Abstract
Wound healing is a complex cascade of events, which diminishes the size of the wound and reestablishes tissue integrity. Secreted frizzled-related protein 1 (SFRP1) contributes to the inhibition of apoptosis in fibroblast populations. We investigated the role of SFRP1 in a mouse wound-healing model; 2.0-mm excisional wounds were created in the scalp and hard palate. Healing responses were measured by histomorphometric analysis, apoptosis assay, and immunohistochemistry. Dermal wounds did not harbor SFRP1, but healed faster than palatal wounds which expressed significant levels of SFRP1. Antibody experiments aimed at blocking SFRP1 in palatal wounds resulted in promotion of wound closure, enhancement of new tissue formation, decrease of inflammatory cell infiltrate, and increase of apoptotic fibroblasts. Analysis of the present data suggests that SFRP1 may be partly responsible for the poorer healing performance of the palatal wounds compared with dermal wounds. Blocking SFRP1 results in improvement of palatal healing outcomes.
Keywords: wound healing, SFRP1, apoptosis, fibroblast
INTRODUCTION
Wound healing of both oral and dermal wounds proceeds through three complex overlapping phases: inflammation, granulation tissue formation, and matrix formation and remodeling (Clark, 1996; Walsh et al., 1996). Wound healing involves a series of specific cell populations tightly controlled by a distinct temporal pattern of cellular apoptosis. Apoptosis serves as a crucial control mechanism not only for organ development and growth, but also for the maintenance of tissue integrity in the mature organism (Greenhalgh, 1998). In general, inflammatory cells undergo apoptosis in early stages of wound healing (Brown et al., 1997), whereas apoptosis eliminates fibroblasts in later stages (Desmouliere et al., 1997).
Our previous investigation demonstrated that the constitutive up-regulation of secreted frizzled-related protein 1 (SFRP1) contributes to the inhibition of apoptosis in periodontal ligament and dermal fibroblasts (Han and Amar, 2004). SFRP1 proteins are involved in apoptosis by negatively modulating wingless/int (WNT) signaling by interacting with either WNTs or Frizzled receptors. The WNT family is a group of secreted glycoproteins mediating embryogenesis, organogenesis, and tumorigenesis through the regulation of cell proliferation, differentiation, and apoptosis by increasing the stability and transcriptional activity of β-catenin (canonical pathway). By sequestering WNTs, SFRP1 removes the stimulus for β-catenin stabilization and mediates various biological processes (Jones and Jomary, 2002; Kawano and Kypta, 2003). Therefore, SFRP1 may be an appropriate target for the modulation of apoptosis.
Given the role of apoptosis in wound healing, the present study was aimed at determining the role of SFRP1 in dermal vs. palatal wound healing and defining whether the modulation of SFRP1 affects wound-healing outcomes.
MATERIALS & METHODS
Animals and Wound Models
Fifty 8-week-old male CD-1 mice, purchased from the Charles River Laboratories (Boston, MA, USA), were used as models for the comparison of dermal and palatal wounds. An additional 10 age-matched mice were used for the antibody-blocking experiment. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Boston University Medical Center. Mice were intraperitoneally anesthetized with a ketamine (80 mg/kg) and xylazine (10 mg/kg) mixture. A palatal excisional wound (2.0 mm) was placed anterior to the soft palate, or a scalp excisional wound was placed at the midline between the ears for each mouse. Mice were killed after 0, 3, 7, 10, and 14 days. Five mice per group were used at each time point.
Blocking SFRP1 with Anti-SFRP1 Antibody
We used anti-SFRP1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) to block the SFRP1 expression in wounded palatal tissues. The control group received IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Five mice per wound group were used. One dose of anti-SFRP1 antibody (30 μg) or IgG (30 μg) was injected submucosally around the wounded area on 6.5, 8, and 9.5 days (a total dose of 90 μg of anti-SFRP1 antibody or IgG). Mice were killed on the 10th day.
