Abstract
Disentangling the myriad determinants of disease, within the context of urban health or health disparities, requires a transdisciplinary approach. Transdisciplinary approaches draw on concepts from multiple scientific disciplines to develop a novel, integrated perspective from which to conduct scientific investigation. Most historic and contemporary conceptual models of health were derived either from the sociobehavioral sciences or the biomolecular sciences. Those models deriving from the sociobehavioral sciences generally lack detail on involved biological mechanisms whereas those derived from the biomolecular sciences largely do not consider socioenvironmental determinants. As such, advances in transdisciplinary characterizations of health in complex systems like the urban environment or health disparities may be impeded. This paper suggests a sociobiologic organizing model that encourages a multilevel, integrative perspective in the study of urban health and health disparities.
Keywords: Urban health, Transdisciplinary, Multilevel, Disparities, Behavior, Genetics
INTRODUCTION
Recently, several researchers have hypothesized pathways that attempt to explain how the sociobehavioral environment is related to health and health disparities.1–9 Historically, these conceptual frameworks have formed a solid foundation upon which science was built. Upon review of these frameworks, it is possible to make at least three general observations. The first is the lack of depth to which they integrate our present understanding of the biology of disease, particularly at the cellular and molecular levels. With the exception of those pathways based on stress (neuroimmunological) mechanisms, the published frameworks in the behavioral sciences and epidemiological literature largely lack clearly stated, causal biologic connections to observed health outcomes.2,4,5,7,10,11 On the other hand, the biologically oriented formulations poorly account for socioenvironmental and behavioral effect modifiers that may affect the pathogenesis of disease and the development of health disparities.12–15
Secondly, the terminology used to characterize the effects of causal agents has not been standardized across disciplines and is derived from toxicology, biostatistics, epidemiology, sociology,16 and the clinical/bench sciences.17,18 Across these fields, the terms cause, mediator, moderator, regulator, effector, interaction, and mechanism of action have vastly different meanings, which may not be readily apparent to all investigators. For example, epidemiologists and statisticians tend to use the terms mediators and moderators to describe distinct aspects of an observed association between two independent variables, largely without reference to the underlying biophysiologic processes. On the other hand, toxicologists and clinical/bench scientists tend to use the terms mediators, moderators, and regulators almost synonymously as descriptors of factors, substances, or agents that alter some characteristic of a known or unknown biophysiologic mechanism. They also tend to reserve the term “cause” or “causal pathway” to describe an agent or series of biophysiologic events that must occur to result in a given outcome.
Finally, scientists and investigators trained in the clinical and bench sciences generally consider discreet, quantitative exposures (viral, bacterial, toxicological, psychological, etc.) as the etiologic agents of disease. Historically, these exposures were studied in isolation from the broader sociobehavioral contexts in which they exist. On the other hand, social scientists often consider more qualitative social factors like poverty, socioeconomic status (SES), and racial segregation as the key determinants of health.19,20 They often assert that other more quantitative exposures are factors, which alter the nature of the association between the social factor and a given health outcome.21 When social scientists are describing causal factors, they draw a distinction between proximal social factors, which they define as the settings in which people live (family, work, school, and neighborhood), and distal social factors, which they define as the pervasive forces in society (culture, SES, and race relations).21
The unique perspectives of each scientific discipline both have strengths and weaknesses. However, as transdisciplinary investigation is increasingly undertaken, the resultant confusion in scientific discourse may hinder scientific inquiry and the advancement of knowledge.
Given this level of complexity, a sociobiologic organizing model or framework could enhance the nascent link between sociobehavioral investigation and biophysiologic or biomolecular mechanisms. Attempts to organize and understand complex biologic systems were attempted in the past. Some researchers have looked to Chaos Theory and Complexity Theory as constructs to facilitate the understanding about health and its relationship to diverse processes and outcomes such as cardiac arrhythmias22,23 and even urban epidemics.24,25 Whereas this approach may have merit, it appears to be beyond the practical usefulness of many clinicians and scientists.
The inherent difficulty of developing a useful transdisciplinary model is demonstrated by the fact that any model detailing all possible biologic pathways through which all possible social and behavioral factors impact all possible health outcomes would be exceedingly complex. Alternatively, an overly simplistic model would likewise be of little value.19 The goal of this paper is to articulate a framework through which multilevel, transdisciplinary work might be collectively organized.
In this paper, an exhaustive analysis or critique of the science is not attempted. Rather, at each step of our model we will provide illustrative examples that suggest how information from disparate fields might be integrated within a single biologically plausible, mechanistically driven, multilevel framework.
THE SBIM
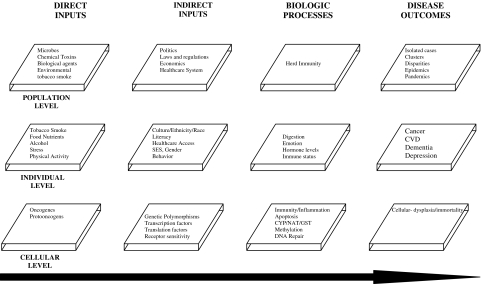
In brief, the sociobiologic integrative model (SBIM) suggests that individuals are constantly being exposed to many health-impacting environmental inputs. These inputs are often modified to increase or attenuate their effects via other “indirect” environmental inputs. Both direct and indirect inputs are, in turn, acted upon by metabolic, digestive, and/or detoxification systems, often producing measurable biologic products (biomarkers). If inputs or metabolic products overwhelm bodily defense or regulatory mechanisms, disease will occur. Because inputs, biologic processes, and outcomes exist on several levels, the model is conceived as operating on the cellular, individual, and population levels, temporally proceeding from input (exposure) to outcome (Figure 1).
