Abstract
A prenylated peptide specific to the C terminal tail of a G protein γ subunit type, γ5, inhibits activation of a G protein by the M2 muscarinic receptor. The γ5 peptide was tested for direct effects on the M2 receptor’s properties. The wild type γ5 peptide reduced the affinity of M2 for the agonist, carbachol, more than 5-fold in an antagonist displacement assay. The peptide was inactive when its amino acid sequence was scrambled or when it was unprenylated. Although the wild type peptide reduced the affinity of M2 for the antagonist QNB, it had no effect on the antagonists NMS or atropine. These results suggest that in the presence of the peptide the M2 receptor adopts a novel conformational state that affects the ligand binding surface. The results also suggest that the G protein γ5 subunit tail interacts with a receptor.
Keywords: Receptor, G protein, γ subunit, Peptide
Activation of a G protein by a receptor is the first step in the transduction of an extracellular signal into an intracellular effect. The molecular mechanisms at the basis of the activation of the G protein by a receptor are unclear. Since the identification of heterotrimeric G proteins, the βγ complex has been known to be a requirement for effective interaction and activation of a G protein by a receptor [1–3]. One reason for this requirement of the βγ complex is the functional interaction between the G protein gamma subunit and a receptor [4]. Different strands of evidence support this role for the G protein γ subunit: a peptide specific to the C terminal tail of the γ1 subunit type inhibits Gt activation by rhodopsin [5]. In addition, in the absence of the G protein, the peptide alone stabilizes the metarhodopsin II state of rhodopsin [6] [7]. A homologous peptide specific to the C terminal domain of the γ5 subunit inhibits G protein activation by the M2 muscarinic receptor and also disrupts the modulation of ion channels by the muscarinic receptor in intact neurons [8]. The direct effect of the γ5 peptide on the M2 receptor in the absence of a G protein has not however been examined. Since the γ1 peptide directly stabilizes a specific state of rhodopsin in the absence of the G protein, homologous peptides could have similar effects on other receptors. Here, we have examined the direct effect of the γ5 peptide on the M2 muscarinic receptor. For these studies, the M2 type was chosen from among the muscarinic receptors because it has been extensively characterized biochemically and pharmacologically [9–11]. The γ5 subunit type was used in these experiments because it is one of the few mammalian γ subunits that are expressed in heart where the M2 receptors are abundant [12,13]. The rationale for examining the effect of a G protein subunit specific peptide on receptor state is based on the well-known ability of a G protein to stabilize the state of a receptor with high affinity for an agonist. To determine whether the γ5 peptide had an effect on the state of the M2 receptor, we examined the relative affinities of the receptor for various ligands in the presence and absence of the peptide. Among the agonist or antagonist ligands tested, the peptide altered the affinities of the M2 receptor for acetylcholine, carbachol, and quinuclidinyl benzilate (QNB). Agonists and antagonists are known to bind to receptor states with specific conformations [14]. These results thus indicate that the G protein γ subunit peptide stabilizes a novel state of the M2 receptor with unique ligand binding properties. The results also imply that the G protein γ subunit tail interacts with a receptor during the activation of a G protein.
Materials and methods
Cells and reagents
[3H]NMS (N-methyl scopolamine) and [35S ]GTPγS were purchased from NEN. Other chemicals were from Sigma. Geranylgeranyl-bromide was purchased from American Radiolabeled Chemicals (St. Louis, MO). Chinese hamster ovary (CHO) cells that over-express M2 receptor protein under the control of a DHFR (dihydrofolate reductase) control element express sufficiently high levels of the receptors for biochemical assays when maintained in culture medium containing methotrexate. These cells and their culture have been described before [8,9]. The level of expression was about 400,000 receptors per cell.
Synthesis and purification of geranylgeranylated peptides
Solid peptide synthesis, mass spectrometry, and amino acid analysis was performed at the Protein and Nucleic Chemistry Laboratory, Washington University School of Medicine. Geranylgeranylation was performed in an organic solvent mixture (1:1:1—butanol:ethanol:water) at pH 9 for 24 h at room temperature in the dark under N2 [15]. The geranylgeranylated peptides were subsequently purified by reverse phase chromatography, freeze-dried, dissolved into butanol:ethanol:water (1:1:1, volume), and frozen at −80 °C. The molecular weight of the prenylated peptide was checked by mass spectrometry. The concentration of the purified peptide was estimated by amino acid analysis. Integrity of peptide stocks was checked periodically by reverse phase chromatography.
Purification of G protein subunits
Recombinant Gαi2 and Gαo were purified from Escherichia coli by expressing rat Gαo and Gαi2 with yeast N-myristoyl transferase [16] (kind gift from Dr. M. Linder). Expression and purification were performed based on this previous published procedure with modifications [15]. Expression of Gα subunit was ~1 mg protein/L of cell culture. Gαi2 was purified as described before [16]. The Gαo protein was purified as previously described except for the use of a gel-exclusion column (Superose 12, Pharmacia) instead of a hydroxyapatite column. In order to ensure Gαo protein stability, bacterial extracts were supplemented with 30 μM aluminium chloride, 1 mM sodium fluoride, and 50 μM GDP. The final purity was ~90%. More than 80% of the protein was found to be functional according to the levels of GTPγS uptake.