Specimen Preparation
Following the animals' death, the calvarial or palatal bone with intact surrounding tissue was dissected and fixed in cold 4% paraformaldehyde for 24 hrs. After fixation, the specimens were decalcified in cold Immunocal (Decal Corporation, Congers, NY, USA) for 7 days, with the solution changed every day. Cryostat sagittal sections were prepared at a thickness of 5 microns.
Quantitative Histologic Analysis
The distance between the edges of the epithelium and connective tissue of the wound, the area of new connective tissue (defined as the new tissue formed between the wound edges), and the percentage of new connective tissue in the defect were measured in H&E-stained sections at the widest part of each wound, with the use of Image-Pro Plus Version 4 software (Media Cybernetics, Silver Spring, MD, USA). We quantified polymorphonuclear neutrophil (PMN) and mononuclear cell infiltrates by identifying their characteristic morphology at 400× magnification. We stained several serial sections adjacent to those H&E-stained with Ly6G for neutrophils and Moma-2 for macrophages at each time-point in both groups. Cell counts obtained with immunostaining or H&E were similar, and the differences were not statistically significant.
In situ Detection of Apoptosis
Apoptotic cells were detected by in situ TUNEL assay by means of an in situ cell death detection kit (Roche Diagnostics, Indianapolis, IN, USA), according to the manufacturer's instructions. At high magnification (400×), TUNEL-positive fibroblasts and inflammatory cells were identified by stringent morphologic characteristics, quantified and presented as percentages of apoptotic cells relative to the total of cell counts in the same field of analysis.
Immunohistochemistry
Immunohistochemical staining was carried out as described previously (Han and Amar, 2004). At high magnification (400×), SFRP1-positive fibroblasts were quantified; only spindle-shaped cells were counted.
Statistical Analysis
Student's t test was performed for statistical analyses.
RESULTS
Healing Responses in Palatal and Dermal Groups
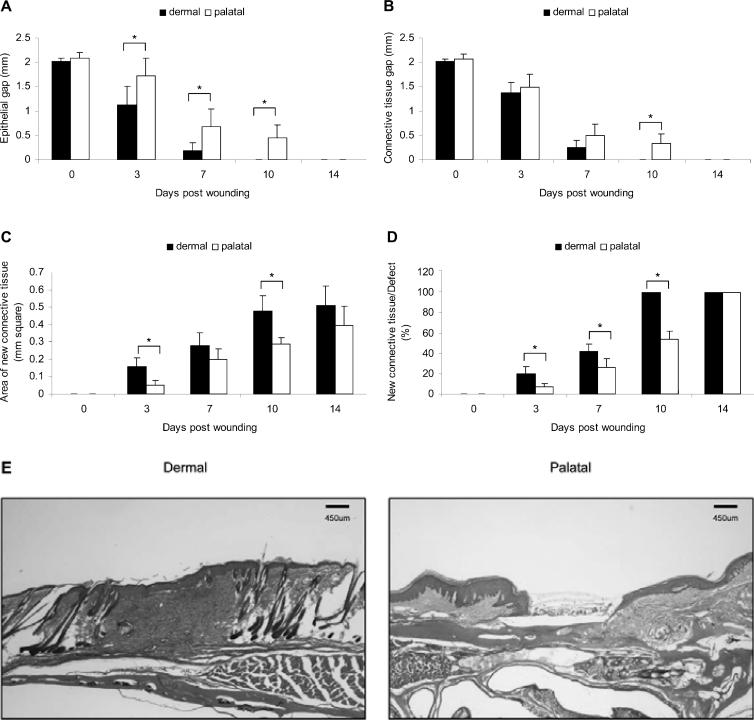
In the dermal group, the epithelial gap was dramatically reduced and completely covered the wounds by day 10. In contrast, intact epithelial coverage was not achieved by day 10 in the palatal group (Figs. 1A, 1E). Connective tissue edges were bridged faster in the dermal group than the palatal group (Figs. 1B, 1E). Connective tissue healing was complete in dermal wounds on day 10, and the amount of new connective tissue was 1.6-fold more than that of palatal wounds (Fig. 1C). By day 10, palatal wounds achieved only partial connective tissue coverage (Fig. 1D).
