Abstract
This study considers the vulnerability of the urban area of the City of Belo Horizonte to dengue. A total number of 89,607 cases registered in the surveillance system from 1996 to 2002 were analyzed. Seven epidemic waves were identified during this period. Cases were grouped into 2,563 census areas, and three risk categories were proposed based on how many times each area reached a threshold established for each epidemic wave. The association between the risk categories and the socioeconomic, demographic and urban-infrastructure characteristics was evaluated. Analysis included Kruskal–Wallis test variance comparisons and multivariate regression using multinomial models. Incidence rates differed significantly among the three risk categories in most of the epidemic waves. The factors that best characterized the areas were low educational level (≤4 years of schooling), low income of the head of the family (≤2 minimum wages per household), household density, and proportion of children and elderly women. Information related to basic sanitation was not enough to discriminate levels of susceptibility to dengue, and study of population density and concentration of establishments considered vulnerable to vector infestation yielded questionable results. It is important to consider different levels of exposure of the population to explain the heterogeneous pattern of distribution of dengue cases in an urban setting. Understanding the dynamics of dengue fever is essential for surveillance purposes, to improve control measures and to avoid epidemics of this disease.
Keywords: Dengue, Epidemics, Risk factors.
Dengue is one of the most important mosquito-borne infectious diseases affecting humans and its occurrence has worsened lately.1 The disease varies from mild to severe forms, or even fatal, hemorrhagic fever. It is caused by a flavivirus that has four closely related groups that are antigenically distinct [DEN-1, DEN-2, DEN-3, DEN-4].
Increased epidemic activity in the past years was associated with geographical expansion of the vectors and the co-circulation of multiple virus serotypes. The virus is maintained in a cycle that involves human and Aedes aegypti, a domestic, day-biting mosquito that prefers to rest inside dwellings.2 It lays its eggs primarily on walls of artificial containers where water can be accumulated, such as vases of plants and tires, often available either inside buildings or in their surroundings.1 The eggs can survive long dry periods. Active mosquito spreading is limited to areas possessing appropriate conditions for feeding, rest, and egg laying. Usually, it does not exceed 50 m.
Currently, vector control is the only way to break the chain of disease transmission, given that there is no effective vaccine nor etiological therapy. However, vector control is not a simple task, especially because of the complexities of urban settings. Failure in control programs was pointed out by several authors.3–5
Many factors are associated with dengue, including those related to the increased likelihood of contact between the vector and the host such as household or population density; factors that promote vector proliferation, including environmental conditions (temperature, humidity and altitude), poor sanitation, or availability of potential breeding sites, besides socioeconomic factors. Several studies have analyzed some of these factors considering different areas, cities or towns,6–8 intra-urban areas,9–11 or even household characteristics.12
The pattern of distribution of dengue cases is well-known to be clustered in certain areas, such as urban spaces, but was also observed as in-house aggregations.1,13 Another characteristic is the systematic repetition of disease transmission in some areas that concentrate a significant part of cases.6,7,14 Some results are controversial, especially those related to living conditions.10 Another issue that is not clearly understood is the minimum level of vector infestation to avoid disease transmission, although it was considered a goal for vector control programs.
The identification of factors associated with disease distribution and the definition of vulnerable areas may contribute to establish priorities not only for vector control but also for surveillance purposes, optimizing resources, which are generally reduced in periods of lower incidence. This knowledge may facilitate interventions aimed at vector control and patient care, minimizing the collective and individual burden of the disease.
This study aims to evaluate the association between socioeconomic, demographic, and urban infrastructure variables and risk areas classified according to dengue occurrence and the persistence of transmission.
METHODS
Area of Study
This is an ecological study carried out in Belo Horizonte, a metropolitan city with 2,238,332 inhabitants living in an area of 335 km2, at an altitude of 894 m, with an annual temperature ranging from 18 to 27°C and annual precipitation of over 1,300 mm.