The βγ complex from bovine brain was purified as described before [17]. Sf9 insect cells were triple infected with His-αi2, β1, and γ5 subunit viruses. Expressed recombinant β1γ5 protein was purified from these cells using modifications of a previously published procedure [18,15]. Before elution of β1γ5 protein with buffer (20 mM Na Hepes (pH 8.0), 0.1 mM EDTA, 50 mM MgCl2, 10 mM β-mercaptoethanol, 50 mM NaCl, 1% cholate, 30 μM AlC13, 10 mM NaF, and 50 μM GDP), the protein bound column was equilibrated at 30 °C in a buffer containing 20 mM Na Hepes (pH 8.0), 100 mM NaCl, 1 mM MgCl2, 10 mM β-mercaptoethanol, 50 μM GDP, and 0.7% sodium cholate. This operation was performed to replace the detergent polyoxyethylene-10-lauryl-ether with sodium cholate. The βγ complex eluted from the Ni-NTA beads (Qiagen, Valencia, CA) was subjected to dialysis and concentration. The purity was about 90%. When higher purity was desired, the purified untagged βγ complex was incubated with 200–500 μl Ni-NTA beads and centrifuged through spin columns. This process significantly reduced the concentration of non-specific contaminant proteins that had been eluted with the heterotrimer earlier due to their affinity for the Ni-NTA beads. The resulting preparation was more than 95% pure. Purified βγ complex was quantitated by laser densitometry using BSA as a standard.
Preparation of M2 receptor containing membranes
CHO cells stably transfected with M2 receptors were freeze-thawed using liquid nitrogen in a hypotonic buffer containing 20 mM Na Hepes, pH 7.4, 1 mM EDTA, 2 mM MgCl2, and a cocktail of protease inhibitors. Lysates were centrifuged at low speed and the supernatant was centrifuged again at 100,000g to obtain membranes. High-speed pellets were resuspended in buffer A and membranes were frozen at −85 °C until further use. Membranes were thawed and incubated for 1 h at 4 °C with buffer A—20 mM sodium phosphate buffer, pH 7.4, 5 mM MgCl2—containing 5 M urea, and 100 μM GTPγS to deplete endogenous G protein subunits. The membranes were then washed twice with buffer A by centrifugation at 100,000g and were frozen at −85 °C until required. Immunoblot analysis with antibodies specific to the β1 subunit type [19], showed a significant decrease in that subunit after this treatment (data not shown). The amount of M2 receptor in our membrane stocks was determined by mixing serially diluted membrane stocks in buffer A with a saturating concentration of [3H]NMS (10 nM, final). After 60 min of incubation at room temperature, the reaction mixtures were filtered through Whatman GF/B glass filters and washed with ice-cold phosphate buffered saline. Liquid scintillation counting was used to measure radio-labeled ligand bound to filters. Typically about 0.2–0.4 nmol of M2 ([3H]NMS binding protein) was obtained from 50 plates of M2-CHO cells with a concentration of 0.1 nmol [3H]NMS-bound M2/mg of protein in the urea-washed membranes.
Effect of peptides on [3H]NMS binding by M2 receptors
Purified peptide in butanol:ethanol:water (from freshly thawed stocks) was mixed with appropriate volume of sodium cholate to obtain a final volume of 20 μl. After thorough mixing, the peptide was dried by centrifugation under a vacuum at room temperature. M2 receptors (5 nM) in membranes were pre-incubated in buffer A for 30 min at 4 °C with the dried peptide (10 μM). The sodium cholate concentration in this M2-peptide suspension was 10 μM. This M2-peptide suspension was diluted 100-fold in buffer A (final M2 concentration ~50 pM) and [3H]NMS binding to the receptor was assayed as described. [3H]NMS was added to the M2-peptide suspension and the reaction mixture incubated for 60 min at room temperature. The concentrations (nM) of [3H]NMS were: 0.02, 0.04, 0.06, 0.1, 0.2, 0.4, 1, 2, and 4. These concentrations span the known Kd for NMS binding to the M2 receptor [20]. The samples were bound to Whatman GF/B glass filters and washed with ice-cold phosphate-buffered saline. Liquid scintillation counting was used to measure radio-labeled ligand bound to filters. Non-specific binding was measured with 1000-fold excess atropine over [3H]NMS and was less than 5% of total counts.
M2 receptor stimulation of GTPγS uptake by the G protein and the effect of a γ5 subunit specific peptide on G protein stimulation
G protein heterotrimer was preformed by incubating Gαi2 + brain βγ for at least 20 min at 4 °C in buffer A: 25 nM M2 in membrane suspension was preincubated with 500 nM of this heterotrimeric G-protein for 30 min at 4 °C in buffer B: 20 mM Hepes, pH 8, 5 mM MgCl2, 1 mM DTT, 100 mM NaCl, and 1 μM GDP. As controls, only the a subunit or the βγ complex alone was incubated with the receptor. In the case of the peptide assays, the M2–G protein incubation mix also contained geranylgeranylated-wild type γ5 (γ5pep-gg) or γ5 with a scrambled sequence (γ5pep-gg-scr). Peptides in butanol:ethanol:water were mixed with sodium cholate as described before. The final concentrations of constituents in the M2–G protein reaction mixture were 5 nM M2, 100 nM αi2βγ, and 10 μM sodium cholate. In the case of the peptides assays, increasing concentrations of γ5pep-gg or γ5pep-scr were as added as shown in the corresponding figure.
Binding reactions (5–10 μl) were started by the addition of 0.2 μM [35S]GTPγS (100–200 cpm/fmol GTPγS) and 1 mM agonist (carbachol) or antagonist (atropine) (final concentrations) at room temperature. Aliquots (1.5 μl) were taken from this reaction mixture at 30s and 3 min. The reaction was stopped by the addition of ice-cold buffer B containing 500 μM GTP and 1 mM atropine. At 3 min when aliquots were examined for GTPγS binding, formation of [35S]GTPγS bound Gαi2 was still increasing linearly with time compared to 30 s. Samples were filtered on nitrocellulose membranes (HAWP-02500, Millipore), washed with ice-cold phosphate-buffered saline, and radioactivity in membranes quantitated by liquid scintillation counting. The accumulated [35S]GTPγS bound protein in the interval between the 30 s and 3 min was divided by this time period to obtain the rates of the individual reactions.