FIGURE 1.
This graphic depicts the SBIM. It shows the general temporal relationship between exposures (direct inputs), factors which can alter these exposures (indirect inputs), biophysiologic mechanisms impacted by these exposures, and potential observed outcomes on the population, individual, and cellular levels.
As applied to our model, the term “environmental” is used in a broad sense. It includes factors such as toxicological agents, microbial pathogens, and sociocultural and geopolitical influences. Because these exposures are extremely varied and emanate from many very different types of sources, we refer to them collectively as inputs. Specifically, we define direct inputs as those exposures that directly alter normal host DNA (directly causal). In contrast to direct inputs, many exposures impact host physiology only indirectly, albeit at times profoundly. Examples of these types of indirectly acting exposures would include culture and SES. We define these indirectly acting factors as indirect environmental inputs.
Population-level direct inputs would include certain carcinogenic emissions from urban factories or diesel exhaust fumes among inner city residents and workers. Individual-level direct inputs would include such things as alcohol- and food-based carcinogen exposures. Finally, cellular direct inputs would include tumor suppressor genes, which confer increased susceptibility of cancer on those who possess the genes.
As with direct inputs, indirect inputs exist on the cellular, individual, and population levels.17,19,21,26–30 For example, tobacco regulatory policies may function as population-level indirect inputs that impact cigarette carcinogen exposure. Thus, people who work in smoke-free environments would potentially be exposed to less work-related tobacco carcinogens than those working in smoke-filled workplaces. Individual-level indirect inputs include gender, age, host immune status, health literacy, and family or social networks. Social networks, for example, impact health outcomes because individuals with larger and more robust social networks tend to engage in health-promoting behaviors and to avoid health-damaging behaviors.31 Finally, other indirect inputs, such as genetic polymorphisms of tumor-associated genes, operate at the cellular or molecular level, impacting gene function or expression (see “LUNG CANCER AND THE SBIM” discussion below).17,32,33,33–39
The specific group of direct and indirect inputs to which an individual is exposed may be highly variable between individuals or populations. Ultimately, it is determined by the sociocultural milieu or surroundings in which the individual lives, works, and socializes.
After being altered by indirect inputs, all inputs (direct and indirect) are acted upon by one or more degradory, detoxification, immune, or metabolic systems or biologic processes within the body. These systems include, but are not limited to, the N-acetyltransferase enzymes, the phase I cytochrome p450 (CYP) system, and the phase II glutathione-S-transferase (GST) system.34 Although there are potentially many such systems operating in the body, the absolute number is finite. Integratively understanding health requires that the combined effects of all inputs (direct and indirect) be understood in the context of their impact on biologic processes.
During these processes, a myriad of excretory, secretory, respiratory, hormonal, and other metabolic substances are produced. Many of these substances are potential biomarkers. Biomarkers have become central to clinical medicine, pathology, and molecular epidemiology.40 Some biomarkers have utility in the diagnosis, treatment, and follow-up of disease. The utility of many others is under active investigation.29,41–46
Finally, the SBIM posits that disease will only occur if the magnitude of impact produced by inputs and metabolic processes is sufficient to overwhelm bodily reparative, restorative, or compensatory mechanisms, causing the accumulation of genotypic, phenotypic, or psychologic abnormalities, which ultimately result in a disease state or health deficit. The challenge then for science is to use this model first to help organize and define the inputs, biologic processes, and outcomes that exist on each of the three suggested levels of exposure. The second challenge is to define how each of these factors relate to each other, again within the framework of a causal schema, to produce the outcome of interest. In other words, elucidate the relationships between inputs, processes and outcomes to produce the individual-level outcome (disease) or population-level outcome (disparity) of interest.
LUNG CANCER AND THE SBIM
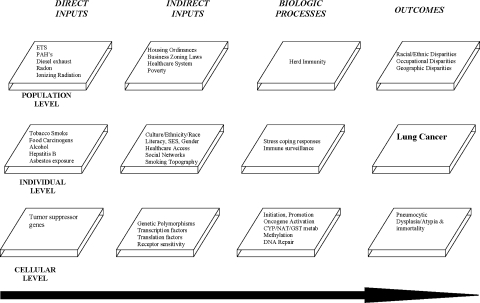
The SBIM framework suggests that the development of lung cancer is preceded by one or more carcinogenic direct inputs (exposures). Environmental, behavioral, and occupational exposures to well known pulmonary carcinogens, including tobacco, asbestos, radon, polycyclic aromatic hydrocarbons (PAHs), and heterocyclic amines are well documented (Figure 2).40,46,47
FIGURE 2.