Figure 1.
Progress in palatal and dermal wound healing. (A) Epithelial gap, (B) connective tissue gap, (C) area of new connective tissue formation, and (D) percentage of new connective tissue formation relative to the area of original excisional defect were measured on H&E-stained wound sections. (E) Wound histology on day 10. Each value represents the mean ± SD (n = 5). *i.e., p < 0.05 by Student's t test.
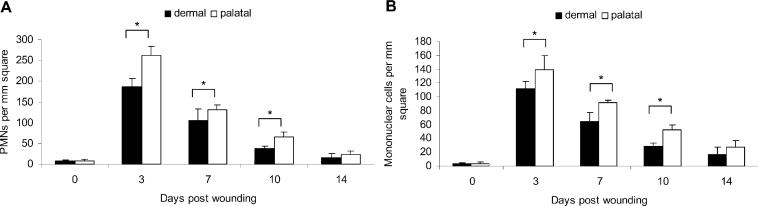
PMN and mononuclear cell infiltration followed the same time-courses in both groups. However, the PMN and mononuclear cell numbers were significantly lower in the dermal group (Figs. 2A, 2B). The inflammatory cell apoptosis peaked on day 3 and then decreased thereafter in both groups. A higher percentage of inflammatory cell apoptosis was observed in the dermal group (Fig. 3A). The fibroblast apoptosis peaked on day 10 and was sustained until day 14 in the dermal group (Figs. 3B, 3C). The fibroblast apoptosis in palatal wounds was relatively low when compared with that of dermal wounds.
Figure 2.
Inflammatory cell infiltrate in palatal and dermal wounds. (A) PMN and (B) mononuclear cell infiltrates were measured by identification of their characteristic appearance on H&E-stained wound sections. Each value represents the mean ± SD (n = 5). *i.e., p < 0.05 by Student's t test.
Figure 3.
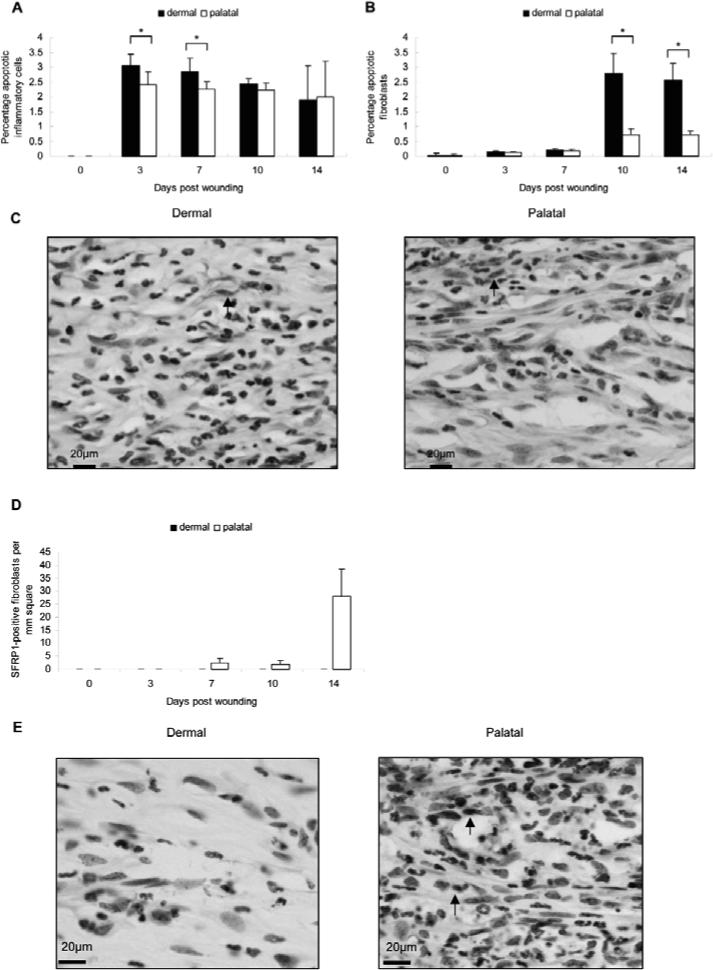
Apoptosis and SFRP1 expression in palatal and dermal wounds. (A) Apoptotic inflammatory cells and (B) fibroblasts were analyzed and quantified, then presented as percentages of apoptotic cells relative to the total cell counts in the same field of analysis by TUNEL assay. Each value represents the mean ± SD (n = 5). *i.e., p < 0.05 by Student's t test. (C) Wound histology on day 10. Arrows show apoptotic fibroblasts. (D) Fibroblasts were analyzed by immunohistochemical staining and quantification of SFRP1-immunopositive cells. Each value represents the mean ± SEM; n = 5). (E) Wound histology on day 14. Arrows show SFRP1-positive fibroblasts.