Interaction of G protein with the M2 receptor
Briefly, 6.25 nM M2 receptors in membrane suspension was preincubated with 200 nM Go heterotrimer (αoβ1γ5) in buffer A supplemented with MgCl2: 20mM sodium phosphate, pH 7.4, 10 mM MgCl2 for 30 min at 4 °C. The mixture was diluted 50-fold in the same buffer to obtain final concentrations of 0.125 nM M2 and 4 nM Go heterotrimer 10 nM [3H]NMS and increasing concentrations of carbachol were added to this diluted mixture. In some cases incubations were performed in the presence of 100 μM GTPγS. Samples (1 ml) were incubated for 1–1.5 h at 23 °C. The low concentration of receptors relative to the Kd of NMS for the M2 receptor and the composition of the buffer used in the experiments were designed to detect the state of the receptor with high affinity for the agonist (R*) [21,22]. Samples were filter-washed on Whatman GF-B glass filters and radioactivity was counted. Non-specific activity was measured in the presence of 10 μM atropine.
Interaction of peptides with M2 receptor
First, 25 nM M2 receptors in membrane suspension were incubated with peptides (prepared as in the assay for measuring peptide effect on NMS binding) for 30 min at 4 °C. The mixture was diluted 5-fold in buffer A to obtain final concentrations of 5 nM M2, 10 μM sodium cholate, 10 nM [3H]NMS, various concentrations of peptides and increasing concentrations of carbachol. This mixture (12.5–25 μl) was incubated for 1–1.5 h at room temperature. [3H]NMS bound to M2 was measured as described before in a filter binding assay.
Statistical analysis
Student’s t test was performed using the online software www.graphpad.com, GraphPad Software, San Diego, CA, USA. Linear regression and non-linear regression for curve-fitting was performed with the program GraphPad Prism version 3.00 for Windows, GraphPad Software, San Diego, CA, USA.
Results and discussion
M2 muscarinic receptors activate Gαi2βγ
Membranes from CHO cells expressing high levels of M2 receptor were depleted of endogenous G protein subunits as described in the Materials and methods and assayed for activity by binding to the radio-labeled muscarinic antagonist [3H]NMS. The receptors bound [3H]NMS with a dissociation constant of 0.2 nM (derived from Fig. 1). In the presence of the agonist carbachol, the M2 receptor in the membranes stimulated GTPγS binding to Gi (αi2βγ). GTPγS binding to Gαi2 was increased 10-fold in the presence of the agonist compared to the antagonist, atropine (Table 1). These results indicated that the M2 receptors in this preparation were functional and had properties similar to those previously reported for native M2 receptors [20].
Fig. 1.
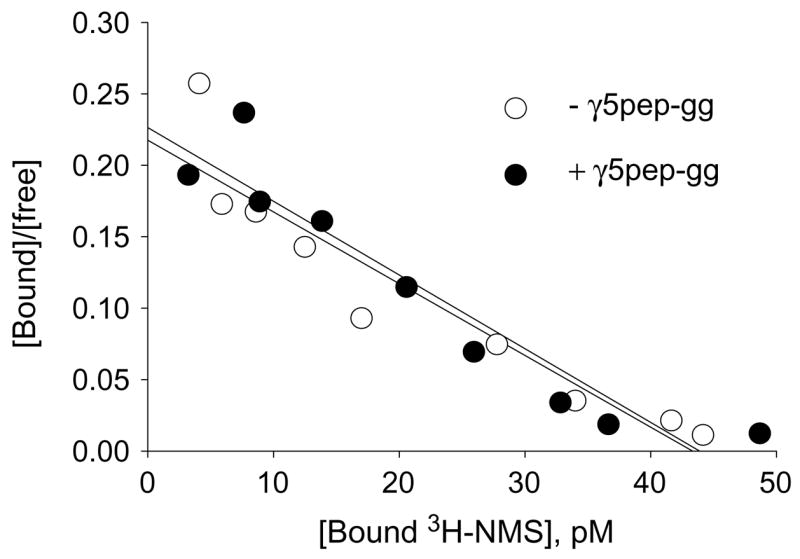
Geranylgeranylated-γ5 peptide (γ5pep-gg) does not affect NMS binding to M2. [3H]NMS binding to G protein depleted CHO cell membranes expressing M2 (50 pM) was assayed in the presence or absence of γ5pep-gg (100 nM) for 60 min at room temperature as described under Materials and methods. [3H]NMS binding was measured in a standard filter binding assay. Representative result is shown from two independent experiments in duplicate. Non-specific binding was measured with 1000-fold excess atropine over NMS and was less than 5% of total counts. Scatchard plot was created with the data and the slope (1/Kd) was calculated by linear regression using the Prism program (GraphPad Software).
Table 1.
Rate of M2 receptor stimulated GTPγS incorporation into Gi2
| fmol GTPγS/mina |
||
|---|---|---|
| Carbachol | Atropine | |
| Vehicle | 0 | 0.03 |
| αi2 (100 nM) | 0.13 | 0.04 |
| βγ(100 nM) | 0 | 0 |
| αi2βγ (100nM) | 1.14 | 0.09 |
GTPγS binding to the Gαi2 subunit was measured as described under Materials and methods. Rates were calculated by measuring [35S]GTPγS incorporation up to 3 min. Representative of three experiments.
Little or no GTPγS was incorporated in the absence of the G protein heterotrimer, in the presence of the a subunit or in the presence of the βγ complex alone (Table 1). These results indicated that the membranes were free of functional G proteins.
