In the heart, depolarization during the action potential activates voltage-dependent Ca2+ channels that mediate a small, localized Ca2+ influx (ICa). This small Ca2+ signal activates specialized Ca2+ release channels, the ryanodine receptors (RyRs), in the sarcoplasmic reticulum (SR). This process is called Ca2+-induced Ca2+ release (CICR). Intuitively, the CICR process should be self-regenerating because the Ca2+ released from the SR should feedback and activate further SR Ca2+ release. However, the CICR process is precisely controlled in the heart and, consequently, some sort of negative control mechanism(s) must exist to counter the inherent positive feedback of the CICR process. Defining the nature of this negative control has been a focus of investigation for decades. Several mechanisms have been suggested including all of the following: Ca2+-dependent inactivation, adaptation, stochastic attrition, “fateful” inactivation, SR Ca2+ depletion, and coupled RyR gating. These mechanisms are generally regarded as being mutually exclusive (i.e., alternative). An emerging and more sophisticated view is that the required negative control is probably provided by a synergy of mechanisms, not a single mechanism.
In this perspective, we focus on the origin of Ca2+-dependent inactivation and adaptation of single cardiac RyR channels. Specific concerns about the adaptation phenomenon are addressed and a comprehensive unifying view of RyR Ca2+ regulation is forwarded. We conclude that the steady-state Ca2+ dependence, high Ca2+ inactivation and low Ca2+ adaptation are three distinct manifestations of the same underlying mechanism, Ca2+-dependent modal RyR channel gating.
Ca2+ -dependent Inactivation
Fabiato 1985 was the first to propose the existence of Ca2+-dependent inactivation. He proposed that inactivation of SR Ca2+ release is due to slow Ca2+ binding to a high affinity inactivation site on the SR Ca2+ release channel. He showed, in a skinned cardiac cell preparation, that SR Ca2+ release was substantially inactivated at steady-state Ca2+ concentrations as low as 60 nM. He argued that this conventional absorbing Ca2+-dependent inactivation leaves the SR Ca2+ release process refractory. Recovery from this refractory state requires removal of the Ca2+ stimulus and time. One important tenet of conventional Ca2+-dependent inactivation is that it should be evident in both the stationary and nonstationary behavior of the channel. Another important tenet of conventional inactivation is that it will be essentially an all-or-none process (i.e., any channel will be either inactivated or not) at the single channel level. Thus, a second incremental Ca2+ stimulus would not be expected to reactivate an inactivated (i.e., refractory) channel regardless of stimulus amplitude or duration.
Historically, studies on intact and permeabilized cells present contradictory evidence concerning the existence of Ca2+-dependent inactivation (Fabiato 1985; Nabauer and Morad 1992). More recently, Lukyanenko and Györke 1999 showed that elevation of resting Ca2+ levels increased the frequency of spontaneous Ca2+ sparks, which are thought to arise from bursts of SR Ca2+ release channel activity. The conclusion was that SR Ca2+ release (RyR) channel activity in cells is not depressed by a high affinity Ca2+-dependent inactivation. There are also no signs of high affinity Ca2+-dependent inactivation (e.g., occurring at <0.1 mM Ca2+) in studies of steady-state single RyR channel behavior (e.g., Györke and Fill 1993; Zahradníková and Zahradník 1995). Interestingly, there is evidence supporting the existence of low affinity inactivation that occurs at very high Ca2+ concentrations (>1 mM; Laver et al. 1995). It is not clear whether such high cytoplasmic Ca2+ concentrations are ever reached in the cell.
Ca2 +-dependent Adaptation
Györke and Fill 1993 were the first to explore the complex dynamics of RyR channel Ca2+ regulation. They showed that fast Ca2+ stimuli rapidly activated (τ ∼1 ms) single RyR channels in planar bilayers to a high open probability (Po) level. The Po then slowly and spontaneously decayed. This was surprising because the Ca2+ stimuli generated a sustained Ca2+ elevation of ∼1 μM, and there was no evidence of steady-state Ca2+-dependent inactivation at that Ca2+ concentration. To test if the spontaneous decay was mediated by conventional Ca2+-dependent inactivation, Györke and Fill 1993 applied a second incremental Ca2+ stimulus to the apparently “inactivated” RyR channels. The second Ca2+ stimulus reactivated the apparently inactivated channels. These data suggested that the spontaneous decay was mediated by some unknown mechanism. This unknown mechanism was not apparent at steady-state and did not leave the channel refractory. Györke and Fill 1993 coined the term adaptation to describe this unexpected RyR channel behavior. Valdivia et al. 1995 confirmed the rate and extent of adaptation using a similar method. There is now substantial experimental evidence that adaptation is a manifestation of Ca2+-dependent modal gating (for details see RyR Adaptation and Modal Gating).
The Györke and Fill 1993 RyR adaptation hypothesis was controversial. Lamb et al. 1994 suggested that the decrease in channel activity might just reflect slow RyR deactivation following the very fast, large Ca2+ spike that was present on the leading edge of the applied Ca2+ stimuli. The merits of this theoretical concern were experimentally addressed. These studies indicate that the fast Ca2+ spike has no impact on the much slower (1,000-fold slower) adaptation phenomenon. They also indicate that the impact of the fast Ca2+ spike is limited to accelerating the closed to open transition of the channel (i.e., essentially super-charging the Ca2+ stimuli). The evidence supporting this conclusion is described later in Ca2 + Spike Concern.
The nonstationary dynamics of RyR Ca2+ regulation have also been explored using other methodologies (Schiefer et al. 1995; Sitsapesan et al. 1995; Laver and Curtis 1996; Laver and Lamb 1998). These studies also report a relatively fast Ca2+ activation followed by a slow spontaneous decay in channel activity. However, the slow spontaneous decay is interpreted as conventional Ca2+-dependent inactivation, not adaptation. Some investigators have evoked the original Lamb et al. 1994 concern to suggest that inactivation, not adaptation, warrants further investigation. We disagree and suggest that our focus should be on reconciling the diverse RyR data sets based on their intrinsic empirical value instead of simply defending a particular interpretation. The merits of each view are discussed later in Mechanical Solution Change Studies.