This graphic employs the SBIM to outline a mechanistically driven framework for understanding socioenvironmentally associated lung cancer development on the cellular, individual, and population levels.
Next, indirect inputs modify these exposures. Potential indirect inputs of pulmonary carcinogens are many and as indicated by the model, operate on the cellular, individual, and population levels. Population-level indirect inputs could include certain geographic factors such as physical proximity of housing to a source of ambient air particulate toxicants. Individuals living in housing units located close to a factory spewing carcinogenic emissions from its smoke stack might be expected to experience higher carcinogenic exposure levels over time compared to ambient air exposures in individuals who live farther away from these sites. In fact, location of urban residence has been associated with increased personal exposure and an increased lifetime risk of cancer.48,49 In addition, carcinogenic exposures from other sources like diesel exhaust fumes may be significantly higher in urban communities than exposures to these same carcinogens in rural environments. Scientific evidence does indeed document that the carcinogenic activity of some PAHs may be related to exposures not only from cigarettes, but also from other environmental sources.50 Coke oven plant workers, commercial printers, truckers (diesel exhaust), and workers from rubber, asphalt, coal, and aluminum plants are all at increased risk of exposure to PAHs.48,51,52 Occupational scientists have shown that increased work-related PAH exposure is associated with an increased risk of morbidity,53 DNA damage,54 abnormal methylation of tumor associated genes,55 and increased risk of lung cancer.56,57 Thus, both proximity of urban residence to a carcinogenic source and ambient air toxicant concentrations likely influence cumulative individual and population PAH exposure.
As one continues to think about potential lung cancer indirect inputs, the role of diet as an important factor must be considered. Whereas diet has often been considered an important carcinogenic exposure, the SBIM encourages a more explicit understanding of diet, dietary constituents, and their level of exposure. As such, it may be that specific dietary nutrients or dietary carcinogens would be considered inputs, whereas other sociocultural and regulatory factors that influence dietary choices of individuals would be considered indirect inputs. As such, “diet” may be considered a culturally influenced (and as such population-level) indirect input whereas the actual dietary constituents consumed may be seen as an individual-level direct or indirect input or depending on the specific constituent in question. For example, cruciferous vegetables of the Brassica family (broccoli, cauliflower, etc.) have been linked to their ability to affect the activity of CYP and GST enzymatic systems, thereby inhibiting phase I bioactivation of carcinogens and inducing phase II carcinogenic detoxification.58–60 Thus, broccoli in the diet would be an individual-level indirect input, whereas the availability of broccoli at local grocery stores, the price of broccoli in those stores, or whether or not individuals would choose to eat broccoli would, on the other hand, represent societal (neighborhood), SES (market forces dictating prices), and cultural (certain cultures tend not to eat certain things) factors, which in the SBIM framework, would all represent population-level indirect inputs.
Local or national regulatory policy may also influence lung carcinogenesis at the population level by either indirectly influencing dietary food choices or by influencing carcinogenic exposures among populations. For example, consider that grocery store location, cost of foodstuffs, and quality of supermarkets have all been linked to dietary choices and nutrient availability.61 In the case of liquor establishments, store location and number of stores in a given community were shown to be associated with amount of alcohol ingested per capita in the local community and associated with dietary nutrient intake by local community residents.62 Regulatory policies then, such as business zoning and liquor licensing laws, may have the unintentional and unrecognized consequences of influencing cancer risk by impacting personal ambient air carcinogenic exposures via regulating the proximity and density of living establishments to urban and occupational particulate carcinogen sources and by influencing dietary nutrient availability of residents living in a given community.
Finally, in terms of indirect inputs, the carcinogenic potential of the PAHs may be impacted by the presence or absence of a given genetic polymorphism operating on the cellular level.18,63–71 Researchers then could seek to elucidate the relationships between these multilevel phenomenon, which all operate along the lung cancer causal chain in individuals, to produce lung cancer or not and also among populations to produce a given lung cancer disparity.
It is true that completely characterizing the independent and joint effects of all potential direct and indirect inputs in complex mixtures, such as ambient air, tobacco smoke, and diet, or completely quantifying the effects of all important genes and polymorphisms impacting all requisite biologic processes is a formidable task.72–74 However, it is clear that as many social and environmental sources as possible must be collectively considered, evaluated, and quantified to most accurately ascertain cumulative individual- and community-level carcinogenic exposure or risk.
Continuing with our model, the physiologic disposition of carcinogenic inputs occurs next. Here, the biomolecular experimental literature is replete with relevant mechanisms and several excellent reviews summarize the expansive current knowledge of biochemical and molecular genetic events involved in the metabolism of tobacco carcinogens.75–79 As such, these will not be detailed. In brief though, over time, prolonged tobacco-related carcinogenic exposure is associated with progressive accumulation of phenotypic and genotypic abnormalities, leading to tumor initiation, promotion, and progression.75–79 Consequent to these processes, many metabolites and byproducts are produced, released, or otherwise given off. Recent advances in molecular biology and genetics have made it possible to identify many of these potential lung cancer biomarkers.45,80 The relative utility of these biomarkers in cancer prevention and prognostication is an active area of research.