γ5 subunit type specific peptide inhibits G protein activation by M2
Our previous studies indicated that a peptide specific to the C terminal domain of the γ subunit of Gt, γ1, interacts with rhodopsin and in the absence of Gt stabilizes the light activated metarhodopsin II state of rhodopsin [6] [7]. To test whether G protein γ subunits in general had similar direct effects on receptors, we tested whether a peptide specific to the γ5 subunit type would similarly interact specifically with M2 and stabilize a particular state of the receptor. Accordingly, we synthesized a 14 amino acid peptide specific to the homologous region of γ5 (VSSSTNPFRPQKVC) and chemically modified it with geranylgeranyl, a C-20 isoprenoid (γ5pep-gg). The γ5 peptide was initially tested for its ability to inhibit G protein activation. If the C terminal tail of the γ subunit in Gi2 interacts with M2, the γ subunit peptide should compete with the heterotrimer for a site on the receptor. Consistent with earlier results from our laboratory [8], γ5pep-gg significantly reduced the rate of agonist-stimulated GTPγS binding to the G protein (Fig. 2). At higher concentrations the inhibitor effect was less probably because of non-specific effects of a lipidated peptide on the membranes. A peptide with the amino acid sequence of the same peptide scrambled (PSRTPVNFSQVSKC) (γ5pep-gg-scr) was significantly less effective, indicating that the activity of the peptide was specific to its amino acid sequence. This result was similar to that obtained in the rhodopsin system with peptides specific to the γ subunit of Gt [5]. The effect of the wild type peptide, γ5pep-gg, on G protein activation by the M2 receptor was also dose dependent. At 1, 3, and 10 μM concentrations, γ5pep-gg reduced G protein activation significantly more than the γ5pep-gg-scr (Fig. 2). Ten micromolar γ5pep-gg is less potent than 3 μM presumably because the hydrophobic C-20 lipid attached to the peptide leads to some aggregation and reduction in effective concentration.
Fig. 2.
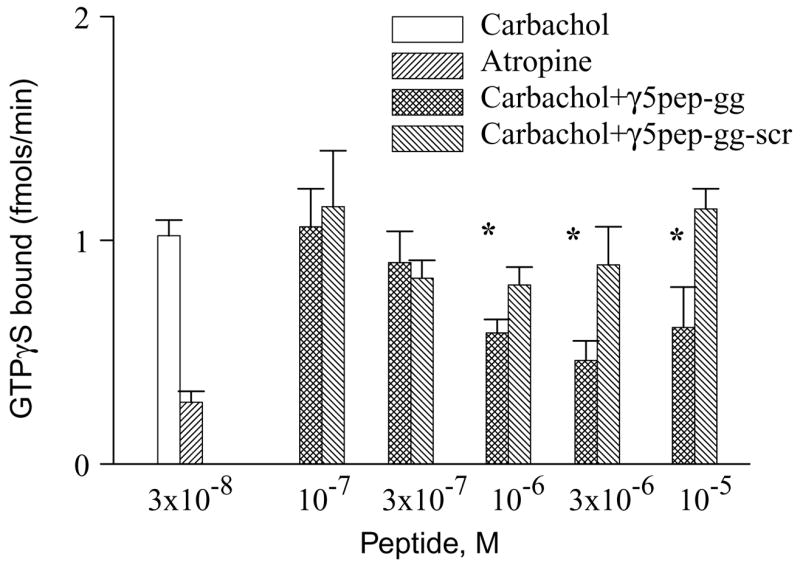
M2 receptor stimulation of GTPγS binding to G protein is inhibited by γ5pep-gg. M2 receptors in membrane suspension were preincubated with G-protein in the presence or absence of geranylgeranylated γ5 peptides as described under Materials and methods. The final concentrations of receptor and G protein were 5 nM M2 and 100 nM Gi2 heterotrimer. Binding reactions were started by the addition of [35S] GTPγS and agonist (carbachol) or antagonist (atropine). Aliquots were taken and Gα in complex with [35S] GTPγS measured as described under Materials and methods. Bars represent mean ± SEM (n ≥ 3). Asterisks denote that the activities in the presence of γ5pep-gg are significantly lower (p < 0.05) than the activities in the presence of γ5pep-gg-scr. Statistical analysis was performed as described under Materials and methods.
Two independently prepared samples of both γ5pep-gg and γ5pep-gg-scr had similar effects on the M2 receptor. We have also previously shown that the effect of the peptide is not due to disruption of the G protein heterotrimer [8]. These results therefore confirm our previous finding [8] that the γ5 subunit peptide competes for a site on the M2 muscarinic receptor with a G protein.
γ5 subunit type specific peptide significantly affects M2 receptor affinity for agonists
We then tested whether γ5pep-gg was capable of stabilizing a particular state of the M2 receptor. Since G proteins stabilize G protein coupled receptors in their high affinity state, we first tested the effect of exogenously added G protein on M2 receptor state by measuring the affinity of the receptors for an agonist (carbachol) in a radio-labeled antagonist ([3H]NMS) displacement assay. A Go heterotrimer was used here instead of the Gi2 heterotrimer used in previous assays because Go is activated more efficiently by the M2 receptor [23]. We therefore anticipated that M2 in the presence of Go would demonstrate a more significant shift in affinity for the agonist compared to Gi2. The results shown in Fig. 3A indicate that the M2 receptor binds less NMS in the presence of the G protein. This result implies that as anticipated the affinity of the M2 receptor for the agonist carbachol increases in the presence of the G protein. In addition, when GTPγS is added to the M2–G protein complex, the affinity of the receptor decreases indicating dissociation of the G protein from the receptor. This result confirms (i) that the properties of the M2 receptor here are similar to the well-known properties of G protein coupled receptors in general and (ii) that the active state of the receptor with high affinity for its agonist can be detected in this preparation.
Fig. 3.