RyR Adaptation and Modal Gating
Under steady-state conditions, the opening of single RyR channels occurs in bursts. These bursts fall into two categories (i.e., high and low open probability; Po). These bursts do not occur randomly, but, instead, they are temporally clustered into distinct modes of RyR channel gating (i.e., high- and low-Po modes). Several groups have now reported the existence of modal RyR channel gating (Zahradníková and Zahradník 1995; Armisén et al. 1996; Villalba-Galea et al. 1998; Saftenku et al. 2000).
Stationary single RyR channel activity exhibits three distinct gating modes (Zahradníková and Zahradník 1995). At intermediate Ca2+ concentrations (∼10 μM), the high Po mode (H-mode) is characterized by periods of frequent and long openings. The low Po mode (L-mode) is marked by periods of infrequent and short openings. The inactivated mode (I-mode) corresponds to periods where no opening events occur. A simple Markovian model of RyR modal gating was proposed by Zahradníková and Zahradník 1996 that predicts that upon a small fast Ca2+ elevation the Po of a RyR channel residing in the H-mode will rapidly increase before the channel has time to relax to a new equilibrium between all three modes. The result is a rapid rise of channel activity that spontaneously decays over time. A second prediction is that the response of a single RyR channel to fast Ca2+ elevation will depend on which mode the channel happens to be in at the moment the stimulus is applied. In other words, a single channel fluctuates spontaneously between modes with different capacities to respond to a Ca2+ stimulus. For example, if a channel happens to be in the L- or I-mode (40% probability at 1 μM Ca), then the probability of a high Po response will be reduced to ∼17% because of the relatively long dwell times of these modes. Another prediction is that a single RyR channel will respond transiently and repeatedly to small fast incremental Ca2+ elevations. This realization lead Zahradníková and Zahradník 1996 to propose that modal gating may explain the adaptation phenomenon observed by Györke and Fill 1993. For years, this proposal was based on theory, not experimental evidence. Recently, Zahradníková et al. 1999a defined the evolving temporal correlations between the three RyR gating modes during the adaptation phenomenon and experimentally established that modal gating and the adaptation phenomenon are likely related.
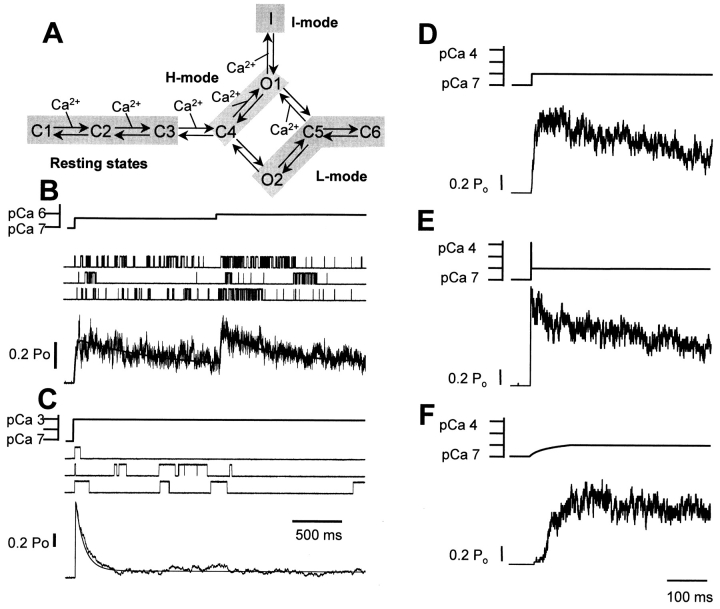
There are certain aspects of the Zahradníková and Zahradník 1996 model, however, that do not entirely reproduce the adaptation phenomenon. Our view is that these discrepancies indicate that the modal gating model needs refinement. Others have viewed these discrepancies as evidence against adaptation. Villalba-Galea et al. 1998 refined and expanded the original Zahradníková and Zahradník 1996 modal gating model to better reproduce various aspects of RyR gating behavior. The Villalba-Galea et al. 1998 form of the model incorporates an additional Ca2+-dependent transition that shifts channel gating to the H-mode as the amplitude of the Ca2+ perturbation becomes greater. In addition, the model includes a Ca2+-dependent transition to the inactivated state to better reproduce high Ca2+ (>1 mM) inhibition. The specific Markovian model presented by Villalba-Galea et al. 1998 is illustrated in Fig. 1 A. The simulated response of a single RyR channel to Ca2+ stimuli like those applied by Györke and Fill 1993 is presented in Fig. 1 B. The Ca2+ stimuli (Fig. 1 B) did not contain the fast Ca2+ spike. The model predicts that single RyR channels display rapid activation followed by a slow, spontaneous decay in channel activity. The spontaneous decay occurs at Ca2+ levels (<1 μM) that are well below those known to induce Ca2+-dependent inactivation under steady-state conditions (e.g., >1 mM). A second incremental Ca2+ stimulus induces a second transient of channel activity. Thus, a specific kinetic scheme (Fig. 1 A) can reproduce the fundamental hallmarks of the adaptation phenomenon (Györke and Fill 1993) in the absence of the fast Ca2+ spike.
Figure 1.
(A) The modal gating kinetic scheme (Villalba-Galea et al. 1998) shows the Ca2+-dependent transitions involved in RyR activation, modal gating (C4-O1-C5 and C5-O2-C4), and inactivation (O1-I, C1-C2, C2-C3, C3-C4, and C4-O1). The rate constants for the transitions between states are listed in Table . (B) Single-channel recordings simulated using the Monte Carlo method. The Ca2+ stimulus, sample single-channel sweeps, and an ensemble record generated from 100 sweeps are shown. These simulations show that each of two consecutive fast incremental Ca2+ elevations (<1 μM) generate transient bursts of channel activity. (C) Single RyR response to a single large Ca2+ elevation to 1 mM. The Ca2+ stimulus, sample single-channel sweeps, and an ensemble record are shown. (D) Single RyR response to a fast small Ca2+ elevation to 1 μM. Only the Ca2+ stimulus and an ensemble record are shown. (E) Single RyR response to a fast small Ca2+ elevation to 1 μM with a large (100 μM) Ca2+ spike at its leading edge. Only the Ca2+ stimulus and an ensemble record are shown. (F) Single RyR response to a 100-ms ramp-like Ca2+ elevation to 1 μM. Only the Ca2+ stimulus and an ensemble record are shown.