Finally, according to the SBIM, lung cancer will occur if the combined effects of all important carcinogenic direct and indirect inputs are sufficient to cause the accumulation of phenotypic and genotypic abnormalities, such that tumor initiation, promotion, and progression will occur in individuals (disease) or populations (disparity). Thus, as can be seen from the preceding example, a single organizing framework such as the SBIM is needed to help organize the myriad of factors involved in lung cancer susceptibility and occurrence among individuals or to comprehensively explain differential outcomes between populations.
Health Disparities, Lung Cancer, and the SBIM
Given that the human genome has proven to be highly conserved, genotypic variation alone cannot adequately explain the existence of health disparities and socioenvironmental factors are now believed to be important in their development. In general, the SBIM model suggests that health disparities (a population-level outcome) occur when direct input–indirect input profiles (the sum total of the effects of all inputs) are sufficient to produce disease (lung cancer) in a higher than expected number of individuals in a given population. With regard to lung cancer specifically, the interplay of environmental factors, geography, smoking, and biology suggested by the SBIM may underlie findings such as those of Pastorino et al.81 who studied the relationship between occupational carcinogen exposure and cigarette smoking in lung cancer. His work found that in a general industrial worker population, occupational exposure to pulmonary carcinogens causes lung cancer in a cooperative and multiplicative fashion with increasing levels of cigarette smoking. Archer et al.82 reported similar findings among uranium miners who smoked. These studies demonstrated that occupational exposures conferred an increased risk of lung cancer in smokers and nonsmokers. This elevated risk, however, was further increased exponentially with increasing levels of cigarette smoking.
Racial and ethnic disparities in lung cancer offer another illustrative case in point. According to the recent Surveillance Epidemiology and End Results (SEER) data, African-American men have significantly elevated lung cancer incidence and mortality rates compared to white men (Table 1). African-American women, on the other hand, have only a slightly elevated lung cancer incidence and essentially the same lung cancer mortality rate compared to white women.83
TABLE 1.
Recent SEER data depicting current racial and ethnic lung cancer disparities between African-Americans and whites in lung cancer incidence and mortality
| US lung cancer incidence and mortality rates, 1992–1999* | ||
|---|---|---|
| White | African-American | |
| Lung cancer incidence | ||
| Men | 82.9 | 124.1 |
| Women | 51.1 | 53.2 |
| Total | 64.3 | 82.6 |
| Lung cancer mortality | ||
| Men | 81.7 | 113.0 |
| Women | 41.1 | 39.6 |
| Total | 57.9 | 68.9 |
At first, no readily apparent, biologically plausible explanation for these findings is evident. The SBIM may suggest some scientific lines of inquiry or indicate some potential pathways. Indeed it is true that many causal pathways likely contribute to the observed population epidemiology. In addition, as stated above, accurate and precise risk characterization can only be accomplished after accounting for all environmental sources of pulmonary carcinogens and major mediators. Finally, causal pathways must work through biological mechanisms.
The SBIM suggests that at a minimum, precise lung cancer risk characterization must include an assessment of tobacco-smoking patterns, geographic factors, occupational exposures, and major potential indirect inputs. With regard to smoking habits, 2001 National Health Interview Survey data reveal that among working age individuals, overall smoking rates do not significantly differ between non-Hispanic African-Americans and non-Hispanic whites. The data also indicate that only relatively small gender differences in smoking prevalence exist (men=24.7, 95% confidence interval [CI] 23.9–25.6; women=20.8, 95% CI 20.1–21.5; whites=24.5%, 95% CI 23.8–25.2; and African-Americans=22.2%, 95% CI 22.1–23.3).84 Epidemiologic data evaluating smoking trends in the US over the last three decades revealed that the prevalence of current smoking has consistently been highest among blacks and in black men in particular, with generally lower rates for women.85,86 Men generally smoked more cigarettes per day than women, but overall, whites smoke more cigarettes than blacks.85,86 Recent increases in smoking by African-American women, however, have led to cigarette consumption rates on par with African-American men.86 Despite the higher number of cigarettes smoked by Caucasians, most African-Americans smoke the brands with higher tar yields per cigarette.86 Finally, in the 1960s and 1970s the age at smoking initiation for women was approximately 4 years later than men. By the 1990s, this difference had been reduced to 2 years.86 Thus, although cigarette smoking is associated with the majority of lung cancer cases today, epidemiologic evaluation of historic smoking patterns in the US do not easily help to explain racial differences in lung cancer incidence and mortality. Other potential factors, including occupation and place of residence, may be important in the genesis of observed lung cancer disparities.
Racial and ethnic minority workers are generally overrepresented in blue collar and service jobs while underrepresented in professional careers.87 In many of these jobs, minority workers are differentially exposed to occupational carcinogens, resulting in disproportionate disease.87 Also, significant proportions of racial and ethnic minority workers live in central cities, in close geographic proximity to industrial plants, or factories. Finally, men comprise the majority of workers who work in exposure-prone industries (miners, steel workers, and chemical industry workers). Individually, these findings do not suggest a unified causal pathway. However, by employing the SBIM to collectively understand how these contributing factors may collectively impact tumor biology, biologically plausible clues begin to emerge.