The City Health Department has a territorial definition that considers census tracts according to the Brazilian Institute of Geography and Statistics Foundation, FIBGE. The Ae. aegypti control program is centralized, and activities fall under the responsibility of each primary health care unit.
The first epidemic began in 1996, and since then, epidemics have occurred every year. The only serotype initially identified was DEN-1. By the end of 1997, an epidemic of great intensity started, characterized by the simultaneous circulation of DEN-1 and DEN-2.15 The two serotypes continued to produce successive epidemics every year. In February 2002, DEN-3 was identified for the first time in the city, and now, the three serotypes co-exist.
Data Sources and Case Definition
Information was obtained from the National System on Reportable Diseases (SINAN), from the Brazilian Ministry of Health, and from the Municipal Surveillance System (SISVE). Data comprised autochthonous cases reported to the City Health Department from 1996 to 2002 and were confirmed by laboratory or clinical/epidemiological criteria (Almeida et al., submitted for publication).16
We used variables to describe surrogate markers of households and neighborhood conditions to identify socioeconomic, demographic, and environmental characteristics related to dengue transmission.9,10,14,17 Most of them were obtained from the national demographic census 2000, but information on business facilities was collected at the municipal taxation system. They are: proportion of households with no piped water; proportion of households with no systematic garbage collection by public or private services; proportion of heads of family with up to 4 years of schooling; proportion of heads of family with income of up to two minimum wages; average number of people per household; population density (inhabitants/km2); proportion of population comprising children aged under 10 years and women over 64 years; and ratio between the number of commercial establishments and the number of households in the census tract.
The proportion of children (younger than 10 years old) and elderly women (older than 64 years) living in the census tract was used as a special group of exposure to dengue, considering their longer permanence at home and surroundings during daytime, having a restricted mobility. Likewise, establishments such as junkyards, car garages, flower markets, building material warehouses, and tire repair shops were classified as highly vulnerable to Ae. aegypti breeding, mirroring the same classification used by the dengue surveillance system. The arguments for this classification were based upon the observation that they may offer appropriate conditions for the mosquito proliferation, given that they have shadowed and dark receptacles that could accumulate and preserve water. Such spaces have deserved special attention and priority actions in the vector control programs in the city.3,4
Cases were georeferenced to the home address, allowing the identification of the corresponding census tract. Green areas were excluded from the area of the census tract to function as a proxy of population density, minimizing the effect of uninhabited sites.
The number of vulnerable establishments comprised those either located in the census tract or in a distance of up to 100 m away from the borders. We used the Geographical Information System, prepared and managed by the Municipal Information Technology and Data Company (Prodabel). Incidence rates were calculated by census tracts considering data from census 2000.
Data Analysis
Seven epidemic waves were identified between 1996 and 2002 by a previous study (Almeida et al., submitted for publication). A threshold was defined as the average number of cases per census tract affected in a respective wave to classify the census tracts according to how many times they reached a threshold for each wave. Then, they were classified into three risk categories, according to how many times they reached a threshold for each wave.
Risk category 1 related to the census tracts that did not reach the respective threshold any time; risk category 2, census tracts that reached the threshold in a single epidemic wave; and risk category 3, the census tracts that reached the threshold two or more times.
Incidence rates, according to the three risk categories, were compared using variance analysis (ANOVA) for each epidemic wave. When the difference was statistically significant, the Tukey’s test was used for multiple comparisons. For variables not normally distributed, the nonparametric Kruskal–Wallis’s test was applied, considering the distribution of variable ranks instead of their effective values.
Multivariate analysis was performed to identify the main ecological factors, using logistic multinomial regression.18 The three risk categories were the dependent variables. Models were fitted separately according to hierarchically structured theory19 especially considering two levels of exposure: local level (within households) and global levels (social and demographic conditions).20
Most of the variables were analyzed in the original format but the variable representing the population with restricted mobility was split in three categories according to the percentiles 25 and 50, to better fit the model.