Recently, the Williams' group has reported highly variable Ca2+-dependent modal RyR gating (Saftenku et al. 2000). They found that elevating the steady-state Ca2+ concentrations increases the Po within the L-mode and also increases the probability of the channel being in the H-mode. They suggest that this observation is inconsistent with the adaptation hypothesis. However, it is not clear, how this behavior might preclude the possibility of adaptation. The Villalba-Galea et al. 1998 model described above, in agreement with the results of Saftenku et al. 2000, clearly predicts a Ca2+-dependent increase in H-mode probability. It also predicts a Ca2+-dependent increase in Po during the H-mode, but not during the L-mode. Like the Saftenku et al. 2000 abstract, Zahradníková et al. 1999a showed a Ca2+-dependent increase in Po during the L-mode but found that this increase was significant but minor (<5% Po) at Ca2+ concentrations where the adaptation phenomenon occurs (i.e., between 0.5 and 20 μM Ca). It is the Ca2+-dependent increase in Po during the H-mode, not during the L-mode, that drives the adaptation phenomenon.
How does Ca2+-dependent modal gating work? At low Ca2+ concentrations (100 nM), the states with the highest probability of being occupied are C1, C2, and C3 and, consequently, the channel will be closed most (97%) of the time. After a small Ca2+ elevation, the channel will move initially into the C4 and O1 states, inducing long openings and short closures (H-mode), and then into the C5, O2, and C6 states, inducing short openings and long closures (L-mode). Interestingly, the larger the Ca2+ stimulus, the longer the channel spends in the H-mode. The Ca2+-dependent transition that connects C5 with O1 shifts the model to the H-mode during large Ca2+ stimuli. At high Ca2+ concentrations (e.g., >1 mM), the Ca2+-dependent transition between the O1 and I states will move the channel into the nonconducting I state (I-mode).
At any steady-state Ca2+ level, there will be a dynamic equilibrium between the high Po, low Po, and inactivated modes. This dynamic equilibrium generates the well-known bell-shaped steady-state Ca2+ dependence of the RyR channel (Laver et al. 1995). As described above, small, fast Ca2+ elevations from a low Ca2+ concentration will upset the existing dynamic equilibrium momentarily in favor of the high Po mode. Consequently, the Po rises to a level above that predicted by steady-state measurements before activity spontaneously decays as the three modes reequilibrate at the new higher Ca2+ concentration. Such suprasteady-state Po followed by a spontaneous decay has been experimentally observed previously (Györke and Fill 1993; Valdivia et al. 1995; Laver and Curtis 1996; Laver and Lamb 1998). Application of a second incremental Ca2+ elevation will induce another transient modal gating shift and a second transient of channel activity like that reported by Györke and Fill 1993. The magnitude of the transient modal gating shift (i.e., adaptation) will depend on Ca2+ stimulus speed and magnitude. Slow and large Ca2+ stimuli will be less effective at generating adaptation. Slow Ca2+ stimuli are less effective because mode reequilibration is time dependent. Large Ca2+ stimuli are less effective because the channel begins to spend time in the inactivated mode. Thus, a relatively large slow Ca2+ stimulus will induce inactivation, not adaptation. This is consistent with the experimental results of Sitsapesan et al. 1995 and Schiefer et al. 1995. If refractory behavior was a characteristic of the inactivated mode, then this modal gating scheme would predict refractory behavior only at high Ca2+ levels. Interestingly, Schiefer et al. 1995 reported clear refractory behavior at 1 mM (following a 72% spontaneous decay), but found no evidence of refractory behavior at 10 μM (after an ∼30% spontaneous decay). In cells, the RyR channel is subjected to very brief (<1 ms) large (>10 μM) Ca2+ stimuli (Stern 1992). The modal gating scheme predicts that such large, fast Ca2+ stimuli will trigger one or two brief opening events followed by fast deactivation. These predictions are also quite consistent with the experimental record (Zahradníková et al. 1999b).
Thus, modal gating reconciles a large body of apparently contradictory experimental results and, thus, provides a useful comprehensive theoretical context for understanding RyR Ca2+ regulation. In this context, RyR inactivation and adaptation should not be viewed as mutually exclusive, but rather two different manifestations of the same underlying mechanism (i.e., modal gating). However, this modal gating scheme is still deficient because the potential impact of certain physiologically important ligands (e.g., luminal Ca, cytosolic Mg, and ATP) is not considered. This deficit, and others, will have to be addressed to correlate single RyR channel data with local Ca2+ release events in cells.
The Fast Ca2+ Spike Concern
Here, the possibility that the fast Ca2+ spike drives the adaptation phenomenon is discussed. It is known that flash photolysis of certain caged Ca2+ compounds (e.g., DM-nitrophen and NP-EGTA) can generate a very fast Ca2+ overshoot (i.e., the Ca2+ spike) at the leading edge of a sustained Ca2+ elevation. Lamb et al. 1994 suggested that the adaptation phenomenon might reflect slow RyR deactivation following a fast Ca2+ spike. The suggestion was that the Ca2+ activation sites on the RyR channel are rapidly occupied during the brief Ca2+ spike (lasting ∼1 ms), resulting in fast RyR activation to a high Po level. As the Ca2+ concentration falls to a substantially lower level after the brief Ca2+ spike, the RyR channel may simply “turn off” (i.e., deactivate) very slowly as occupancy of Ca2+ activation sites falls. This concern was clearly legitimate considering the vacuum of knowledge that existed in 1994.