One possible pathway that is suggested by the SBIM is that differential exposures related to urban residence and occupation across racial and ethnic groups may act cooperatively to influence the genesis of lung cancer disparities. For example, at baseline, men smoke more than women and blacks smoke higher tar-yielding cigarettes compared to whites. In addition, this smoking-related risk elevation in African-Americans might be further heightened via ambient air carcinogenic exposures among African-Americans who live in the urban inner city. This could further elevate the risk of lung cancer in African-Americans above that of white Americans who are less likely to live in neighborhoods with elevated baseline ambient air carcinogen levels or smoke high tar cigarettes. Among men, African-American incidence and mortality rates may still be further increased through occupational carcinogenic exposures, which biologically act synergistically with smoking patterns and geographic exposures. Women, both African-American and Caucasian American, who comprise a substantially smaller proportion of the workers in exposure-prone industries would not have this additional exposure and thus may not be expected to have lung cancer incidence and mortality rates at par with African-American men. Finally, indirect inputs including insurance status, healthcare access, dietary factors, genetic polymorphisms, or regulatory policy may attenuate or potentiate this pathway as outlined in previous sections.
Discussion
It is interesting to note that scientific evidence suggests that disease causation in general and health disparities in particular result from complex interactions of many factors that simultaneously and often cooperatively act across more than one level of influence over time. An integrated understanding of disease or disparities causation would likely facilitate research and breakthroughs in treatments and interventions. Achieving such a goal in the current state of scientific inquiry is itself a difficult task, with several factors mitigating against such an accomplishment.
We first outline a multilevel, transdisciplinary organizing model and define the terminology used in reference to this model. Then using lung cancer as a case in point, we attempt to illustrate that this model provides a population-oriented, biologically grounded framework for understanding cancer etiology and pathogenesis. We also use the model to provide a mechanistic framework for understanding lung cancer disparities.
This model facilitates cross-disciplinary investigation and communication by providing a common conceptual model and terminology while articulating a biologically driven construct employing both sociobehavioral and biologic variables that influence disease pathogenesis. We acknowledge that some investigators will favor further subdivisions at each of the proposed levels of organization presented in this model. For example, social scientists may prefer that the population level be subdivided into family, neighborhood, and community levels. On the other hand, clinical scientists may want the individual level to be further subdivided into an “organ” level, whereas molecular scientists may seek a submolecular level to be added to the model. Whereas each of these modifications may make sense to a given investigator, they also may have no meaning to an investigator who works largely on a different level. For example, social scientists may not see a reason or value of further subdividing the individual or cellular levels of the model whereas molecular scientists may see no need for multiple subdivisions at the population level. Indeed, further subdivisions of the basic model may increase confusion across disciplines. As such, this model presents a basic three-level framework. Yet, the authors vigorously encourage individual scientists and groups to further subdivide the model as deemed appropriate to facilitate their investigations. In this way, it is hoped that this model may help science to move beyond only attempting to identify isolated “causes” of disease or isolated causes of health disparities (be they behavioral, biologic, or environmental), to also seeking to uncover patterns of behavior-biology interaction that positively or negatively affect individuals and populations. In so doing, we may then improve our understanding of health and disease at the interface of biology, behavior and the environment.
Acknowledgment
This research was supported by funding from the National Institute of Aging (5U01AG018033), National Cancer Institute (5U01CA086308), and National Institute of Environmental Health Sciences (ES03819). Dr. Alberg is a recipient of a KO7 award from the National Cancer Institute (CA73790).
Footnotes
Gibbons and Fox are with the Johns Hopkins Urban Health Institute, Baltimore, MD, USA; Brock, Baylin, and Levine are with the Johns Hopkins School of Medicine, Baltimore, MD, USA; Gibbons, Alberg, LaVeist, Levine, and Fox are with the Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Brock, Alberg, and Baylin are with the Johns Hopkins Oncology Center, Baltimore, MD, USA; Gibbons and LaVeist are with the Johns Hopkins Center for Health Disparities Studies, Baltimore, MD, USA; Glass is with the Johns Hopkins Bloomberg School of Public Health Center on Aging and Health, Baltimore, MD, USA.
References
- 1.Birch S. The 39 steps: the mystery of health inequalities in the UK. Health Econ. 1999;8(4):301–308. [DOI] [PubMed]
- 2.Macintyre S. The black report and beyond: what are the issues? Soc Sci Med. 1997;44(6):723–745. [DOI] [PubMed]
- 3.Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;55(1):125–139. [DOI] [PubMed]
- 4.Acheson D. Independent Inquiry into Inequalities in Health. London: The Stationery Office; 1998.
- 5.Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Ann N Y Acad Sci. 1999;896:3–15. [DOI] [PubMed]
- 6.Fuhrer R, Shipley MJ, Chastang JF et al. Socioeconomic position, health, and possible explanations: a tale of two cohorts. Am J Public Health. 2002;92(8):1290–1294. [DOI] [PMC free article] [PubMed]
- 7.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896:173–188. [DOI] [PubMed]
- 8.Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann N Y Acad Sci 1999;896:131–144. [DOI] [PubMed]
- 9.Capitman J, Bhalotra SM, Calderon-Rosado V, Gibbons MC. Cancer Prevention and Treatment Demonstration for Racial and Ethnic Minorities; Evidence Report and Evidence-based Recommendations. USA: US Department of Health and Human Services; 2003:500-00-0031.