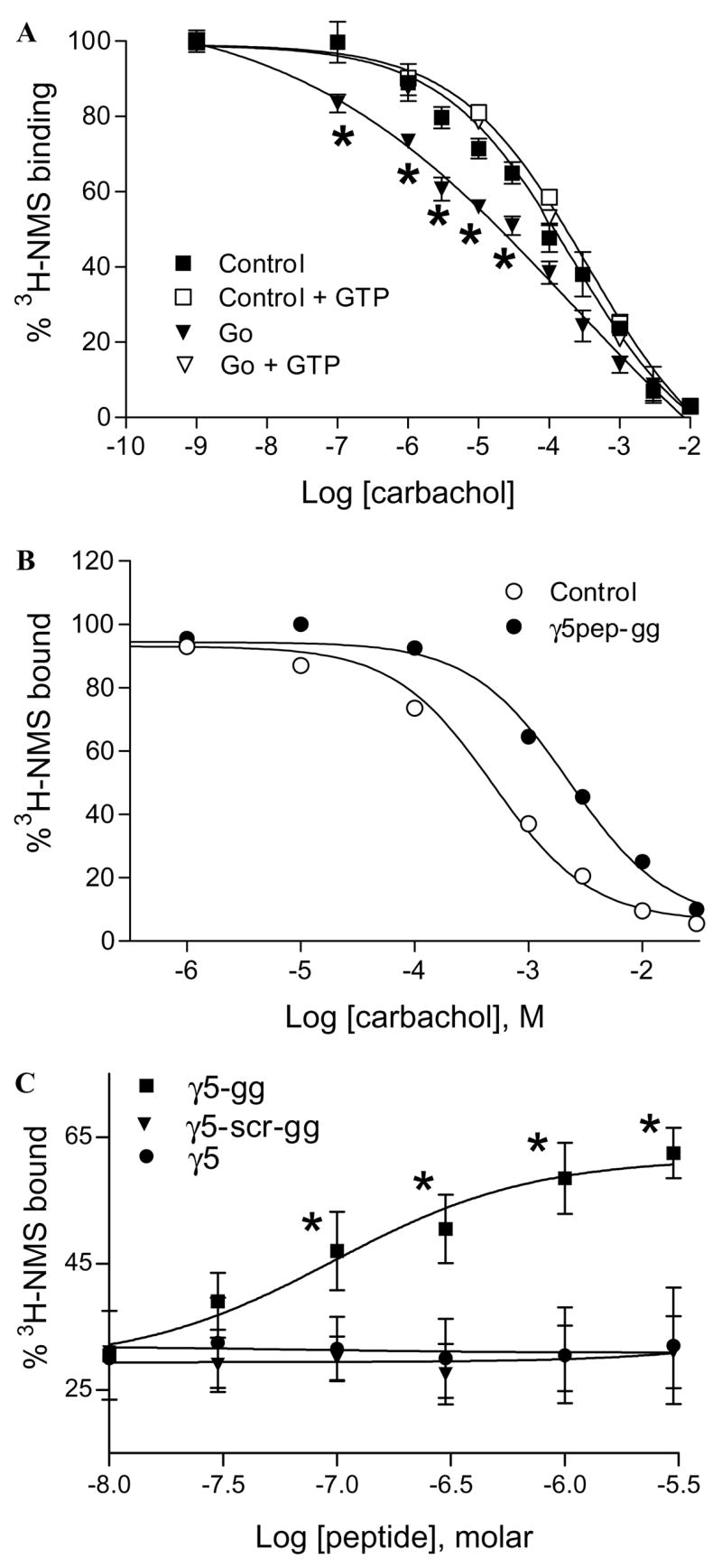
Effects of G protein and γ5 peptides on M2 affinity for carbachol. (A) G protein increases the affinity of M2 for carbachol. M2 in membrane suspension was incubated with or without the Go heterotrimer as described under Materials and methods. Samples were diluted and incubated with [3H]NMS and carbachol. Some samples were treated with 100 μM GTPγS to dissociate the G protein/receptor complexes. Samples were washed on glass filters and radioactivity was counted as described earlier. Non-specific binding was measured in the presence of 10 μM atropine. Points represent mean ± SEM (n ≥ 5). Asterisks denote that the [3H]NMS binding to the M2–Go complex is significantly lower (p < 0.05) compared to the free receptor. Data points were curve-fitted by non-linear regression using GraphPad Prism. (B) γ5pep-gg affects M2 affinity for carbachol. M2 receptors in membranes were preincubated with γ5 peptides. Samples after dilution were incubated with [3H]NMS and carbachol as described before. The final concentrations were 5 nM M2, 10 μM sodium cholate, 1 μM γ5pep-gg, 10 nM [3H]NMS, and increasing concentrations of carbachol. Representative result is shown from at least four independent experiments in duplicate. Data points were curve-fitted as described before. (C) Dose dependence of M2 receptor affinity for carbachol in the presence of γ5 peptides. The effects of peptides specific to γ5 on M2 receptor affinity for carbachol were measured as in (B). Increasing concentrations of peptides were added at 1 mM constant concentration of carbachol. Points represent means ± SEM (n ≥ 3). Asterisks denote that the M2 receptors bind significantly higher concentrations of [3H]NMS (p < 0.05) in the presence of the wild type peptide compared to the mutant peptide. The dose–response curves were generated by non-linear regression using Prism.
To test the effect of the γ5 peptide on the receptor directly, the affinity of M2 for an agonist, carbachol, was measured in the presence of γ5pep-gg and γ5pep-gg-scr in an antagonist displacement assay. As shown in Fig. 3B, in striking contrast to the G protein, γ5pep-gg significantly reduced displacement of [3H]NMS by carbachol. A different preparation of CHO cell membranes as well as purified M2 receptors reconstituted into artificial membranes provided similar results (data not shown), indicating that the effect of the γ5pep-gg on M2 receptor state was not peculiar to the M2 preparation used here. At the mole ratios of receptor:peptide used in experiments here (Figs. 2–4), γ5pep-gg did not have any effect on the receptor’s ability to bind NMS alone (Fig. 1). This indicates that the affinity of the receptor for carbachol is significantly less in the presence of the peptide. When the effect of various concentrations of γ5pep-gg was examined, a dose dependent effect of the peptide was observed on M2 receptor affinity with an EC50 of 200 nM (Fig. 3C). γ5pep-gg-scr or γ5pep (unprenylated peptide) was inactive (Fig. 3C). In addition, peptides specific to other G protein γ subunit types—γ2, γ7, and γ12—were significantly less active (data not shown). Thus, as in the case of rhodopsin [5], both the primary structure and the lipid group are individually necessary but not sufficient in themselves for peptide activity. To test whether the effect of the peptide was peculiar to carbachol, we assayed the receptor with acetylcholine in a similar assay. NMS displacement by acetylcholine was significantly reduced in the presence of γ5pep-gg (data not shown). This result indicated that γ5pep-gg reduced the affinity of M2 for a similar but distinct agonist.