Lamb et al. 1994 estimated the properties of the fast Ca2+ spike using the available published properties of the Ca2+ cage complex. Györke and Fill 1994 began to experimentally evaluate the potential impact of the Ca2+ spike on the adaptation phenomenon. They reported no compelling evidence to support the theoretical contentions of Lamb et al. 1994. Subsequently, it became clear that the previous estimates of Ca2+ spike properties were wrong. The experiments of Escobar et al. 1997 revealed that the Ca2+ spike was considerably faster and smaller than previously thought. This supported the intuitive assumption of Györke and Fill 1994 that it was very unlikely that such a brief Ca2+ signal (lasting <0.2 ms) could drive the slow adaptation phenomenon (lasting >1,000 ms). Another interesting insight gained from the Escobar et al. 1997 study was that the fast Ca2+ spike is actually a reasonable representation of the fast Ca2+ signals that may occur in the dyadic cleft during openings of single dihydropyridine receptor (DHPR) channels (Stern 1992). This latter insight prompted Zahradníková et al. 1999b to further explore the potential impact of the fast Ca2+ spikes on single RyR channel activity. They improved the experimental recording conditions (i.e., increasing bandwidth, minimizing flash artifacts, etc.), so that they could define the impact of a Ca2+ spike alone (with little or no sustained Ca2+ elevation) during the first few milliseconds following its application. The rationale was that bandwidth and flash artifacts may have masked the impact of the Ca2+ spike in previous studies (Györke and Fill 1993; Györke et al. 1994; Valdivia et al. 1995). They showed that a fast Ca2+ spike alone, albeit slightly smaller than those applied previously, induced one or two brief opening events during the first few milliseconds after the flash. When Zahradníková et al. 1999b duplicated the Györke and Fill 1993 stimuli (i.e., spike plus sustained Ca2+ elevation), the classical adaptation phenomenon was observed. Thus, the RyR channel's response to a Ca2+ spike alone is dramatically different than its response to af Ca2+ spike followed by a sustained Ca2+ elevation. Zahradníková et al. 1999b concluded that Ca2+ spikes are simply too brief to drive the very slow adaptation phenomenon.
Lamb et al. 2000 have a different interpretation of the Zahradníková et al. 1999b data with adaptation again being driven by the Ca2+ spike and not by the sustained Ca2+ elevation. This interpretation is based, in part, on the fact that the Zahradníková et al. 1999b Ca2+ spikes were somewhat smaller (≤30 μM peak or estimated Ca2+ time integrals of 2–7 μMms) than those applied by Györke and Fill 1993(30–60 μM peaks or estimated Ca2+ time integrals of 8–30 μMms). The Lamb group suggests that the Zahradníková et al. 1999b data simply show that there is some sort of critical threshold for the Ca2+ spike to induce the adaptation phenomenon. In other words, the RyR channel distinguishes a Ca2+ spike peaking at 40 μM (with a Ca2+ time integral of 8 μMms) from one peaking at 30 μM (with a Ca2+ time integral of 6 μMms) and its response to each is different (slow or fast deactivation, respectively). They speculate that the action of large Ca2+ spikes is mediated by some hypothetical very low affinity Ca2+ binding sites that are preferentially occupied only during large Ca2+ spikes. However, this new hypothesis is inconsistent with common kinetic principles of receptor–ligand interaction and directly contradicts existing experimental evidence regarding RyR behavior.
Common kinetic principles dictate that very low affinity Ca2+ binding sites typically have fast kinetics (conversely high affinity sites typically have slow kinetics). It is difficult to understand how the hypothetical low affinity sites could mediate a very slow phenomenon like adaptation. In numerical terms, the low affinity sites would have a K d near 50 μM and an off-rate of 1 s−1 (i.e., the published time constant of RyR adaptation). Consequently, the probability of that site being occupied during a fast 50-μM Ca2+ spike lasting <1 ms will be <0.001; thus, the spike is unlikely to have a dramatic impact on such a site (Escobar et al. 1997). Additionally, the probability of a state of being occupied in any kinetic reaction is typically described by a probability distribution and not by a particular triggering level. Lamb et al. 2000(this issue) acknowledge the difficulty of explaining their new hypothesis in specific kinetic terms, and offer no specific kinetic scheme to support their point of view. Instead, they simply suggest that large Ca2+ spikes must drive the RyR channel into some unusual configuration and/or induce some unknown mechanisms. On the other hand, we have forwarded a specific and relatively simple Markovian gating scheme (Fig. 1) that describes adaptive behavior arising from Ca2+-dependent modal gating changes driven by the sustained component of the photolytic Ca2+ stimuli.
As described earlier, the Lamb et al. 2000 predicts that adaptation of a single RyR channel occurs when some critical Ca2+ spike threshold is exceeded (i.e., ≥30 μM peak or Ca2+ time integral ≥8 μMms). However, there is experimental evidence that suggest otherwise. Györke and Fill 1993 and Valdivia et al. 1995 used the same method but different caged-Ca2+ compounds, DM-nitrophen and NP-EGTA, respectively. Consequently, the applied Ca2+ stimuli had a similar sustained Ca2+ elevation, but fast Ca2+ spikes of dramatically different amplitude (and time integral). The similarity of RyR adaptation in both works suggested that adaptation is not highly dependent on subtle features of the fast Ca2+ spike. More recently, our group (Zahradníková, A., and S. Györke, unpublished results) has also explored the impact of large Ca2+ spikes on single RyR channel function. In this work, large Ca2+ spikes (80–150 μM peak, Ca2+ time integrals of up to 15 μMms) were generated by photolysis of NP-EGTA (3 mM). The large Ca2+ spikes triggered only solitary RyR openings, not the slow adaptation phenomenon. The openings were reminiscent of the spike-activated events previously reported by Zahradníková et al. 1999b. Thus, it does not appear that adaptation depends on the amplitude or Ca-time integral of the fast Ca2+ spike.
The premise that the RyR channel undergoes slow deactivation (lasting seconds) after the fast Ca2+ spike is also inconsistent with existing experimental evidence. The rate of RyR deactivation in response to a fast Ca2+ reduction has been measured (Schiefer et al. 1995; Vélez et al. 1997; Zahradníková et al. 1999b). These experimental data show that RyR deactivation from high Ca2+ concentrations is fast (τ's <10 ms), not slow (lasting seconds). Thus, the slow adaptation process is not likely due to very slow RyR deactivation.