No detectable SFRP1 expression was observed in the dermal group (Figs. 3D, 3E). In contrast, SFRP1 was expressed on day 7 and peaked on day 14 in the palatal group. The expression of SFRP1 in palatal wounds, and not in dermal wounds, may suggest its tissue-specific role in wound healing.
Blocking SFRP1 with Anti-SFRP1 Antibody in Palatal Wounds
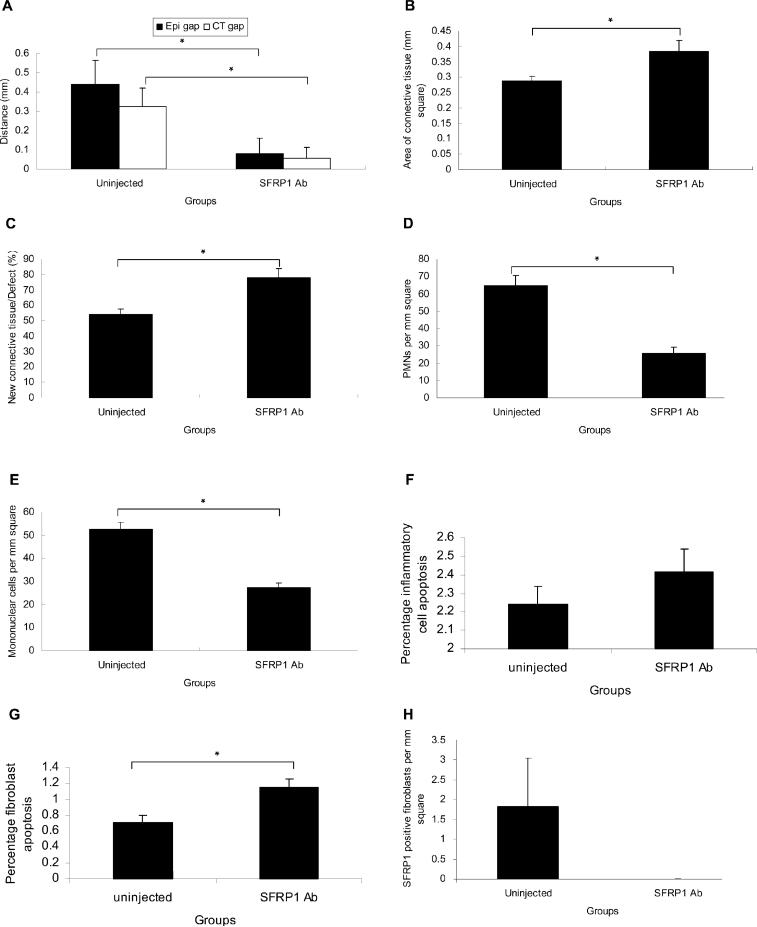
There were more apoptotic fibroblasts in dermal wounds than in palatal wounds. To investigate the role of SFRP1 in wound healing, we injected anti-SFRP1 antibody into the palatal wound edge. SFRP1 expression was substantially reduced in the anti-SFRP1-injected group (Fig. 4H). A reduction in epithelial and connective tissue gaps (Fig. 4A), an increase in new connective tissue formation (Figs. 4B, 4C), a lower inflammatory cell infiltrate (Figs. 4D, 4E), as well as an increase in fibroblast apoptosis (Fig. 4G), were observed in the anti-SFRP1 antibody-injected group compared with those of the palatal group on day 10 (uninjected group). There was no significant difference in inflammatory cell apoptosis in both groups (Fig. 4F). IgG-injected mice behaved similarly to uninjected mice, with no statistical difference in both groups. No statistical differences were observed when the anti-SFRP1 antibody-injected group was compared with IgG-injected mice or with uninjected mice (data not shown).
