Abstract
Airway epithelia are confronted with distinct signals emanating from the luminal and/or serosal environments. This study tested whether airway epithelia exhibit polarized intracellular free calcium (Ca2+ i) and anion secretory responses to 5′ triphosphate nucleotides (ATP/UTP), which may be released across both barriers of these epithelia. In both normal and cystic fibrosis (CF) airway epithelia, mucosal exposure to ATP/UTP increased Ca2+ i and anion secretion, but both responses were greater in magnitude for CF epithelia. In CF epithelia, the mucosal nucleotide–induced response was mediated exclusively via Ca2+ i interacting with a Ca2+-activated Cl− channel (CaCC). In normal airway epithelia (but not CF), nucleotides stimulated a component of anion secretion via a chelerythrine-sensitive, Ca2+-independent PKC activation of cystic fibrosis transmembrane conductance regulator. In normal and CF airway epithelia, serosally applied ATP or UTP were equally effective in mobilizing Ca2+ i. However, serosally applied nucleotides failed to induce anion transport in CF epithelia, whereas a PKC-regulated anion secretory response was detected in normal airway epithelia. We conclude that (1) in normal nasal epithelium, apical/basolateral purinergic receptor activation by ATP/UTP regulates separate Ca2+-sensitive and Ca2+-insensitive (PKC-mediated) anion conductances; (2) in CF airway epithelia, the mucosal ATP/UTP-dependent anion secretory response is mediated exclusively via Ca2+ i; and (3) Ca2+ i regulation of the Ca2+-sensitive anion conductance (via CaCC) is compartmentalized in both CF and normal airway epithelia, with basolaterally released Ca2+ i failing to activate CaCC in both epithelia.
Keywords: cystic fibrosis transmembrane conductance regulator, purinergic receptors, triphosphate nucleotides, protein kinase C, anion secretion
INTRODUCTION
The pathogenesis of cystic fibrosis (CF) lung disease is complex, but likely involves abnormal regulation of the airway surface liquid. Airway surface liquid volume regulation reflects the integrated function of cystic fibrosis transmembrane conductance regulator (CFTR) as a Cl− channel and as a regulator of the epithelial Na+ channel. Further, there is compelling evidence that a second, calcium-activated Cl− channel (CaCC) pathway exists in the apical membrane of airway epithelia (Al-Bazzaz and Jayaram 1981; Barthelson et al. 1987; Welsh 1987; Boucher et al. 1989; Willumsen and Boucher 1989; Hartmann et al. 1992), which is regulated by increases in intracellular free Ca2+ (Ca2+ i). In the airway of the CFTR(−/−) knockout mouse (Snouwaert et al. 1992), the CaCC pathway is preserved, and, in some regions, upregulated (Grubb and Boucher 1999). Similarly, in human CF airway epithelia, this Ca2+ i-regulated Cl− conductance may also be upregulated, as initially revealed by studies with Ca2+ ionophores (Boucher et al. 1989; Willumsen and Boucher 1989).
Extracellular triphosphate nucleotides are released in response to local stresses in the airways and may exert autocrine/paracrine effects on ion transport (Leuba et al. 1996; Taylor et al. 1998; Homolya et al. 2000). Triphosphate nucleotides have been reported to stimulate Cl− secretion through non-CFTR, CaCC-dependent mechanisms (Mason et al. 1991), both in normal and CF airway epithelia (Knowles et al. 1991; Clarke and Boucher 1992). External ATP and UTP mediate their effects in airway epithelia, at least in part, via interactions with the P2Y2 purinergic receptor (P2Y2-R; Parr et al. 1994). In human airway epithelia, P2Y2-R is linked to PLC-generated inositol(1,4,5) trisphosphate (IP3)–mediated release of Ca2+ i (Brown et al. 1991). However, the transduction pathways that link occupancy of airway P2Y2-Rs to Cl− secretion are complex, and previous reports raise questions with respect to differences in the polarity of nucleotide responses in normal airway epithelia and differences in the pattern of nucleotide responses exhibited by normal and CF airway epithelia (Mason et al. 1991; Clarke and Boucher 1992). The following three examples, juxtaposing independent observations from electrophysiologic (Cl− secretion) and imaging studies (ΔCa2+ i), illustrate these questions.