What then is the impact of the Ca2+ spike on RyR channel function? The fast Ca2+ spike essentially supercharged the Ca2+ stimulus in the flash photolysis studies (Györke and Fill 1993). Its impact is best illustrated using the RyR scheme presented above. Simulated RyR channel response to a fast Ca2+ elevation with and without a fast Ca2+ spike at its leading edge is shown in Fig. 1E and Fig. D, respectively. The sustained component of the Ca2+ stimulus elevated Ca2+ from 0.1 to 1.0 μM. The fast Ca2+ spike peaked at ∼50 μM and lasted 0.2 ms. The rate and extent of the slow spontaneous decay (i.e., adaptation) is identical in the presence or absence of the fast Ca2+ spike. The presence of the fast Ca2+ spike, however, introduces a brief peak in channel activity that rapidly decays (i.e., deactivates). This brief peak is generated by the spike-activated events as described experimentally by Zahradníková et al. 1999b. In essence, the impact of the fast Ca2+ spike is limited to accelerating the closed to open transition of the channel. It does not impact the rate or extent of the much slower adaptation phenomenon. This is consistent with the original assumption of Györke and Fill 1993 that the fast Ca2+ spike is simply too fast to impact the adaptation phenomenon.
Mechanical Solution Change Studies
Several studies have explored the complex dynamics of single RyR channel Ca2+ regulation using the flash photolysis methodology (Györke and Fill 1993; Györke et al. 1994; Valdivia et al. 1995; Vélez et al. 1997; Zahradníková et al. 1999b). The data are quite consistent between studies, and are generally interpreted in terms of adaptation, not inactivation. Other studies have explored the dynamics of RyR Ca2+ regulation using Ca2+ stimuli generated by different mechanical solution change methodologies (Schiefer et al. 1995; Sitsapesan et al. 1995; Laver and Curtis 1996). These studies report RyR activation followed by either a slow spontaneous decay or no decay at all. When RyR channel activity spontaneously decayed, the decay was interpreted as inactivation, not adaptation. This interpretation and the absence of fast Ca2+ spikes in these latter studies have been construed as evidence against the adaptation phenomenon.
The following questions are addressed below. First, are the mechanical solution change data internally consistent enough to dismiss data collected using a different methodology? Second, are the stimuli in the mechanical solution change studies “true” [Ca2+] steps and the only ones suitable for defining the nonstationary behavior of RyR? Finally, is conventional Ca2+-dependent inactivation the only mechanism consistent with the mechanical solution change data? We believe the answer to each of these questions is no.
Three different mechanical solution change strategies have been applied to study single RyR channel function in planar bilayers (Schiefer et al. 1995; Sitsapesan et al. 1995; Laver and Curtis 1996; Laver and Lamb 1998). Schiefer et al. 1995 used a piezo-based method and achieved complete solution exchange over a 1-ms period. Sitsapesan et al. 1995 used a solenoid-based method that exchanged solutions over a period of 10 ms. Laver and Curtis 1996 and Laver and Lamb 1998 used a “puffing” method that exchanged solutions over a period ranging from 20 to 110 ms. Each of these mechanical solution change studies also applied different size Ca2+ stimuli. Some applied small Ca2+ stimuli (<10 μM; Schiefer et al. 1995, Laver and Curtis 1996). All applied very large Ca2+ stimuli (≥100 μM; Schiefer et al. 1995; Sitsapesan et al. 1995; Laver and Curtis 1996; Laver and Lamb 1998). The reported rates of Ca2+ activation varied from 0.2 to 20 ms, whereas the reported rates of inactivation varied from 200 to 15,000 ms. Inactivation was highly voltage-dependent in one study (Sitsapesan et al. 1995), but was voltage-independent in another (Schiefer et al. 1995). Two of the studies report inactivation only at Ca2+ concentrations (≥100 μM) known to inactivate channels under steady-state conditions (Schiefer et al. 1995; Sitsapesan et al. 1995). Two other studies report inactivation at Ca2+ concentrations (1 μM) that do not inactivate channels under steady-state conditions (Laver and Curtis 1996; Laver and Lamb 1998). The variability in results between the different solution exchange studies probably reflects the different methodologies used and ultimately the types of Ca2+ stimuli applied (see below). Another potential contributing factor may be a limited sample size in some cases (Schiefer et al. 1995; Laver and Lamb 1998). We suggest that the data presented in the solution exchange studies is important, but is not internally consistent enough to categorically dismiss results collected using a different methodology.
Recently, studies of RyR Ca2+ dynamics performed with improved time resolution show that RyR Ca2+ activation is very fast (activation time constant 0.1 ms; Zahradníková et al. 1999b). Such a rapid response time is consistent with the local control theory (Stern 1992). In vivo, the RyR channel may be activated by very fast, brief local Ca2+ changes associated with single openings of a DHPR channel. Considering the fast kinetics of RyR channel Ca2+ activation, the Ca2+ stimuli applied by the mechanical solution change studies described above cannot be regarded as true instantaneous Ca2+ steps. The possible exception is the Schiefer et al. 1995 study. However, the other studies applied relatively slow Ca2+ changes that required 10–110 ms to complete due to considering the large unstirred layers that exist immediately adjacent to the bilayer (Laver and Curtis 1996). Consequently, the Ca2+ stimuli applied in all but one of the mechanical solution change studies can be more accurately described as Ca2+ ramps instead of steps. The point is that the same critical scrutiny that was applied to the photolytic Ca2+ stimuli has not yet been applied to the mechanical Ca2+ stimuli.
The dynamics of RyR channel behavior in response to a very fast Ca2+ step or a relatively slow Ca2+ ramp are likely to be different. The potential impact of Ca2+ stimulus speed on single RyR channel gating can be seen in simulated channel data presented in Fig. 1. Not surprisingly, RyR behavior to a fast Ca2+ step (rise time ≤100 μs) or a ramp-like Ca2+ stimulus (rise time 100 ms; Laver and Curtis 1996) was quite different. Changes in Ca2+ stimulus speed impacted both Ca2+ activation and spontaneous decay (Fig. 1, D–F). The point is that Ca2+ stimuli with such slow rise times (10 to 100 ms) can have rather dramatic impact on RyR channel function. This may explain the tight parallel between the dynamic and stationary behavior of single RyR channels in certain mechanical solution change studies (Sitsapesan et al. 1995). The high sensitivity to Ca2+ stimulus speed (Zahradníková et al. 1999b) and the very fast Ca2+ stimuli thought to occur in vivo (Stern 1992) suggest that the relatively slow Ca2+ stimuli available in most mechanical solution change studies may not be appropriate to study the dynamics of RyR regulation especially in the physiologically relevant range of Ca2+ stimuli rates (<1 ms).