Fig. 4.
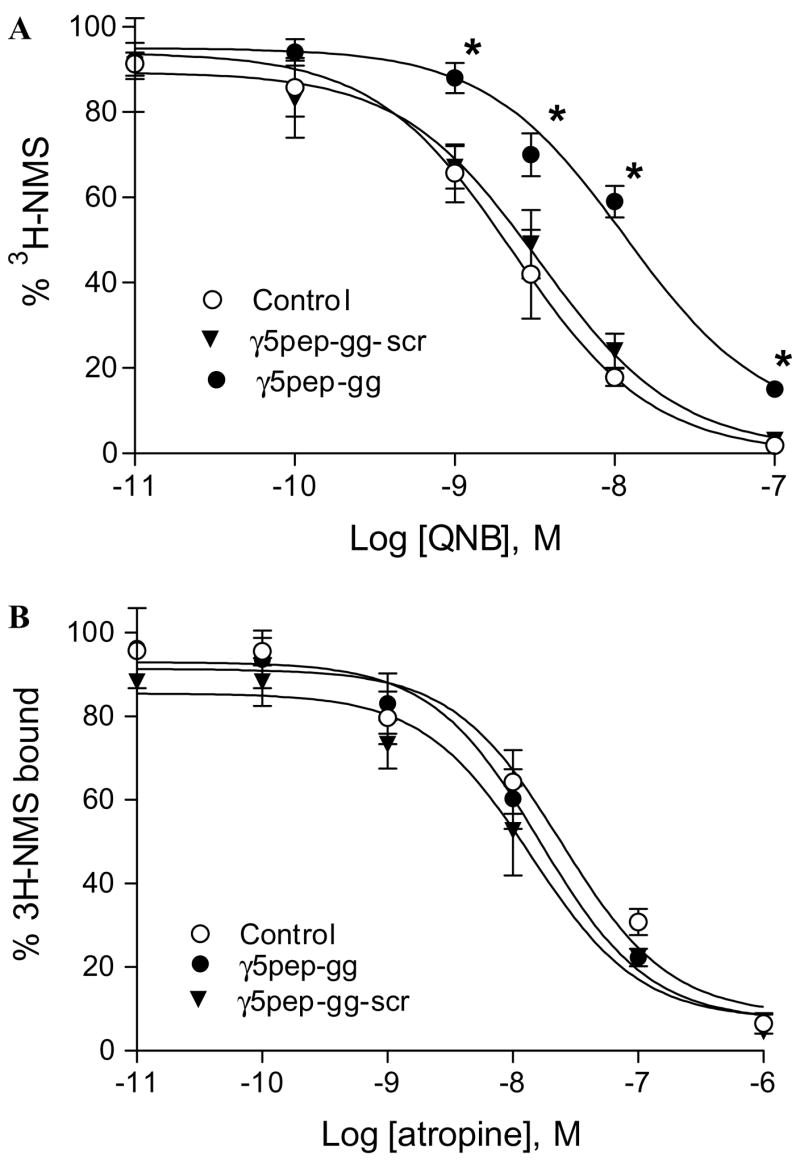
Effect of γ5 peptides on antagonist binding affinity of M2 receptor. (A) Effect of γ5pep-gg on M2 receptor binding affinity for QNB. The antagonist binding assay was performed as described in Fig. 3B legend and Materials and methods. The concentration of peptide used was 3 μM. Control represents experiments performed with the vehicle (sodium cholate). Data are expressed as means ± SEM (n = 5). Asterisks denote that NMS binding was significantly more in the presence of γ5pep-gg at these concentrations of QNB compared to either control or receptor + γ5pep-gg-scr (p < 0.05). (B) Effect of γ5pep-gg on M2 receptor binding affinity for atropine. Assay conditions were similar to those in (A). The concentration of peptide used was 3 μM. Control represents experiments performed with the vehicle (sodium cholate). Data points are expressed as means ± SEM (n = 5).
γ5pep-gg had no effect on the affinity of the β2-adrenergic receptor for an agonist (isoproterenol) and an antagonist (ICI 118–551) in a displacement assay using radio-labeled alprenolol (data not shown). This result indicates that the action of γ5pep-gg on M2 receptors is not due to a general non-specific effect on membranes or receptor conformation.
A peptide from the C terminus of the Gt γ subunit can stabilize the light activated form of rhodopsin, metarhodopsin II (MII) [5]. It has been thought that MII is the equivalent of the high affinity form of the neurohormonal receptors. The simple expectation that the homologous γ5pep-gg would stabilize the high affinity form of M2 receptors is however belied by these results. This highlights a lack of information about the precise relationship between the various states of rhodopsin and the neurotransmitter/hormone receptors.
The γ5 peptide reduces affinity for QNB but not atropine
To test whether the effect of γ5pep-gg was restricted to agonists, we assayed the M2 receptor’s affinity for two antagonists, quinuclidinyl benzilate (QNB) and atropine, again in a [3H]NMS displacement assay. NMS displacement by QNB is significantly reduced in the presence of γ5pep-gg (Fig. 4A). Since γ5pep-gg has no effect on NMS binding (Fig. 1), this result indicates that affinity for QNB is reduced in the presence of the peptide. In contrast to its effect on M2 affinity for QNB, the peptide has no effect on the affinity for a different antagonist, atropine (Fig. 4B).
