we speculate their involvement based on studies previously discussed which demonstrate that TLRs are important mediators of neuroinflammation and tissue damage during infectious and non-infectious CNS disease.
Conclusions
Although the CNS is no longer considered to be entirely immune-privileged, there are significant obstacles to mounting immune responses in the CNS; notably the limited immune surveillance and general immunosuppressive properties (Bailey et al., 2006). These properties make it critical that CNS resident cells be able to recognize and efficiently respond to infection, and coordinate an immune response capable of clearing the infection without causing extensive tissue damage. Since the recognition of mammalian TLRs as critical pattern-recognition receptors of the innate immune system, defining their expression and function in the CNS has been a priority in the field of neuroimmunology. Glial cells, primarily microglia and astrocytes, express a wide complement of TLRs, leaving them poised to respond to a variety of pathogens. Importantly, recent studies confirm the role of TLRs in promoting innate immune functions of glia in response to microbial products. It appears that in many cases, a single TLR is not responsible for activating glial responses to whole microbes. Rather, a complement of TLRs or other pattern recognition receptors may be able to activate similar immune functions, a redundancy with clear advantages in light of constantly evolving microbes. Although the activation of the innate immune system is unquestionably critical for the clearance of a pathogen, the CNS is a particularly fragile tissue with limited regenerative capacity. Several examples have now been reported where the activation of innate immunity via TLRs plays a role in pathology acquired after an infection. Similarly, recent studies implicate TLRs in autoimmune and neurodegenerative diseases, where they may initiate or amplify neuroinflammation via their activation by endogenous ligands. Future studies in our lab and others will further clarify the roles of TLRs specifically in the CNS during infection and autoimmunity. Careful targeting of TLRs in specific compartments may prove necessary in order to effectively balance the ability of the immune system to clear a CNS infection and its ability to cause significant tissue damage and ensuing autoimmunity.
Acknowledgments
Work in the Miller Lab is supported by grants from the NIH, the National Multiple Sclerosis Society, and the Myelin Repair Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arimoto T, Bing G. Up-regulation of inducible nitric oxide synthase in the substantia nigra by lipopolysaccharide causes microglial activation and neurodegeneration. Neurobiol Dis. 2003;12:35–45. doi: 10.1016/s0969-9961(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Carpentier PA, McMahon EJ, Begolka WS, Miller SD. Innate and adaptive immune responses of the central nervous system. Crit Rev Immunol. 2006;26:149–188. doi: 10.1615/critrevimmunol.v26.i2.40. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4(+) T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21:6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Williams BR, Miller SD. Distinct roles of protein kinase R and toll-like receptor 3 in the activation of astrocytes by viral stimuli. Glia. 2007;55:239–252. doi: 10.1002/glia.20450. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem. 1998;70:1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbridge LM, O’Riordan MX. Innate recognition of intracellular bacteria. Curr Opin Immunol. 2007;19:10–16. doi: 10.1016/j.coi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem. 2004;88:746–758. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- Glezer I, Lapointe A, Rivest S. Innate immunity triggers oligodendrocyte progenitor reactivity and confines damages to brain injuries. FASEB J. 2006 doi: 10.1096/fj.05-5234fje. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Hoffmann O, Braun JS, Becker D, Halle A, Freyer D, Dagand E, Lehnardt S, Weber JR. TLR2 mediates neuroinflammation and neuronal damage. J Immunol. 2007;178:6476–6481. doi: 10.4049/jimmunol.178.10.6476. [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005a;49:567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Haney A, Mayes PM, Garg S, Esen N. Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect Immun. 2005b;73:7428–7435. doi: 10.1128/IAI.73.11.7428-7435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Phulwani NK, Esen N, Syed MM, Haney AC, McCastlain K, Johnson J. MyD88-dependent signals are essential for the host immune response in experimental brain abscess. J Immunol. 2007;178:4528–4537. doi: 10.4049/jimmunol.178.7.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- Koedel U, Angele B, Rupprecht T, Wagner H, Roggenkamp A, Pfister HW, Kirschning CJ. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J Immunol. 2003;170:438–444. doi: 10.4049/jimmunol.170.1.438. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J. 2001;15:155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Soucy G, Rivest S. Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. J Neurochem. 2001;79:648–657. doi: 10.1046/j.1471-4159.2001.00603.x. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Henneke P, Lien E, Kasper DL, Volpe JJ, Bechmann I, Nitsch R, Weber JR, Golenbock DT, Vartanian T. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006;177:583–592. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimmie CS, Fazakerley JK. In response to pathogens, glial cells dynamically and differentially regulate Toll-like receptor gene expression. J Neuroimmunol. 2005;169:116–125. doi: 10.1016/j.jneuroim.2005.08.006. [DOI] [PubMed] [Google Scholar]
- McKimmie CS, Johnson N, Fooks AR, Fazakerley JK. Viruses selectively upregulate Toll-like receptors in the central nervous system. Biochem Biophys Res Commun. 2005;336:925–933. doi: 10.1016/j.bbrc.2005.08.209. [DOI] [PubMed] [Google Scholar]
- McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- Melton LM, Keith AB, Davis S, Oakley AE, Edwardson JA, Morris CM. Chronic glial activation, neurodegeneration, and APP immunoreactive deposits following acute administration of double-stranded RNA. Glia. 2003;44:1–12. doi: 10.1002/glia.10276. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Prehaud C, Megret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K, Piesche M, Schroers R, Weiss E, Kirschning CJ, Rochford CD, Bruck W, Becher B. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Li G, Qian X, Liu Y, Wu X, Liu B, Hong JS, Block ML. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52:78–84. doi: 10.1002/glia.20225. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scumpia PO, Kelly KM, Reeves WH, Stevens BR. Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes. Glia. 2005;52:153–162. doi: 10.1002/glia.20234. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- Town T, Jeng D, Alexopoulou L, Tan J, Flavell RA. Microglia recognize double-stranded RNA via TLR3. J Immunol. 2006;176:3804–3812. doi: 10.4049/jimmunol.176.6.3804. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006;84:712–725. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- van Noort JM. Toll-like receptors as targets for inflammation, development and repair in the central nervous system. Curr Opin Investig Drugs. 2007;8:60–65. [PubMed] [Google Scholar]
- Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Zekki H, Feinstein DL, Rivest S. The clinical course of experimental autoimmune encephalomyelitis is associated with a profound and sustained transcriptional activation of the genes encoding toll-like receptor 2 and CD14 in the mouse CNS. Brain Pathol. 2002;12:308–319. doi: 10.1111/j.1750-3639.2002.tb00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Schluesener HJ. Mammalian toll-like receptors: from endogenous ligands to tissue regeneration. Cell Mol Life Sci. 2006;63:2901–2907. doi: 10.1007/s00018-006-6189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lapointe BM, Clark SR, Zbytnuik L, Kubes P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol. 2006;177:8103–8110. doi: 10.4049/jimmunol.177.11.8103. [DOI] [PubMed] [Google Scholar]