First, whereas activation of apical P2Y2-Rs by nucleotides evoked smaller changes in Ca2+ i than basolateral P2Y2-R activation (Paradiso et al. 1995), experiments by Clarke and Boucher 1992 showed that the relationship for Cl− secretion was inverse, i.e., the magnitude of the Cl− secretory response was greater after apical than after basolateral additions of extracellular nucleotides. These studies raised the issue of whether the membrane location (apical/basolateral) of nucleotide-induced Ca2+ release, and hence local Ca2+ i concentration, are important for Cl− secretion in airway epithelia. Second, in cultured normal airway epithelial cells prestimulated with ionomycin to maximally raise Ca2+ i activity, treatment with the endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin evoked no additional Cl− secretion, whereas luminal ATP activated an additional Cl− secretory response without a further rise in Ca2+ i (Stutts et al. 1994). This finding suggested the possibility that extracellular nucleotides regulate Cl− secretion via Ca2+-independent pathways (e.g., PKC) and, perhaps, via a different Cl− channel (e.g., CFTR). Third, in normal human airways, basolateral application of ATP induced a Cl− secretory response, whereas this response could not be detected in CF (Clarke and Boucher 1992) despite similar changes in Ca2+ i (Mason et al. 1991), raising the possibility of compartmentalization of Ca2+ i signaling in columnar airway epithelial cells (Paradiso 1997).
In the present study, we sought to test the relative roles of polarized Ca2+ i mobilization and PKC activation in response to mucosal versus serosal nucleotide administration in normal and CF airway epithelia. We specifically explored the hypotheses that (1) in normal airway epithelia, luminal addition of nucleotides activates both CaCC and CFTR, whereas basolateral addition activates only CFTR; (2) in CF airway epithelia, apical P2Y2-R activation is effective in activating only CaCC; and (3) Ca2+ i signals evoked by serosal nucleotides in both normal and CF are functionally confined to the basolateral domain. For these experiments, we developed the necessary measurement systems that permitted simultaneous measurements of Ca2+ i and anion secretion.
MATERIALS AND METHODS
Tissue Samples
Nasal epithelial cells were obtained from 10 normal subjects (34 ± 7 yr old [four males and six females]) undergoing elective surgery for standard medical indications (e.g., sleep apnea secondary to nasal obstruction), and eight CF patients (16 ± 5 yr old [four males and four females]). All procedures were approved by the University of North Carolina Committee for the Rights of Human Subjects.
Chemicals and Solutions
Acetoxymethyl ester (AM) of Fura-2, 4,4′ diisothiocyanatodihydrostilbene-2,2′-disulfonic acid (H2DIDS), and BAPTA were purchased from Molecular Probes. Chelerythrine chloride and PMA were purchased from Alexis Biochemical. ATP and UTP were obtained from Boehringer Mannheim Biochemical. Ionomycin and thapsigargin were obtained from Calbiochem-Novabiochem. All other chemicals were obtained from Sigma-Aldrich.
Stock solutions of Fura-2/AM, BAPTA-AM, ionomycin, PMA, chelerythrine, and thapsigargin, all at 1 mM, were dissolved in DMSO and stored up to 30 d at −20°C without a loss of potency of the drugs. Stock solutions of N-methyl-d-glucamine (NMG) gluconate and NMG-Cl (both at 0.5 M, pH 7.0) were prepared by mixing NMG base with equimolar concentrations of d-gluconic acid lactone and HCl, respectively, and stored up to 1 wk at 4°C. For Na+-free, HCO3 −-containing solutions, a stock solution of NMG-HCO3 (1 M, pH 7.0) was prepared by bubbling NMG base (1 M) with 100% CO2.
The standard Kreb's bicarbonate Ringer (KBR) solution contained (in mM): 125 NaCl, 2.5 K2HPO4, 1.3 CaCl2, 1.3 MgCl2, 25 NaHCO3, and 5 d-glucose (5% CO2/95% O2). For NMG-Cl Ringer solution, NaCl and NaHCO3 were replaced mole for mole by NMG-Cl and NMG-HCO3, respectively. For NMG-gluconate (low Cl−) Ringer, NaCl and NaHCO3 were replaced mole for mole with NMG-gluconate and NMG-HCO3 -, respectively (final Cl− = 2.6 mM) and 2 mM CaSO4 was added to the solution to compensate for Ca2+ chelation by gluconate, as previously reported (Clarke and Boucher 1992).