The reduction in affinity of the M2 receptor for a known antagonist, QNB, in the presence of the peptide suggests that the conformation of the receptor state stabilized by the peptide is distinct from the inactive state. In an earlier report, inhibition of cAMP synthesis by over-expressed M2 receptors in CHO cells was relieved by the addition of the antagonists, NMS and atropine but not by QNB [24]. These results imply that a proportion of the receptors in cells over-expressing the M2 receptor may be in the state similar to that bound to the peptide labeled the R′ state (Fig. 5). Since the results here indicate that the R′ state binds NMS/atropine with the same affinity as the inactive R state, NMS will inhibit ‘constitutive’ signaling from M2. QNB will be much less effective because of its altered affinity.
Fig. 5.
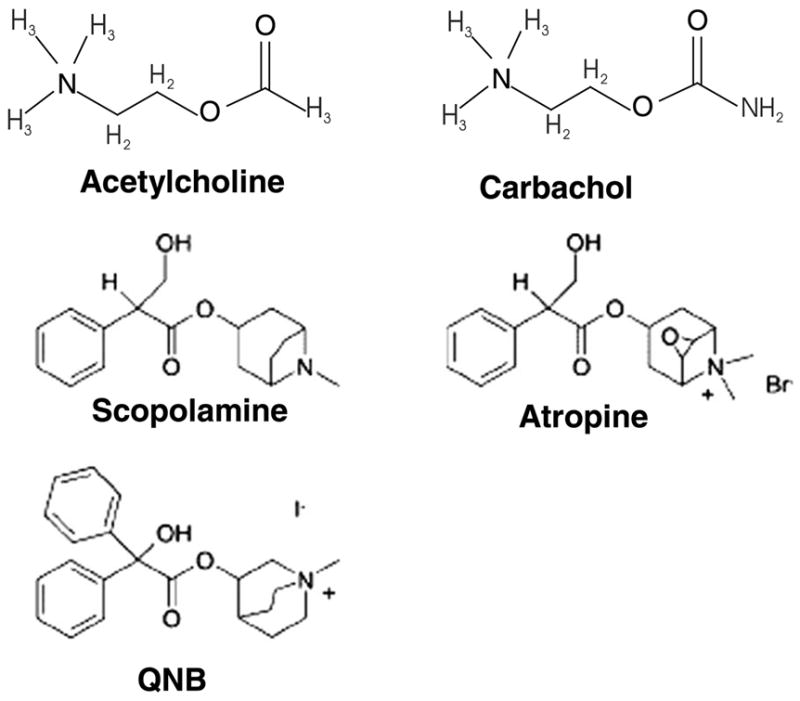
Chemical structures of agonists and antagonists of M2 receptors. NMS and atropine have similar structures. As shown carbachol is an analog of acetylcholine.
The results presented here also indicate that the R′ state of the M2 receptor must possess a distinct conformation at the ligand binding site in comparison to the inactive R state or the activated R* state. The chemical structures for the various ligands used in the experiments here are shown in Fig. 6. Inspection of the chemical structures indicates that recognition of the ligands by M2 in the presence of the peptide is influenced by the structures of the ligands. Recent models of G protein coupled receptor function have invoked the idea that they are more fluid than thought in terms of conformations with multiple potential states [14]. The results here are consistent with such a model.
Fig. 6.
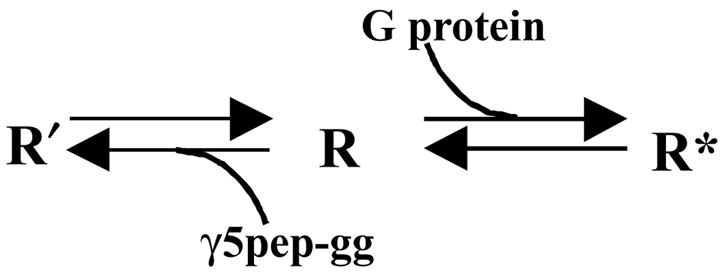
Model depicting the different states of the M2 receptor. G protein stabilizes the high affinity R* form of the receptor that initiates nucleotide exchange in the G protein α subunit. The γ5 subunit peptide stabilizes a unique conformation of the receptor R′ that has distinct properties compared to the R state.
These results also indicate that the M2 muscarinic receptor possesses a site(s) that interacts specifically with the G protein γ subunit C terminal tail confirming previous reports [4]. Consistent with the earlier reports both the prenyl moiety and the amino acid sequence are important for this interaction. The interaction is also specific to a particular γ subunit type–γ5. Peptides specific to the C termini of other γ subunit types—γ7 and γ12—do not effectively stabilize the R′ form. The ability of the γ5 subunit peptide to stabilize a particular state of the receptor suggests that potentially, the γ subunit tail interacts with a specific conformation of the receptor.
The ability to stabilize a novel state of the M2 receptor by the addition of a peptide specific to a G protein γ subunit raises the possibility of isolating drugs that bind specifically to the R′ form of the M2 receptor by screening in the presence of G protein γ subunit specific peptides. Such drugs can show a pattern of specificity for muscarinic receptor types that is different from known drugs and can also have unique effects on signaling in a cell.
Acknowledgments
This was supported by National Institutes of Health Grants GM46963 and GM69027.