We also believe that the mechanical solution change results provide evidence for both conventional inactivation and adaptation. Schiefer et al. 1995 and Sitsapesan et al. 1995 report a spontaneous decay in RyR activity at Ca2+ concentrations (i.e., ≥100 μM) where inactivation is observed under steady-state conditions. Thus, we agree that it is appropriate to conclude that the spontaneous decay was due to inactivation. In other cases, it is more difficult to justify the inactivation interpretation. For example, Laver and Curtis 1996(see their figure 3B) report that RyR activity decays following a Ca2+ elevation to 1 μM Ca, a Ca2+ concentration where there is no evidence of inactivation under steady-state conditions. Laver and Curtis also report that the peak Po reached was well above that expected under steady-state conditions (see Figure 4 in Laver and Curtis 1996). This unusual behavior is difficult to understand if the decay was due to conventional inactivation. More recently, Laver and Lamb 1998 applied a 1-μM Ca2+ “puff” to induce a burst of RyR channel activity that decayed over time. They applied a second sustained Ca2+ elevation to 100 μM after the spontaneous decay in channel activity was complete. The single RyR channel did not reactivate in the presence of 100 μM Ca. Laver and Lamb 1998 interprets these results as evidence that the RyR channel becomes refractory in response to the 1-μM Ca2+ stimulus, and suggests that conventional Ca2+ inactivation regulates single RyR channels at such low Ca2+ concentrations. However, this interpretation is inconsistent with the classical steady-state Ca2+ dependence of the RyR channel. First, there is no evidence of conventional inactivation at 1 μM Ca2+ under steady-state conditions. Second, there is substantial spontaneous RyR channel activity at 100 μM Ca2+ and, thus, the channel should have reactivated at some point during their second sustained Ca2+ stimulus. It is difficult to understand how the spontaneous decay at 1 μM Ca2+ could be due to conventional inactivation. Instead, we suggest that a spontaneous shift to a low (or zero) Po mode may have been mistaken for conventional absorbing inactivation.
Schiefer et al. 1995 report a nonrefractory decrease in Po after 500 ms steps to 1–100 μM Ca. There was no or only a barely detectable decrease of Po (<10%) for steps to 0.3–3 μM Ca. However, several of their observations are consistent with the RyR adaptation hypothesis. First, they reported a K d for Ca2+ activation of 0.7 μM. This is closer to the 0.3-μM K d reported for the adaptation response (Györke and Fill 1993) than the 1.5–4-μM K d reported under steady-state conditions (Györke and Fill 1993; Györke et al. 1994; Zahradníková and Zahradník 1995; Laver et al. 1995). Second, Schiefer et al. 1995 observed only a single open time component with a time constant of 3 ms. This is similar to that reported for H-mode openings (Zahradníková and Zahradník 1995; Zahradníková et al. 1999a), openings during the first phase of adaptation (Zahradníková et al. 1999a), as well as solitary spike-activated openings (Zahradníková et al. 1999b). Under steady-state conditions, the RyR channel displays at least two distinct open times (Laver et al. 1995; Zahradníková and Zahradník 1995; Armisén et al. 1996; Laver and Lamb 1998). Third, Schiefer et al. 1995 estimated a Po of 0.2 at 0.3 μM Ca. This is considerably higher than the Po (<0.05) reported under steady-state conditions (Györke and Fill 1993; Laver et al. 1995), but quite close to the peak Po (∼0.3) after Ca2+ elevation to 0.5 μM Ca2+ (Zahradníková et al. 1999a). Lack of refractory behavior at low Ca, the K d for Ca2+ activation, the single open time constant, and the high Po can all be easily explained evoking the adaptation hypothesis.
In summary, the mechanical solution change studies present clear evidence supporting the existence of inactivation at high Ca2+ concentrations (i.e., ≥100 μM). However, these same studies also present data that are consistent with adaptation at low Ca2+ concentrations (<10 μM). We believe that the mechanical solution change studies show evidence that inactivation and adaptation coexist. In any event, the dynamics of RyR Ca2+ regulation are complex and simple conventional interpretations are likely to be insufficient.
Correlating Single RyR Behavior to Ca2+ Release Phenomena in Cells
The identity of the negative control mechanism(s) that counters the inherent positive feedback of CICR in heart has been debated over the last 15 years. Two candidate mechanisms are conventional Ca2+-dependent inactivation and adaptation. However, it appears that neither of these mechanisms alone is sufficient to explain the properties of CICR in cells. It is clear that a more comprehensive understanding of the mechanisms that govern intracellular Ca2+ release is required.
Fabiato 1985 was the first to propose that Ca2+-dependent inactivation is the negative control mechanism that regulates CICR in the heart. This proposal was controversial because early patch-clamp studies found no evidence of inactivation of cell-averaged (global) Ca2+ release in experiments using a conventional two-pulse protocol. Subsequent patch-clamp studies, using a sustained trigger Ca2+ stimulus, showed that SR Ca2+ release does, indeed, “turn-off” (Yasui et al. 1994). Interestingly, the “turned-off” Ca2+ release could be reactivated by a further increase in the trigger Ca2+ stimulus. Other groups have reported such paradoxical behavior in variety of preparations (e.g., Pizarro et al. 1997) and found it to be analogous to quantal or incremental Ca2+ release observed from IP3-sensitive Ca2+ stores. This is interesting because the RyR and IP3-receptor are homologous intracellular Ca2+ release channels. Each IP3 increment of an incremental IP3 stimulus induces a small transient Ca2+ release. The mechanism that turns-off Ca2+ release after an incremental IP3 stimulus is not desensitization because a second incremental IP3 stimulus could reactivate Ca2+ release. This property of IP3-sensitive Ca2+ stores is interesting here for the following two reasons: (1) it is reminiscent of the RyR adaptation phenomenon; and (2) it could be related to the apparent quantal or incremental Ca2+ release observed from ryanodine sensitive Ca2+ stores (Yasui et al. 1994; Pizarro et al. 1997). The IP3 literature has focused on two potential explanations of quantal Ca2+ release. First, quantal release phenomenon could be generated by Ca2+ stores with heterogeneous IP3 sensitivity, so that each stimulus increment recruits a new segment of the Ca2+ store population. Second, a homogenous Ca2+ store population could generate quantal release if each release was governed by an adaptation-like phenomenon. The RyR-IP3R homology, the homogeneity of IP3 binding affinity, and the existence of RyR adaptation supports the second hypothesis. Therefore, it is possible that much of the complexity of global Ca2+ signaling in cells may be attributable to properties of individual Ca2+ release channels.