- 10.LaLonde M. A New Perspective on the Health of Canadians. Canada: Government of Canada; 1981.
- 11.Evans RG, Stoddart GL. Producing health, consuming health care. Soc Sci Med. 1990;31(12):1347–1363. [DOI] [PubMed]
- 12.Burger R, Gimelfarb A. Genetic variation maintained in multilocus models of additive quantitative traits under stabilizing selection. Genetics 1999;152(2):807–820. [DOI] [PMC free article] [PubMed]
- 13.Phillips TJ, Belknap JK. Complex-trait genetics: emergence of multivariate strategies. Nat Rev Neurosci. 2002;3(6):478–485. [DOI] [PubMed]
- 14.Sharma AM. The thrifty-genotype hypothesis and its implications for the study of complex genetic disorders in man. J Mol Med. 1998;76(8):568–571. [DOI] [PubMed]
- 15.Meyer JM, Breitner JC. Multiple threshold model for the onset of Alzheimer’s disease in the NAS-NRC twin panel. Am J Med Genet. 1998;81(1):92–97. [DOI] [PubMed]
- 16.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed]
- 17.Minamoto T, Mai M, Ronai Z. Environmental factors as regulators and effectors of multistep carcinogenesis. Carcinogenesis. 1999;20(4):519–527. [DOI] [PubMed]
- 18.Stucker I, Hirvonen A, de Waziers I et al. Genetic polymorphisms of glutathione S-transferases as modulators of lung cancer susceptibility. Carcinogenesis 2002;23(9):1475–1481. [DOI] [PubMed]
- 19.Berkman LF, Kawachi I. Social Epidemiology. New York: Oxford University Press; 2000.
- 20.LaVeist TA. Race and Health. San Francisco: Jose-Bass; 2002.
- 21.Amick BC, Levine S, Tarlov AR, Walsh DC. Society and Health. New York: Oxford University Press; 1995.
- 22.Garfinkel A, Spano ML, Ditto WL, Weiss JN. Controlling cardiac chaos. Science 1992;257(5074):1230–1235. [DOI] [PubMed]
- 23.Weiss JN, Garfinkel A, Spano ML, Ditto WL. Chaos and chaos control in biology. J Clin Invest. 1994;93(4):1355–1360. [DOI] [PMC free article] [PubMed]
- 24.Olsen LF, Schaffer WM. Chaos versus noisy periodicity: alternative hypotheses for childhood epidemics. Science 1990;249(4968):499–504. [DOI] [PubMed]
- 25.Tidd CW, Olsen LF, Schaffer WM. The case for chaos in childhood epidemics. II. Predicting historical epidemics from mathematical models. Proc Biol Sci. 1993;254(1341):257–273. [DOI] [PubMed]
- 26.Pargament KI, Maton KI, Hess RE. Religion and Prevention in Mental Health. Binghamton, NY: Hawthorne Press; 1992.
- 27.Evans RG, Barer ML, Marmor TR. Why are Some People Healthy and Others Not? The Determinants of Population Health. New York: Aldine De Gruyter, 1994.
- 28.Wadsworth ME. Health inequalities in the life course perspective. Soc Sci Med. 1997;44(6):859–869. [DOI] [PubMed]
- 29.Christenson RH, Azzazy HM. Biochemical markers of the acute coronary syndromes. Clin Chem. 1998;44(8 Pt 2):1855–1864. [PubMed]
- 30.Braunwald E, Jameson JL, Longo DL, Hauser SL, Kasper DL, Fauci AS. Harrisson’s Textbook of Medicine 15th Ed. New York: McGraw-Hill Publishers, 2001.