Wald statistic and likelihood ratio test were used for verifying, respectively, the relevance of each variable and the improvement of the models. Interaction terms were investigated. Variables that demonstrated statistical significance (p ≤ 0.05) and plausibility in each final model were further used to build the most parsimonious final model.18
MapInfo software21 was used for data treatment and mapping. Statistical analysis was performed using SPSS/PC.22
RESULTS
A total of 99,469 cases of dengue were identified during the seven epidemic waves; of those, 89,607 cases (90.1%) were geocoded. Median age ranged from 26 to 32 years old in all waves, and a higher incidence among women was observed.
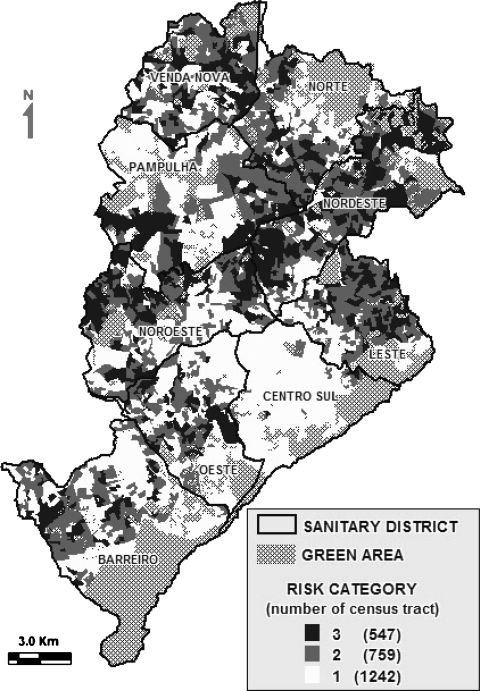
From the 2,563 census tracts in the city, 2,548 were analyzed (99.4%), after the exclusion of special tracts comprised by asylums, orphanages, and military housing. The risk areas classification showed the following proportion: category of risk 1 with 1,242 census tracts (48.7%); category 2 with 759 census tracts (29.8%), and category 3 with 547 census tracts (21.5%).
Table 1 presents the distribution of dengue cases stratifying risk categories and number of census tract by total and average number of cases, population and households. A clear difference and a gradient among the averages were observed, in which the category of risk 1 is below the average in all of them. The average of cases for category 3 was 5.3 times greater than in category 1. Incidence rates for each epidemic wave and the relative risks between risk categories 2 and 3 compared to 1 are shown in Table 2. Category 2 showed increased relative risks, except in the second and fifth waves, whereas for category 3, they were remarkably higher, ranging from 1.42 in the second up to 5.26 in the sixth wave.
TABLE 1.
Distribution of dengue cases, population, and households of the census tract according to risk categories, Belo Horizonte, 1996–2002
| Risk category | Number of census tract | Cases* | Population* | Households* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Mean | Standard deviation | Total | Mean | Standard deviation | Total | Mean | Standard deviation | ||
| 1 | 1,242 | 16,368 | 13.18 | 10.49 | 946,436 | 762.03 | 260.99 | 283,170 | 228.00 | 69.17 |
| 2 | 759 | 35,110 | 46.26 | 30.30 | 723,310 | 952.98 | 266.33 | 198,638 | 261.71 | 69.43 |
| 3 | 547 | 38,129 | 69.71 | 34.59 | 567,760 | 1,037.95 | 260.04 | 153,708 | 281.00 | 67.73 |
| Total | 2,548 | 89,607 | 35.17 | 33.35 | 2,237,506 | 878.14 | 287.27 | 635,516 | 249.42 | 72.34 |
*P < 0.001 (ANOVA/Tukey’s test for multiple comparisons)
TABLE 2.