Is this the case for RyR channels in heart cells? One caveat here is that the conditions that produce RyR adaptation in vitro may not exist in vivo. In the heart, the RyR channels operate in local Ca2+ microdomains, where even small Ca2+ stimuli are thought to elevate local Ca2+ concentrations to high levels (Stern 1992). Additionally, RyR channels in vivo operate in essentially all-or-none clusters (Stern 1992), where activation of one activates all. Thus, the small incremental Ca2+ stimuli, known to induce RyR adaptation in vitro, may not occur in the local control environment of the cell. A second caveat is that termination of local Ca2+ release events in cells is ∼10 times faster than both RyR adaptation (Györke and Fill 1993) and inactivation (Schiefer et al. 1995; Sitsapesan et al. 1995), as defined in bilayers (Györke and Fill 1993; Valdivia et al. 1995). Thus, the impact of RyR adaptation and/or inactivation in the heart is not yet clear.
Recently, Sham et al. 1998 extended the findings of Yasui et al. 1994 to the local Ca2+ release level. Not surprisingly, considering the caveats described above, they found only weak evidence for quantal/adaptive behavior of local Ca2+ release units. They activated a subset of local Ca2+ release units and applied a second incremental Ca2+ stimulus. The second incremental Ca2+ stimulus preferentially activated release units that had not been activated by the first stimulus. Their interpretation was that local Ca2+ release is terminated by an inactivation-like mechanism, not an adaptation-like process. However, there is one interesting problem here. Intuitively, the initial small, but sustained, Ca2+ stimulus should have continued to recruit (i.e., activate/inactivate) additional release units throughout its duration. In contrast, Sham et al. 1998 showed that the sustained Ca2+ stimulus activated/inactivated a subset of release units only at the beginning of the Ca2+ stimulus. This rather paradoxical behavior was unexplained. It appears as if local release units were sensitive to the rate of Ca2+ change during the stimulus and not necessarily stimulus amplitude. Such rate-sensing behavior has been considered a hallmark of quantal Ca2+ release behavior (Pizarro et al. 1997). The point is that unconventional behavior is difficult to reconcile with conventional mechanisms. Consequently, the Sham et al. 1998 data clearly illustrate the complexity of local SR Ca2+ release regulation more than they identify the mechanism(s) that may underlie it. Interestingly, Callamaras and Parker 2000 recently showed that such rate sensing, or quantal behavior of local Ca2+ release events may occur during IP3-dependent Ca2+ signaling in nonmuscle cells.
At this point, there is an insufficient understanding of the mechanisms that regulate local Ca2+ signaling in heart. It is becoming clear that none of the candidate negative control mechanisms by themselves is sufficient. In efforts to define the mechanisms that underlie complex Ca2+ signaling phenomena in cells, two general themes are developing. The first evolves around defining the extent to which single Ca2+ release channel gating mechanisms govern the complexity of local Ca2+ release events. We believe that defining RyR modal gating dynamics under relatively simple ionic conditions (i.e., no Mg2+ and ATP) is an important first step in this direction. The second evolves around defining how interactions between Ca2+ release channels contribute to the complexity of local Ca2+ signaling in cells. Recently, Stern et al. 1997 has forwarded a local control model of RyR-mediated Ca2+ release that predicts quantal behavior as a collective phenomenon of stochastically interacting RyR channels. This model describes Ca2+ release in the well defined spatial RyR geometry in skeletal muscle and, thus, may not fully apply to other less ordered systems. However, it shows that groups of interacting RyR channels can indeed exhibit collective phenomena (i.e., “group dynamics”) that are qualitatively different from those predicted solely from the properties of individual RyR channels.
Concluding Remarks
In this perspective, we attempt to forward the concept that there will be no simple answers to the complex questions concerning RyR-mediated Ca2+ signaling in the heart. An emphasis on reconciling and/or unifying apparently contradictory data sets is clearly the path that will advance knowledge. In this context, we believe there has been substantial progress. We suggest that Ca2+-dependent modal gating may be the mechanism that underlies the complex dynamics of single RyR channel Ca2+ regulation, and we have presented a specific kinetic modal gating scheme that reconciles a large body of apparently contradictory results. This scheme suggests that RyR adaptation and inactivation are two distinct manifestations of the same underlying mechanism (i.e., modal gating).
Table 1.