- 31.Cattell V. Poor people, poor places, and poor health: the mediating role of social networks and social capital. Soc Sci Med. 2001;52(10):1501–1516. [DOI] [PubMed]
- 32.Wilson SH, Jones L, Coussens C, Hanna K, eds. Cancer and the Environment; Gene–Environment Interaction. Washington, DC: National Academy Press, 2002. [PubMed]
- 33.Ulrich CM, Kampman E, Bigler J et al. Colorectal adenomas and the C677T MTHFR polymorphism: evidence for gene-environment interaction? Cancer Epidemiol Biomarkers Prev. 1999;8(8):659–668. [PubMed]
- 34.Mucci LA, Wedren S, Tamimi RM, Trichopoulos D, Adami HO. The role of gene-environment interaction in the aetiology of human cancer: examples from cancers of the large bowel, lung and breast. J Intern Med. 2001;249(6):477–493. [DOI] [PubMed]
- 35.Slattery ML, Curtin K, Ma K et al. Diet activity, and lifestyle associations with p53 mutations in colon tumors. Cancer Epidemiol Biomarkers Prev. 2002;11(6):541–548. [PubMed]
- 36.Friedman EM, Lawrence DA. Environmental stress mediates changes in neuroimmunological interactions. Toxicol Sci. 2002;67(1):4–10. [DOI] [PubMed]
- 37.Kelada SN, Kardia SL, Walker AH, Wein AJ, Malkowicz SB, Rebbeck TR. The glutathione S-transferase-mu and -theta genotypes in the etiology of prostate cancer: genotype-environment interactions with smoking. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1329–1334. [PubMed]
- 38.Kramer MS, Goulet L, Lydon J et al. Socio-economic disparities in preterm birth: causal pathways and mechanisms. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):104–123. [DOI] [PubMed]
- 39.Goldman R, Shields P. Food mutagens. J Nutr. 2003;133:965s–973s. [DOI] [PubMed]
- 40.Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123(1 Suppl):21S–49S. [DOI] [PubMed]
- 41.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163(7):1693–1722. [DOI] [PubMed]
- 42.Montuschi P, Collins JV, Ciabattoni G et al. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1175–1177. [DOI] [PubMed]
- 43.De Servi B, La Porta CA, Bontempelli M, Comolli R. Decrease of TGF-beta1 plasma levels and increase of nitric oxide synthase activity in leukocytes as potential biomarkers of Alzheimer’s disease. Exp Gerontol. 2002;37(6):813–821. [DOI] [PubMed]
- 44.Andreasen N, Blennow K. Beta-amyloid (Abeta) protein in cerebrospinal fluid as a biomarker for Alzheimer’s disease. Peptides 2002;23(7):1205–1214. [DOI] [PubMed]
- 45.Meyer JM, Ginsburg GS. The path to personalized medicine. Curr Opin Chem Biol. 2002;6(4):434–438. [DOI] [PubMed]
- 46.Pitot HC. The host-tumor relationship. In: Pitot HC, ed. Fundamentals of Oncology. New York: Marcel Dekker, Inc.; 2002:743–781.
- 47.Franceschi S, Bidoli E. The epidemiology of lung cancer. Ann Oncol. 1999;10(Suppl 5):S3–S6. [DOI] [PubMed]
- 48.Morello-Frosch R, Pastor M, Porras C, Sadd J. Environmental justice and regional inequality in southern California: Implications for future research. Environ Health Perspect. 2002;110(Suppl 2):149–154. [DOI] [PMC free article] [PubMed]
- 49.Kinney P, Chillrud S, Ramstrom S, Ross J, Stansfeld SA. Exposure to multiple air toxics in New York city. Environ Health Perspect. 2002;110(Suppl 4):539–546. [DOI] [PMC free article] [PubMed]
- 50.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91(14):1194–1210. [DOI] [PubMed]
- 51.Benowitz NL. Smoking and occupational health. In: Ladou J, ed. Occupational and Environmental Medicine. Stamford: Appleton and Lange; 1997:713–722.
- 52.Fielding JE, Husten CG, Eriksen MP. Tobacco: health effects and control. In: Wallace RB, ed. Public Health and Preventive Medicine. Stamford, CT: Appleton and Lange; 1998:817–846.
- 53.Kyle A, Woodruff T, Buffler P, Davis D. Use of an index to reflect the aggregate burden of long-term exposure to criteria air pollutants in the United States. Environ Health Perspect. 2002;110(Suppl 1):95–102. [DOI] [PMC free article] [PubMed]
- 54.Sorenson M, Autrup H, Hertel O, Wallin H, Knudson E, Logan RA. Personal exposure to PM2.5 and biomarkers of DNA damage. Cancer Epidemiol Biomarkers Prev. 2003;12:191h–196h. [PubMed]
- 55.Belinsky SA, Snow SS, Nikula KJ, Finch GL, Tellez CS, Palmisano WA. Aberrant CpG island methylation of the p16(INK4a) and estrogen receptor genes in rat lung tumors induced by particulate carcinogens. Carcinogenesis 2002;23(2):335–339. [DOI] [PubMed]
- 56.Zmirou D, Masclet P, Boudet C, Dor F, Dechenaux J. Personal exposure to atmospheric polycyclic aromatic hydrocarbons in a general adult population and lung cancer risk assessment. J Occup Environ Med. 2000;42(2):121–126. [DOI] [PubMed]
- 57.Pope C, Burnett R, Thun M et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(1132):1141. [DOI] [PMC free article] [PubMed]
- 58.Zhao B, Seow A, Lee EJ et al. Dietary isothiocyanates, glutathione S-transferase-M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2001;10(10):1063–1067. [PubMed]