Incidences and relative risks according to epidemic waves, Belo Horizonte, 1996–2002
| Risk category | 1st Wave | 2nd Wave | 3rd Wave | 4th Wave | 5th Wave | 6th Wave | 7th Wave |
|---|---|---|---|---|---|---|---|
| Incidencea | |||||||
| 1 | 3.24 | 6.58 | 148.46 | 1.08 | 0.49 | 6.53 | 6.56 |
| 2 | 6.66 | 5.96 | 436.01 | 2.36 | 0.72 | 19.34 | 14.35 |
| 3 | 16.80 | 9.33 | 570.95 | 5.16 | 1.96 | 34.33 | 33.04 |
| RR2b | 2.05 | 0.91 | 2.94 | 2.19 | 1.48 | 2.96 | 2.19 |
| (CI 95%) | (1.78–2.37) | (0.80–1.02) | (2.88–3.00) | (1.72–2.80) | (0.99–2.20) | (2.69–3.26) | (1.98–2.42) |
| RR3b | 5.18 | 1.42 | 3.85 | 4.79 | 4.02 | 5.26 | 5.04 |
| (CI 95%) | (4.56–5.89) | (1.26–1.59) | (3.77–3.92) | (3.82–6.00) | (2.85–5.67) | (4.80–5.75) | (4.60–5.51) |
aIncidence rates by 10,000 inhabitants
bRR (CI 95%): relative risk with 95% confidence interval for risk categories 2 and 3, respectively. Category 1 was the reference.
Figure 1 displays the distribution of census tracts according to risk categories, where a pattern with concentration of census tracts into the same category over the city is verified. Comparing the mean ranks of the incidence rates for each risk category in each wave, by Kruskal–Wallis test, a significant difference was observed among the three categories with an ascending gradient for all the waves (data not shown).
FIGURE 1.
Distribution of census tracts according to risk categories for dengue transmission, Belo Horizonte.
The analysis of the mean rank of variables, according to risk category, is presented in Table 3. A statistically significant difference was observed, except for the ratio between vulnerable establishments and household in the census tract. A gradient from category 1 to 3 was observed, except for population density, with an inverted gradient. To pinpoint the differences in these distributions, the comparison among several categories was performed, presenting statistically significant differences between categories 1 and 2 for all the variables and between categories 1 and 3, except for the vulnerable establishments. The variables referring to sanitation, such as water supply and garbage collection did not discriminate categories 2 and 3. The same happened with population density. The difference between risk categories for variables regarding schooling level, income of family’s head, mean residents per household, and proportion of children and elderly women were statistically significant and confirmed the observed gradients (data not shown).
TABLE 3.
Distribution of the mean ranks of the variables, according to risk categories and census tractsa, Belo Horizonte, 1996–2002
| Variable | Risk category | Mean rank | Chi-squareb | P |
|---|---|---|---|---|
| Proportion of households with no piped water | 1 | 1,201.91 | 40.706 | <0.001 |
| 2 | 1,326.53 | |||
| 3 | 1,367.14 | |||
| Proportion of households without systematic garbage collection by public or private services | 1 | 1,198.43 | 33.523 | <0.001 |
| 2 | 1,344.12 | |||
| 3 | 1,350.62 | |||
| Proportion of heads of family with up to 4 years of schooling | 1 | 995.49 | 364.886 | <0.001 |
| 2 | 1,469.92 | |||
| 3 | 1,636.85 | |||
| Proportion of heads of family with income of up to two minimum wages | 1 | 997.11 | 362.921 | <0.001 |
| 2 | 1,464.10 | |||
| 3 | 1,641.25 | |||
| Average number of people per household | 1 | 1,030.70 | 270.237 | <0.001 |
| 2 | 1,471.25 | |||
| 3 | 1,555.07 | |||
| Population density (inhabitants/km2) | 1 | 1,341.55 | 21.130 | <0.001 |
| 2 | 1,228.02 | |||
| 3 | 1,186.75 | |||
| Proportion of population comprising children aged under 10 years and women over 64 years | 1 | 1,102.95 | 141.696 | <0.001 |
| 2 | 1,383.17 | |||
| 3 | 1,513.24 | |||
| Ratio between the number of commercial establishments considered highly vulnerable to Aedes aegypti proliferation and the number of households in the census tractc | 1 | 1,308.04 | 5.804 | 0.055 |
| 2 | 1,227.96 | |||
| 3 | 1,262.92 |
aNumber of census tract by risk categories: category 1 = 1,242; category 2 = 759; category 3 = 547
bKruskal–Wallis test with 2 degrees of freedom
cConsidered junkyards, car garages, flower markets, building material warehouses, and tire repair shops
Using risk category 1 as reference, the final multinomial logistic model showed that for categories 2 and 3, the following variables were significant: 17% or higher of vulnerable population (children and elderly women) in the census tract and average number of people per household. Low income was only associated with the worst risk category (Table 4). Because of the high correlation (0.906) between low schooling level and low income, only the latter was kept in the model. No interaction term was found statistically significant.