References
- 1.Fung BK. Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of the subunits. J Biol Chem. 1983;258:10495–10502. [PubMed] [Google Scholar]
- 2.Hekman M, Holzhofer A, Gierschik P, Im MJ, Jakobs KH, Pfeuffer T, Helmreich EJ. Regulation of signal transfer from beta 1-adrenoceptor to adenylate cyclase by beta gamma subunits in a reconstituted system. Eur J Biochem. 1987;169:431–439. doi: 10.1111/j.1432-1033.1987.tb13630.x. [DOI] [PubMed] [Google Scholar]
- 3.Florio VA, Sternweis PC. Mechanisms of muscarinic receptor action on Go in reconstituted phospholipid vesicles. J Biol Chem. 1989;264:3909–3915. [PubMed] [Google Scholar]
- 4.Gautam N. A conformational switch regulates receptor-g protein interaction. Structure (Camb) 2003;11:359–360. doi: 10.1016/s0969-2126(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 5.Kisselev OG, Ermolaeva MV, Gautam N. A farnesylated domain in the G protein gamma subunit is a specific determinant of receptor coupling. J Biol Chem. 1994;269:21399–21402. [PubMed] [Google Scholar]
- 6.Kisselev O, Pronin A, Ermolaeva M, Gautam N. Receptor-G protein coupling is established by a potential conformational switch in the beta gamma complex. Proc Natl Acad Sci USA. 1995;92:9102–9106. doi: 10.1073/pnas.92.20.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kisselev O, Ermolaeva M, Gautam N. Efficient interaction with a receptor requires a specific type of prenyl group on the G protein gamma subunit. J Biol Chem. 1995;270:25356–25358. doi: 10.1074/jbc.270.43.25356. [DOI] [PubMed] [Google Scholar]
- 8.Azpiazu I, Cruzblanca H, Li P, Linder M, Zhuo M, Gautam N. A G protein gamma subunit-specific peptide inhibits muscarinic receptor signaling. J Biol Chem. 1999;274:35305–35308. doi: 10.1074/jbc.274.50.35305. [DOI] [PubMed] [Google Scholar]
- 9.Peralta EG, Winslow JW, Peterson GL, Smith DH, Ashkenazi A, Ramachandran J, Schimerlik MI, Capon DJ. Primary structure and biochemical properties of an M2 muscarinic receptor. Science. 1987;236:600–605. doi: 10.1126/science.3107123. [DOI] [PubMed] [Google Scholar]
- 10.Wess J, Bonner TI, Dorje F, Brann MR. Delineation of muscarinic receptor domains conferring selectivity of coupling to guanine nucleotide-binding proteins and second messengers. Mol Pharmacol. 1990;38:517–523. [PubMed] [Google Scholar]
- 11.Peterson GL, Toumadje A, Johnson WC, Jr, Schimerlik MI. Purification of recombinant porcine m2 muscarinic acetylcholine receptor from Chinese hamster ovary cells. Circular dichroism spectra and ligand binding properties. J Biol Chem. 1995;270:17808–17814. doi: 10.1074/jbc.270.30.17808. [DOI] [PubMed] [Google Scholar]
- 12.Peralta EG, Ashkenazi A, Winslow JW, Smith DH, Ramachandran J, Capon DJ. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987;6:3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen CA, Schroering AG, Robishaw JD. Subunit expression of signal transducing G proteins in cardiac tissue: implications for phospholipase C-beta regulation. J Mol Cell Cardiol. 1995;27:471–484. doi: 10.1016/s0022-2828(08)80043-0. [DOI] [PubMed] [Google Scholar]
- 14.Perez DM, Karnik SS. Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev. 2005;57:147–161. doi: 10.1124/pr.57.2.2. [DOI] [PubMed] [Google Scholar]
- 15.Hou Y, Azpiazu I, Smrcka A, Gautam N. Selective role of G protein gamma subunits in receptor interaction. J Biol Chem. 2000;275:38961–38964. doi: 10.1074/jbc.C000604200. [DOI] [PubMed] [Google Scholar]
- 16.Linder ME, Kleuss C, Mumby SM. Palmitoylation of G-protein alpha subunits. Methods Enzymol. 1995;250:314–330. doi: 10.1016/0076-6879(95)50081-2. [DOI] [PubMed] [Google Scholar]
- 17.Sternweis PC, Robishaw JD. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- 18.Kozasa T, Gilman AG. Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits. Characterization of alpha 12 and inhibition of adenylyl cyclase by alpha z. J Biol Chem. 1995;270:1734–1741. doi: 10.1074/jbc.270.4.1734. [DOI] [PubMed] [Google Scholar]
- 19.Pronin AN, Gautam N. Characterization of antibodies for various G-protein beta and gamma subunits. Methods Enzymol. 1994;237:482–498. doi: 10.1016/s0076-6879(94)37085-0. [DOI] [PubMed] [Google Scholar]
- 20.Hulme EC, Birdsall NJ, Buckley NJ. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- 21.Hirschberg BT, Schimerlik MI. A kinetic model for oxotremorine M binding to recombinant porcine m2 muscarinic receptors expressed in Chinese hamster ovary cells. J Biol Chem. 1994;269:26127–26135. [PubMed] [Google Scholar]
- 22.Shiozaki K, Haga T. Effects of magnesium ion on the interaction of atrial muscarinic acetylcholine receptors and GTP-binding regulatory proteins. Biochemistry. 1992;31:10634–10642. doi: 10.1021/bi00158a028. [DOI] [PubMed] [Google Scholar]
- 23.Parker EM, Kameyama K, Higashijima T, Ross EM. Reconstitutively active G protein-coupled receptors purified from baculovirus-infected insect cells. J Biol Chem. 1991;266:519–527. [PubMed] [Google Scholar]
- 24.Jakubik J, Bacakova L, el-Fakahany EE, Tucek S. Constitutive activity of the M1–M4 subtypes of muscarinic receptors in transfected CHO cells and of muscarinic receptors in the heart cells revealed by negative antagonists. FEBS Lett. 1995;377:275–279. doi: 10.1016/0014-5793(95)01360-1. [DOI] [PubMed] [Google Scholar]