- 59.Yang CS, Chhabra SK, Hong JY, Smith TJ. Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. J Nutr. 2001;131(3s):1041S–1045S. [DOI] [PubMed]
- 60.Weisburger JH, Chung FL. Mechanisms of chronic disease causation by nutritional factors and tobacco products and their prevention by tea polyphenols. Food Chem Toxicol. 2002;40(8):1145–1154. [DOI] [PubMed]
- 61.Morland K, Wing S, Diez RA. The contextual effect of the local food environment on residents’ diets: the atherosclerosis risk in communities study. Am J Public Health. 2002;92(11):1761–1767. [DOI] [PMC free article] [PubMed]
- 62.LaVeist TA, Wallace JM, Jr. Health risk and inequitable distribution of liquor stores in African American neighborhood. Soc Sci Med. 2000;51(4):613–617. [DOI] [PubMed]
- 63.Alexandrov K, Cascorbi I, Rojas M, Bouvier G, Kriek E, Bartsch H. CYP1A1 and GSTM1 genotypes affect benzo[a]pyrene DNA adducts in smokers’ lung: comparison with aromatic/hydrophobic adduct formation. Carcinogenesis. 2002;23(12):1969–1977. [DOI] [PubMed]
- 64.Haugen A, Ryberg D, Mollerup S, Zienolddiny S, Skaug V, Svendsrud DH. Gene–environment interactions in human lung cancer. Toxicol Lett. 2000;112–113:233–237. [DOI] [PubMed]
- 65.Miller DP, Liu G, De Vivo I et al. Combinations of the variant genotypes of GSTP1, GSTM1, and p53 are associated with an increased lung cancer risk. Cancer Res. 2002;62(10):2819–2823. [PubMed]
- 66.Lewis SJ, Cherry NM, Niven RM, Barber PV, Povey AC. GSTM1, GSTT1 and GSTP1 polymorphisms and lung cancer risk. Cancer Lett. 2002;180(2):165–171. [DOI] [PubMed]
- 67.Kamataki T, Nunoya K, Sakai Y, Kushida H, Fujita K. Genetic polymorphism of CYP2A6 in relation to cancer. Mutat Res. 1999;428(1–2):125–130. [DOI] [PubMed]
- 68.Sunaga N, Kohno T, Yanagitani N et al. Contribution of the NQO1 and GSTT1 polymorphisms to lung adenocarcinoma susceptibility. Cancer Epidemiol Biomarkers Prev. 2002;11(8):730–738. [PubMed]
- 69.Song N, Tan W, Xing D, Lin D. CYP 1A1 polymorphism and risk of lung cancer in relation to tobacco smoking: a case-control study in China. Carcinogenesis. 2001;22(1):11–16. [DOI] [PubMed]
- 70.Itoga S, Nomura F, Makino Y, Tomonaga T, Shimada H, Ochiai T et al. Tandem repeat polymorphism of the CYP2E1 gene: an association study with esophageal cancer and lung cancer. Alcohol Clin Exp Res. 2002;26(8 Suppl):15S–19S. [DOI] [PubMed]
- 71.Shields PG. Molecular epidemiology of lung cancer. Ann Oncol. 1999;10(Suppl 5):S7–11. [DOI] [PubMed]
- 72.Samet JM. What can we expect from epidemiologic studies of chemical mixtures? Toxicology. 1995;105:307–314. [DOI] [PubMed]
- 73.Feron V, Groten J. Toxicologic evaluation of chemical mixtures. Food Chem Toxicol. 2002;40:825–839. [DOI] [PubMed]
- 74.Mauderly J. Toxicological approaches to complex mixtures. Environ Health Perspect. 1993;101(Suppl 4):155–165. [DOI] [PMC free article] [PubMed]
- 75.Pitot HC. Fundamentals of Oncology, 4th edn. New York, NY: Marcel Dekker, Inc.; 2002.
- 76.Medelsohn J, Howley PM, Isreal MA, Liotta LA. The Molecular Basis of Cancer, 2nd edn. Philadelphia, PA: WB Saunders; 2001.
- 77.Terry L. Smoking and Health: A report of the Surgeon General. USA: DHHS; 1964.
- 78.Zochbauer-Muller S, Gazdar AF, Minna JD. Molecular pathogenesis of lung cancer. Annu Rev Physiol. 2002;64:681–708. [DOI] [PubMed]
- 79.Schuller HM. Mechanisms of smoking-related lung and pancreatic adenocarcinoma development. Nat Rev Cancer. 2002;2(6):455–463. [DOI] [PubMed]
- 80.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23(6):907–922. [DOI] [PubMed]
- 81.Pastorino U, Berrino F, Gervasio A, Pesenti V, Riboli E, Crosignani P. Proportion of lung cancers due to occupational exposure. Int J Cancer. 1984;33(2):231–237. [DOI] [PubMed]
- 82.Archer VE, Wagoner JK, Lundin FE. Lung cancer among uranium miners in the United States. Health Phys. 1973;25(4):351–371. [DOI] [PubMed]
- 83.Cancer Facts and Figures 2003. Washington, DC: American Cancer Society; 2003.
- 84.National Center for Health Statistics. Early Release of Selected Estimates Based on Data from the 2001 National Health Interview Survey. Available at http://www.cdc.gov/nchs/about/major/nhis/released200207.htm#early. USA: CDC, National Center for Health Statistics; 2003.
- 85.Garfinkel L. Trends in cigarette smoking in the United States. Prev Med. 1997;26(4):447–450. [DOI] [PubMed]
- 86.Zang EA, Wynder EL. Smoking trends in the United States between 1969 and 1995 based on patients hospitalized with non-smoking-related diseases. Prev Med. 1998;27(6):854–861. [DOI] [PubMed]
- 87.Murray LR. Sick and tired of being sick and tired: scientific evidence, methods, and research implications for racial and ethnic disparities in occupational health. Am J Public Health. 2003;93(2):221–226. [DOI] [PMC free article] [PubMed]