TABLE 4.
Multinomial logistic regression of the risk categories for dengue on selected socioeconomic variables, Belo Horizonte, 1996–2002
| Variables | Risk category 2 | Risk category 3 | ||
|---|---|---|---|---|
| ORa | 95% CIb | ORa | 95% CIb | |
| Average number of people per household | 3.32 | 2.42–4.55 | 3.07 | 2.11–4.47 |
| Proportion of heads of family with income of up to two minimum wages | 1.89 | 0.91–3.91 | 4.79 | 2.10–10.92 |
| Proportion of population comprising children aged under 10 years and women over 64 years. | ||||
| Percentile 25 (0–17.1%) | 1.00 | 1.00 | ||
| Percentile 50 (17.2–19.2%) | 1.52 | 1.12–2.06 | 2.42 | 1.65–3.57 |
| Percentile 75 (≥19.3%) | 1.59 | 1.20–2.11 | 2.78 | 1.93–4.01 |
| 2 Log likelihood of the final model | 4,899.60 | |||
| Chi-square 407.00 (P < 0.001) | ||||
For all models, the comparative group was Risk Category 1 and all variables are simultaneously in the model.
aOdds ratio
b95% Confidence interval
DISCUSSION
This study classifies Belo Horizonte City areas according to their vulnerability to the transmission of dengue and characterizes them based on some socio-demographic variables, indicating heterogeneity of the urban space. Factors significantly associated to higher risk areas were lower income of the head of the family, higher household density, and larger proportion of children and elderly women. Other factors such as basic sanitation, concentration of establishments vulnerable to vector proliferation, and population density did not reach significant statistics to characterize risk areas.
The classification of the census tracts according to vulnerability to dengue transmission, established after 7 years of epidemics, was enough to retrospectively discriminate these areas, even for the first epidemic. This corroborates the hypothesis that factors inherent to these sites may facilitate or restrict the transmission and/or maintenance of the disease.
Differences in dengue transmission are determined by the interactions among human being, vectors, and the environment, and this interaction defines the contact among mosquitoes and susceptible and infected individuals.6 In 2000, Barrera et al.14 analyzed a hyperendemic city in Venezuela and found a high correlation between the occurrence of classic dengue and hemorrhagic dengue fever in the neighborhoods. Thus, it is possible that areas classified at higher risk are more likely to present serious cases of the disease.
Lower income, high household density and a larger proportion of children and elderly women in the census tract were the factors that best characterized the risk areas for dengue. These results are similar to those observed by Costa and Natal, in Sao José do Rio Preto, in the Southern region of Brazil, in 1995,9 who reported an association between the occurrence of the disease and schooling and income of the head of family, water supply and garbage collection, but disagreeing for household density.
The apparent contradiction in the associations observed between population and household density with the risk categories could be explained by the type of occupation of the urban space, with areas of vertical dwellings where the population density (population/area) is high, which does not mean that people are gathered. Other areas, with population density equally high, comprise agglomerations of one- or two-storey houses, mostly in precarious conditions. Thus, considering the different patterns of urban occupation, population density, as the ratio of the number of individuals/area, seemed not to be appropriate to characterize the risk areas for dengue in the city area. On the other hand, household density might be a better indicator.