Figure 4.
Blocking SFRP1 in palatal wounds. (A) Epithelial and connective tissue gaps, (B) area of new connective tissue formation, (C) percentage of new connective tissue formation relative to the area of original excisional defect, (D) the number of PMNs, and (E) mononuclear inflammatory cells, (F) the percentage of apoptotic inflammatory cells and (G) fibroblasts, and (H) SFRP1 expression in uninjected (palatal group on day 10) and anti-SFRP1 antibody-injected groups. Each value represents the mean ± SEM (n = 5). *i.e., p < 0.05 by Student's t test.
DISCUSSION
Hair follicular stem cells located in the skin are important sources for reepithelialization, since they lessen overall healing time (Taylor et al., 2000). In addition, the formation of a wound bed matrix is important for re-epithelialization (Woodley, 1996). Thus, increased amounts of new connective tissue formation in dermal wounds may accelerate the migration rate of the epithelium to reduce overall healing time. Our study, supported by others (Nooh and Graves, 2003), demonstrated that palatal healing is delayed relative to dermal healing.
Reduced inflammatory infiltrates correlate with an accelerated healing rate (Bullard et al., 2003). Our study showed that the increased infiltration of inflammatory cells with a low percentage of inflammatory cell apoptosis in palatal wounds might lead to a delayed healing process. This impediment may be caused by environmental factors and/or by intrinsic characteristics of palatal tissue.
Reduced fibroblast apoptosis was found to correlate with enhanced scarring (Linge et al., 2005). Previous studies have demonstrated that scar formation in the palate was responsible for the resistance to dental arch expansion or transverse and anterior-posterior growth of alveolar bone after palatal surgery in orthodontic treatment or cleft palate patients (Ishikawa et al., 1998; Chu et al., 2000). Consistent with the literature, our study showed that palatal wounds had a reduced fibroblast apoptosis compared with dermal wounds, which may enhance scarring.
Analysis of our data showed that SFRP1 was up-regulated in palatal wounds on day 7−14 (most notably on day 14). During the initial phase of wound repair, β-catenin-mediated signaling (canonical WNT pathway), a known inhibitor of SFRP1, is activated and prevents SFRP1 expression (Cheon et al., 2004). However, the later phase of wound healing (7−14 days) is marked by cell de-activation and scar maturation, correlating with the heightened SFRP1 up-regulated expression. This, in turn, interferes with the WNT pathway to regulate fibroblast apoptosis. Furthermore, analysis of current data points to the fact that SFRP1 may not play a significant role in inflammatory cell apoptosis, and that its role is mediated through fibroblasts (Ijiri et al., 2002). This may explain the differences in terms of the number of inflammatory cells observed at earlier time points.
Silencing SFRP1 enhanced osteoblast proliferation and bone formation (Bodine et al., 2004). In this study, we have shown that blocking SFRP1 enhanced new connective tissue formation, the up-regulation of which might lead to a prompt closure of palatal wounds. However, little is known about the role of SFRP1 in wound healing. The exact mechanism should be further investigated.