Cell Culture, Perfusion Chamber, and Bioelectric Measurements
Nasal epithelial cells were harvested from polyps by enzymatic digestion (Protease XIV [Sigma-Aldrich] for 24-48 h at 4°C] as previously described (Wu et al. 1985). Nasal epithelial cells were plated on porous Transwell Col filters (pore diam = 0.4 μm; Costar) affixed to O-rings and maintained in serum-free Ham's F12 medium supplemented with insulin (10 μg/ml), transferrin (5 μg/ml), 3,3′,5-triiodothyronine (3 × 10−8 M), endothelial cell growth supplement (3.75 μg/ml), hydrocortisone (5 × 10−9 M), and CaCl2 (10−3 M). Polarized monolayers were studied 7–12 d after seeding, a time coincident for the development of the maximal transepithelial potential difference (Vt). For normal nasal epithelium Vt = −9.8 ± 1.2 mV mucosal side negative and chord resistance (RT) = 408 ± 32 Ω·cm2 (n = 90; 10 different individuals); for CF Vt = −21.7 ± 1.4 mV, and RT = 396 ± 38 Ω·cm2 (n = 85; 8 different individuals).
After confluency was achieved, polarized monolayers of normal or CF epithelium were loaded with Fura-2 (5 μM at 37°C for 25 min) or coloaded with 100 μM BAPTA-AM ± 1 μM chelerythrine chloride before being mounted in a miniature Ussing chamber over an objective (Zeiss LD Achroplan 40×, NA 0.6; working distance 1.8 mm) of a Zeiss Axiovert 35 microscope. The chamber is similar in design to that described by Larsen et al. 1990 for sweat duct epithelial cells in primary culture. In brief, a perfusion chamber was constructed in which solutions bathing apical and basolateral surfaces could be changed rapidly and independently. The chamber consists of top (mucosal) and bottom (serosal) half-chambers (volume = 250 μl each) made from light-absorbing polyacetal. The Transwell wafer containing the cell monolayer was mounted apical surface up, between the two half-chambers. Effective sealing is achieved by means of rubber O-rings embedded in grooves of the two half-chambers, which are screwed tightly together. A glass coverslip is affixed to the bottom of the serosal chamber with dental sticking wax (model Deiberit-502; Ludwig Bohme). The mucosal chamber is open to the atmosphere. Both half-chambers have inlet and outlet ports for solution flow. When mounted and tightly sealed, the monolayer is ∼1.5 mm from the glass coverslip. For passing current, two circular Ag/AgCl electrodes are placed in the two half-chambers to secure a uniform density of current through the preparation. For measurements of the Vt, polyethylene bridges containing 2 M KCl in 2% agar are positioned in the two half-chambers and connected to calomel electrodes (Radiometer).
The chamber system also has a perfusion incubator with eight mucosal and eight serosal reservoirs containing Ringer solution, which are uniformly prewarmed to ∼42°C with a small heater and fan. The prewarmed Ringer solutions are delivered to the chamber by gravity flow (rate = 3–5 ml/min) through tubing that is kept as short as possible (∼20 cm) to minimize heat loss. Serosal and mucosal solutions are changed by using two eight-port valves (Hamilton). The half-time for solution exchange is <3 s.
For bioelectric measurements, Vt was monitored by a voltage clamp/pulse generator (model VCC600; Physiologic Instruments) and the signal was recorded on a two-channel recorder (model L2005; Linseis). All experiments were performed under open circuit conditions. To calculate the equivalent current from changes in Vt in response to purinergic receptor activation, a defined (1 or 2 μA) 1-s current pulse was delivered across the tissue every 5 s (see Fig. 1), and from the magnitude change in the deflection of Vt, the chord resistances, and subsequent equivalent currents, were calculated using Ohm's law. To convert the tissue from its native Na+ absorptive state and increase the transepithelial Cl− driving force, nasal monolayers were exposed to Na+-free/low Cl− Ringer in the mucosal bath with KBR remaining constant in the serosal perfusate.
Figure 1.