The rationale of using children and elderly women as a special group of exposure to dengue, considering their longer permanence at home and surroundings during daytime, having a restricted mobility, is based on two arguments. In Brazil, children usually attend school located in the same neighborhood where they live, and school time only lasts for 4 h a day. Similarly, elderly women are usually retired and spend most of their time at home or surroundings. Thus, given the favorable conditions, we assumed that both groups were at higher risk of infection than the other adults, as analyzed in a previous study (Almeida et al., submitted for publication). What remains to be understood are the higher incidence rates observed among young adults in Belo Horizonte, and in Brazil, contrary to what were described in Southeast Asia.23
A critical level of vector infestation may not exist as a single measure to characterize a metropolitan area as a whole. As pointed out by Gómez-Dantés, in 1995,6 a combination of factors is related to the transmission of dengue. Considering the complex structure and heterogeneity of urban areas, a low level of vector infestation could lead to an epidemic in a densely populated area, whereas a similar infestation is not enough to maintain the transmission in an area with larger population dispersion.
The variables regarding basic sanitation, water supply, and garbage collection were not enough to differentiate categories 2 and 3. These services have a good coverage in the city, and water storage in small containers is not a common practice. However, the storage system in house water containers may be inadequate and badly covered, allowing proliferation of Ae. aegypti. Furthermore, the indicator of garbage collection cannot reflect the local situation, as many areas have illegal dumping despite provision of regular services. In spite of the wide coverage of garbage collection service, it is irregularly provided in some areas regarding frequency. Unfortunately, the indicator used in this study is not refined for such precision.
The variable that encompasses the distribution of commercial establishments, which are considered strategic sites for vector proliferation, demonstrated unexpected results, for such establishments were more concentrated in lower risk areas. Classifying an establishment as vulnerable does not necessarily mean inappropriate storage of utensils, such as tires or other containers that accumulate water. Moreover, this variable was not able to detect such qualitative characteristics.
Before we mention some possible sources of bias in this study, we have to consider the relative absence of cases of dengue hemorrhagic fever (DHF). Up to the censored date of this analysis, a relatively few cases were reported in Brazil and in Belo Horizonte. Several reasons were stated, related to either the low virulence of DEN-2 strain circulating in the Americas or the possible underreporting of such cases all over Brazil. After the recent introduction of DEN-3 in 2002, an increase in DHF was observed and is subject of another investigation. This issue was revised recently by some Brazilian authors.23
Regarding the possible bias, besides the ones related to ecological studies, the first refers to the use of secondary data from the official surveillance system, regarding misclassification and underreporting cases. The increased availability of laboratory testing for diagnostic confirmation along the years might have reduced the misclassification in the last waves. In a seroepidemiological survey carried out by our group in three Sanitary Districts of Belo Horizonte, during the year 2000, a ratio of 5.4:1 was found between the number of seropositive individuals (clinical and subclinical cases) and the number of reported cases, with a very small variation among the areas.24
A second point to be considered is the classification of census tracts using an arbitrary threshold. This criterion should increase the specificity for the real occurrence of cases in the area. In other words, cases infected in places different from their household census tract or even misdiagnosis could have spread distribution, resulting in a misclassification of the tracts with sporadic occurrence. However, areas where the infection might have occurred outside the case’s household, such as workplace or other (factories, hospitals, or commercial establishments) would be underestimated in this evaluation.
A third source of bias is presented when calculating incidence rates. We did not consider the reduction of exposed susceptibles in each census tract over time. The variety of circulating serotypes and the population flows hindered this estimation. In either process, the procedure would result in an underestimated incidence.
A fourth consideration refers to losses because of no location of cases (9.9%). Again, this fact must have contributed to underestimation of some specific areas, as these losses are well-known to be more frequent in the slums of the city, considering that the municipal geographical database lacks data.
One caution refers to the use of different scales adopted in epidemiological studies. Census tracts present a high resolution and socioeconomic homogeneity, although they are subject to larger random fluctuations.25 The scale adopted in this study—census tracts—seemed to be appropriate for the study of dengue transmission in the city, given that the high frequency of the outcome and the number of inhabitants by area has little variation. Besides, this territorial delimitation is consistent with the organization of the City Health Department, allowing for the planning for focal interventions.