ACKNOWLEDGMENT
This study was supported by National Institute of Dental and Craniofacial Research grant #RO1 15989 to S. Amar.
REFERENCES
- Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- Brown DL, Kao WW, Greenhalgh DG. Apoptosis down-regulates inflammation under the advancing epithelial wound edge: delayed patterns in diabetes and improvement with topical growth factors. Surgery. 1997;121:372–380. doi: 10.1016/s0039-6060(97)90306-8. [DOI] [PubMed] [Google Scholar]
- Bullard KM, Longaker MT, Lorenz HP. Fetal wound healing: current biology. World J Surg. 2003;27:54–61. doi: 10.1007/s00268-002-6737-2. [DOI] [PubMed] [Google Scholar]
- Cheon SS, Nadesan P, Poon R, Alman BA. Growth factors regulate beta-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp Cell Res. 2004;293:267–274. doi: 10.1016/j.yexcr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Chu S, Ishikawa H, Kim T, Yoshida S. Analysis of scar tissue distribution on rat palates: a laser Doppler flowmetric study. Cleft Palate Craniofac J. 2000;37:488–496. doi: 10.1597/1545-1569_2000_037_0488_aostdo_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Clark RAF. Overview and general considerations of wound repair. In: Clark RAF, editor. The molecular and cellular biology of wound repair. Springer; New York: 1996. pp. 3–50. [Google Scholar]
- Desmouliere A, Badid C, Bochaton-Piallat ML, Gabbiani G. Apoptosis during wound healing, fibrocontractive diseases and vascular wall injury. Int J Biochem Cell Biol. 1997;29:19–30. doi: 10.1016/s1357-2725(96)00117-3. [DOI] [PubMed] [Google Scholar]
- Greenhalgh DG. The role of apoptosis in wound healing. Int J Biochem Cell Biol. 1998;30:1019–1030. doi: 10.1016/s1357-2725(98)00058-2. [DOI] [PubMed] [Google Scholar]
- Han X, Amar S. Secreted frizzled-related protein 1 (SFRP1) protects fibroblasts from ceramide-induced apoptosis. J Biol Chem. 2004;279:2832–2840. doi: 10.1074/jbc.M308102200. [DOI] [PubMed] [Google Scholar]
- Ijiri K, Nagayoshi R, Matsushita N, Tsuruga H, Taniguchi N, Gushi A, et al. Differential expression patterns of secreted frizzled related protein genes in synovial cells from patients with arthritis. J Rheumatol. 2002;29:2266–2270. [PubMed] [Google Scholar]
- Ishikawa H, Nakamura S, Misaki K, Kudoh M, Fukuda H, Yoshida S. Scar tissue distribution on palates and its relation to maxillary dental arch form. Cleft Palate Craniofac J. 1998;35:313–319. doi: 10.1597/1545-1569_1998_035_0313_stdopa_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Jones SE, Jomary C. Secreted frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signaling pathway. J Cell Sci. 2003;116(Pt 13):2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Linge C, Richardson J, Vigor C, Clayton E, Hardas B, Rolfe K. Hypertrophic scar cells fail to undergo a form of apoptosis specific to contractile collagen—the role of tissue transglutaminase. J Invest Dermatol. 2005;125:72–82. doi: 10.1111/j.0022-202X.2005.23771.x. [DOI] [PubMed] [Google Scholar]
- Nooh N, Graves DT. Healing is delayed in oral compared to dermal excisional wounds. J Periodontol. 2003;74:242–246. doi: 10.1902/jop.2003.74.2.242. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Walsh LJ, L'Estrange PR, Seymour GJ. High magnification in situ viewing of wound healing in oral mucosa. Aust Dent J. 1996;41:75–79. doi: 10.1111/j.1834-7819.1996.tb05916.x. [DOI] [PubMed] [Google Scholar]
- Woodley DT. Reepithelialization. In: Clark RAF, editor. The molecular and cellular biology of wound repair. Springer; New York: 1996. pp. 339–354. [Google Scholar]