Modal Gating Scheme Rate Constants
| Transition | Value | Units | Transition | Value | Units |
|---|---|---|---|---|---|
| C1-C2 | 0.5 | mM−1ms−1 | C2-C1 | 1 | ms−1 |
| C2-C3 | 0.8 | mM−1ms−1 | C3-C2 | 0.525 | ms−1 |
| C3-C4 | 0.8 | mM−1ms−1 | C4-C3 | 0.2 | ms−1 |
| C4-O1 | 0.8 | mM−1ms−1 | O1-C4 | 0.25 | ms−1 |
| O1-C5 | 0.0025 | ms−1 | C5-O1 | 0.01 | μM−1ms−1 |
| C5-C6 | 0.5 | ms−1 | C6-C5 | 0.01 | ms−1 |
| C5-O2 | 1 | ms−1 | O2-C5 | 2 | ms−1 |
| O2-C4 | 0.001 | ms−1 | C4-O2 | 0.001 | ms−1 |
| O1-I | 0.00001 | μM−1ms−1 | I-O1 | 0.001 | ms−1 |
References
- Armisén R., Sierralta J., Vélez P., Naranjo D., Suárez-Isla B.A. Modal gating in neuronal and skeletal muscle ryanodine-sensitive Ca2+ release channels. Am. J. Physiol. 1996;271:C144–C1153. doi: 10.1152/ajpcell.1996.271.1.C144. [DOI] [PubMed] [Google Scholar]
- Callamaras N., Parker I. Phasic characteristic of elementary Ca2+ release sites underlies quantal responses to IP. EMBO (Euro. Mol. Biol. Organ.) J. 2000;19:3608–3617. doi: 10.1093/emboj/19.14.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar A.L., Velez P., Kim A.M., Cifuentes F., Fill M., Vergara J.L. Kinetic properties of DM-nitrophen and calcium indicatorsrapid transient response to flash photolysis. Pflügers Arch. 1997;434:615–631. doi: 10.1007/s004240050444. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 1985;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptationcontrol mechanism of Ca2+ -induced Ca2+ release in heart. Science. 1993;260:807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Györke S., Fill M. Ca2+-induced Ca2+ release in response to flash photolysis. Science. 1994;263:987–988. doi: 10.1126/science.263.5149.987. [DOI] [PubMed] [Google Scholar]
- Györke S., Vélez P., Suarez-Isla B., Fill M. Activation of single cardiac and skeletal ryanodine receptor channels by flash photolysis of caged Ca2+ . Biophys. J. 1994;66:1879–1886. doi: 10.1016/S0006-3495(94)80981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G.D., Fryer M.W., Stephenson D.G. Ca2+-induced Ca2+ release in response to flash photolysis. Science. 1994;263:986–988. doi: 10.1126/science.8310298. [DOI] [PubMed] [Google Scholar]
- Lamb G.D., Laver D.R., Stephenson D.G. Question about adaptation in ryanodine receptors. J. Gen. Physiol. 2000;116:883–890. doi: 10.1085/jgp.116.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D.R., Curtis B.A. Response of ryanodine receptor channels to Ca2+ steps produced by rapid solution exchange. Biophys. J. 1996;71:732–741. doi: 10.1016/S0006-3495(96)79272-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D.R., Lamb G.D. Inactivation of Ca2+ release channels (ryanodine receptors RyR1 and RyR2) with rapid steps in [Ca2+] and voltage. Biophys. J. 1998;74:2352–2364. doi: 10.1016/S0006-3495(98)77944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D.R., Roden L.D., Ahern G.P., Eager K.R., Junankar P.R., Dulhunty A.F. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J. Membr. Biol. 1995;147:7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V., Györke S. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J. Physiol. 1999;521:575–585. doi: 10.1111/j.1469-7793.1999.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabauer M., Morad M. Ca2(+)-induced Ca2+ release as examined by photolysis of caged Ca2+ in single ventricular myocytes. Am. J. Physiol. 1992;258:C189–C193. doi: 10.1152/ajpcell.1990.258.1.C189. [DOI] [PubMed] [Google Scholar]
- Pizarro G., Shirokova N., Tsugorka A., Rios E. “Quantal” calcium release operated by membrane voltage in frog skeletal muscle. J. Physiol. 1997;501:289–303. doi: 10.1111/j.1469-7793.1997.289bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftenku E., Williams A.J., Sitsapesan R. Ca2+-dependence of low and high activity levels of cardiac ryanodine receptor channels Biophys. J. 78 2000. 312A(Abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer A., Meissner G., Isenberg G. Ca2+ activation and Ca2+ inactivation of canine reconstituted cardiac sarcoplasmic reticulum Ca2+-release channels. J. Physiol. 1995;489:337–348. doi: 10.1113/jphysiol.1995.sp021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham J.S., Song L.S., Chen Y., Deng L.H., Stern M.D., Lakatta E.G., Cheng H. Termination of Ca2+ release by a local inactivation of ryanodine receptors in cardiac myocytes. Proc. Natl. Acad. Sci. USA. 1998;95:15096–15101. doi: 10.1073/pnas.95.25.15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R., Montgomery R.A., Williams A.J. New insights into the gating mechanisms of cardiac ryanodine receptors revealed by rapid changes in ligand concentration. Circ. Res. 1995;77:765–772. doi: 10.1161/01.res.77.4.765. [DOI] [PubMed] [Google Scholar]
- Stern M.D. Theory of excitation-contraction coupling in cardiac muscle. Biophys. J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D., Pizarro G., Rios E. Local control model of excitation-contraction coupling in skeletal muscle. J. Gen. Physiol. 1997;110:415–440. doi: 10.1085/jgp.110.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia H., Kaplan J.H., Ellis-Davies G.C.R., Lederer W.J. Rapid adaptation of cardiac ryanodine receptorsmodulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez P., Györke S., Escobar A.L., Vergara J., Fill M. Adaptation of single cardiac ryanodine receptor channels. Biophys. J. 1997;72:691–697. doi: 10.1016/s0006-3495(97)78705-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Galea C.A., Suarez-Isla B.A., Fill M., Escobar A.L. Kinetic model for ryanodine receptor adaptation Biophys. J. 74 1998. A58(Abstr.) [Google Scholar]
- Yasui K., Palade P., Györke S. Negative control mechanism with features of adaptation controls Ca2+ release in cardiac myocytes. Biophys. J. 1994;67:457–460. doi: 10.1016/S0006-3495(94)80501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradníková A., Zahradník I. Description of modal gating of the cardiac calcium release channel in planar lipid membranes. Biophys. J. 1995;69:1780–1788. doi: 10.1016/S0006-3495(95)80048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradníková A., Zahradník I. A minimal gating model for the cardiac calcium release channel. Biophys. J. 1996;71:2996–3012. doi: 10.1016/S0006-3495(96)79492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradníková A., Dura M., Györke S. Modal gating transitions in cardiac ryanodine receptors during increases of Ca2+ concentration produced by photolysis of caged Ca2+ Pflügers Arch. 438 1999. 283 288a [DOI] [PubMed] [Google Scholar]
- Zahradníková A., Zahradník I., Györke I., Györke S. Rapid activation of the cardiac ryanodine receptor by sub-millisecond calcium stimuli J. Gen. Physiol. 114 1999. 787 798b [DOI] [PMC free article] [PubMed] [Google Scholar]