The last methodological thought is related to the risk classification used in this study based upon geographical areas. It has the disadvantage for being applied only where a report of the occurrence of the dengue has already been done. To understand the dynamics of such a complex disease, with an immense range of factors interacting, further studies considering the specificities of each area are still needed, as well as the construction of predictive models that are able to identify the risk in areas not affected yet. These issues notwithstanding, the classification of dengue risk areas presented here can be useful to organize epidemic surveillance, enhancing knowledge of the distribution of the disease, and thus, enabling early control actions and preventing the severe forms of the disease.
As long as a vaccine is still not available and local interventions are very welcome, our findings could contribute for pointing out vulnerable areas for environmental interventions and identifying strategic groups for increased community participation, aiming to reduce the amount of Ae. aegypti breeding sites. Moreover, considering that the traditional indicator of mosquito infestation, based on larval survey, was not accurate enough to estimate dengue risk,23 this study may provide one way to overlap this limitation as introducing other variables to classify risk areas, especially for surveillance purposes.
Acknowledgments
We would like to thank Paulo Roberto Lopes Corrêa for reviewing data from 1996 to 2001, after data checking and validation.
This study is part of a Master’s degree dissertation, of the Master’s degree course in Public Health, Universidade Federal de Minas Gerais. It was approved by the Institutional Review Board/Research Ethics Committee of the Universidade Federal de Minas Gerais (COEP No. 233/03). The last three authors are recipients of Brazilian National Council for Scientific and Technological Development (CNPq) scholarships.
Footnotes
de Mattos Almeida is with the Municipal Health Secretariat of Belo Horizonte, Belo Horizonte, Minas Gerais, Brazil; de Mattos Almeida, Caiaffa, and Proietti are with the Urban Health Observatory, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, Minas Gerais, Brazil; Caiaffa and Proietti are with the Research Group in Epidemiology (GPE/CNPq), Department of Social and Preventive Medicine, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, Minas Gerais, Brazil; Assunc¸a˜o is with the Department of Statistics, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, Minas Gerais, Brazil.
References
- 1.Kouri G. Dengue, a growing problem of health in the Americas. Rev Panam Salud Publica. 2006 Mar;19(3):143–145. [DOI] [PubMed]
- 2.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever: a global public health problem in the 21st century. Dengue Bull [serial online] 1997;21:1–15. Available at: http://w3.whosea.org/en/Section10/Section332/Section519_2380.htm. Accessed on January 30, 2004.
- 3.Tauil PL. Aspectos críticos do controle do dengue no Brasil. Cad Saúde Pública. 2002;18(3):867–871. [DOI] [PubMed]
- 4.Teixeira MG, Barreto ML, Guerra Z. Epidemiologia e medidas de prevenção do dengue. Inf Epidemiol SUS. 1999;8(4):5–33.
- 5.Pinheiro F, Nelson M. Re-emergence of dengue and emergence of dengue haemorrhagic fever in the Americas. Dengue Bull [serial online] 1997;21:16–24. Available at: http://w3.whosea.org/en/Section10/Section332/Section519_2381.htm. Accessed on January 30, 2004.
- 6.Gómez-Dantés H, Ramos-Bonifaz B, Tapia-Conyer R. El riesgo de transmisión del dengue: um espacio para la estratificación. Salud Pública Méx. 1995;37(suppl):88–97. [PubMed]
- 7.Escobar-Mesa J, Gómez-Dantés H. Determinantes de la transmisión de dengue en Veracruz: un abordaje ecológico para su control. Salud Pública Méx. 2003;45(1):43–52. [DOI] [PubMed]
- 8.Koopman JS, Prevots DR, Vaca-Marin MA, Gomez-Dantés H, Dorate AML, Longini MI. Determinants and predictors of dengue infection in Mexico. Am J Epidemiol. 1991;133(11):1168–1178. [DOI] [PubMed]
- 9.Costa AIP, Natal D. Distribuição espacial da dengue e determinantes socioeconômicos em localidade urbana no Sudeste do Brasil. Rev Saúde Pública. 1998;32(3):232–236. [DOI] [PubMed]
- 10.Teixeira MG, Barreto ML, Costa MCN, Ferreira LDA, Vasconcelos P. Dinâmica de circulação do vírus da dengue em uma área metropolitana do Brasil. Epidemiol Serv Saúde. 2003;12(2):87–97.
- 11.Medronho R. Geoprocessamento e saúde: uma nova abordagem do espaço no processo Saúde Doença. Rio de Janeiro: Fundação Oswaldo Cruz; 1995.
- 12.Bohra A, Adrianasolo H. Application of GIS in modeling of dengue risk based on socio-cultural data: case of Jalor, Rajasthan, Indian. Proceedings of the 22nd Asian Conference on Remote Sensing 2001 Singapore. Available at: http://www.crisp.nus.edu.sg/~acrs2001/pdf/096bohra.pdf. Accessed on December 2, 2003.
- 13.Morrison AC, Getis A, Santiago M, Rigau-Perez JG, Reiter P. Exploratory space-time analysis of reported dengue cases during an outbreak in Florida, Puerto Rico, 1991–1992. Am J Trop Med Hyg. 1998;58(3):287–298. [DOI] [PubMed]
- 14.Barrera R, Delgado N, Jiménez M, Villalobos I, Romero I. Estratificación de una ciudad hiperendémica en dengue hemorrágico. Rev Panam Salud Pública. 2000;8(4):225–233. [DOI] [PubMed]
- 15.Correa PRL, França E, Bogutchi TF. Aedes aegypti infestation and occurrence of dengue in the city of Belo Horizonte, Brazil. Rev Saúde Pública. 2005;39(1):33–40. [DOI] [PubMed]
- 16.Brasil. Ministério da Saúde, Fundação Nacional de Saúde, Departamento de Operações. Manual de dengue-vigilância epidemiológica e atenção ao doente. Brasília (Brasil): DEOPE; 1995.
- 17.Barcellos C, Pustai AK, Weber MA, Brito MRV. Identificação de locais com potencial de transmissão de dengue em Porto Alegre através de técnicas de geoprocessamento. Rev Soc Bras Med Trop. 2005, maio/jun; 38(3):246–250. ISSN 0037-8682. [DOI] [PubMed]
- 18.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley and Sons; 2000.
- 19.Victora CG, Huttly SR, Fuchs SC, Olinto MTA. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol. 1997 Feb;26(1):224–227. [DOI] [PubMed]
- 20.Favier C, Schmit D, Muller-Graf CDM, et al. Influence of spatial heterogeneity on an emerging infectious disease: the case of dengue epidemics. In: Proc R Soc B. 2005 Jun 7;272(1568):1171–1177. [DOI] [PMC free article] [PubMed]
- 21.MapInfo Professional [computer program] MapInfo Corporation: New York: Troy Applied innovations; 2001.
- 22.SPSS [computer program] SPSS Inc; 1999.
- 23.Teixeira MG, Costa, MCN, Barreto ML, Mota E. Dengue and dengue hemorrhagic fever epidemics in Brazil: what research is needed based on trends, surveillance, and control experiences? Cad Saúde Pública. 2005;21(5):1307–1315. [DOI] [PubMed]
- 24.Pessanha JEM, DiLorenzo C, Costa MA, et al. Dengue seroprevalence in Belo Horizonte: detecting participation bias through spatial analysis methods for small-area studies. In: The Second Annual International Conference on Urban Health, October 15–18, 2003. J Urban Health. 2003;80(Suppl 2)ii29.
- 25.Carvalho MS, Cruz OG. Análise espacial por microáreas: métodos e experiências. In: Veras RP, Barreto ML, Filho PA, Barata RB, eds. Epidemiologia contextos e pluralidade. Rio de Janeiro: Fiocruz/Abrasco; 1998:79–89.