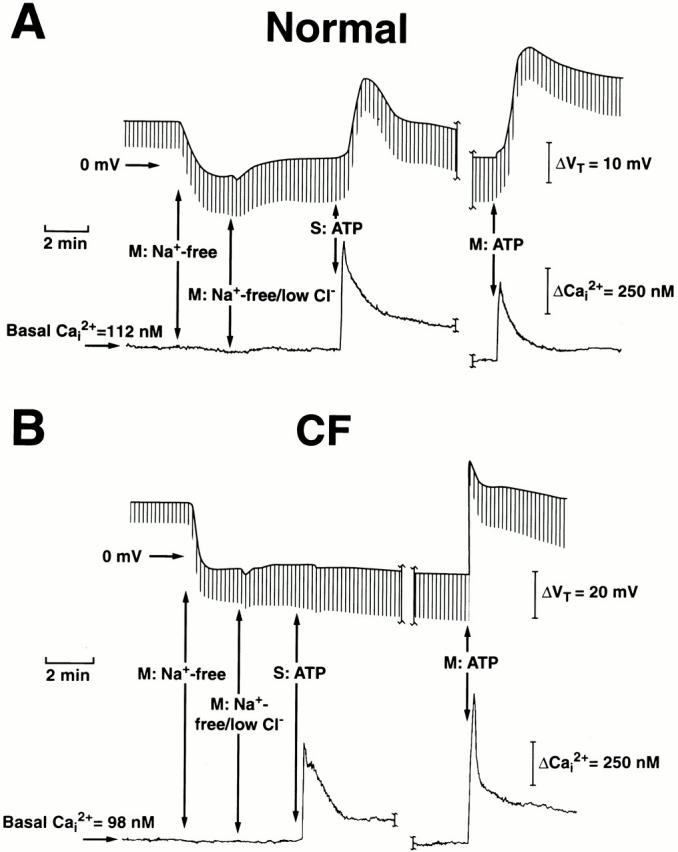
Simultaneous measurements of intracellular Ca2+ (Ca2+ i) and transepithelial potential difference (Vt) in normal (A) and cystic fibrosis (CF; B) nasal epithelia. Monolayers of nasal epithelial cells were initially perfused bilaterally in Kreb's bicarbonate Ringer before changing to mucosal Na+-free (N-methyl-d-glucamine [NMG]) Cl−), and then Na+-free/low Cl− (2.6 mM; NMG gluconate) Ringer. 100 μM ATP was added to the serosal (S) or mucosal (M) bath as indicated. The break in the traces represents a washout period (∼30 min) for serosal ATP before adding mucosal ATP to the perfusate. At 100 μM ATP, each trace is representative of at least 85 independent experiments (eight or more different individuals).
Fluorimeter and Measurements of Ca2+ i
Measurements of Ca2+ i in monolayers were obtained using a RadioMaster fluorimeter (Photon Technology International) coupled via fiber optics to the microscope. Fura-2 fluorescence from 30–40 cells (spot diameter ∼65 μm) was acquired alternately at 340 and 380 nm (emission > 450 nm). Excitation slit widths were minimized to reduce photodamage to cells and bleaching of the dye. At a given excitation wavelength (340 or 380 nm), background light levels were measured by exposing cells to digitonin (15 μM) and MnCl2 (10−3 M) and subtracted from the corresponding signal measured in Fura-2–loaded cells before taking the ratio (340/380). The corrected ratio was converted to Ca2+ i by using external Ca2+ standards as described previously (Paradiso et al. 1995).
Data Analysis
Where applicable, data are presented as the mean ± SEM for a given experimental condition. All of the data presented in summary form are expressed as the absolute change (Δ) in Ca2+ i and anion secretion (peak basal values) before and after the addition of ATP/UTP to monolayers. Negative equivalent currents refer to the luminal side of the monolayer, negative with respect to the serosal side (Clarke and Boucher 1992). The mean effective dose (ED50) was calculated from the Boltzman model (Willard et al. 1974). Statistical significance was determined using the t test, with P < 0.05 being considered significant.
RESULTS
Simultaneous Measurements of Cell Ca2+ and Anion Transport in Nasal Monolayers
To compare the effects of apical versus basolateral triphosphate nucleotides on changes in Ca2+ i and anion secretion, we chose experimental conditions that maximized the signal generated by anion movement through the apical membrane by bathing the preparations with Na+-free, low Cl− Ringer. Representative tracings depicting simultaneous measurements of anion secretion and changes in Ca2+ i induced by serosal or mucosal administration of ATP (100 μM) in normal and CF epithelial cell preparations are shown in Fig. 1 (A and B). The downward deflections shown in each tracing (Fig. 1A and Fig. B) were produced by passing a current pulse across the tissue as described in materials and methods, and the break in the tracings represents a 30-min washout period after serosal ATP treatment before exposing monolayers to mucosal ATP.
Under these conditions, there exist chemical gradients across the intercellular shunt for Na+ and Cl−. These gradients generate electromotive forces that contribute to basal Vt, but previous equivalent circuit analyses have shown that ATP (Clarke and Boucher 1992) or ionomycin (Willumsen and Boucher 1989) elicited no significant changes in shunt resistance. Thus, the change from basal Vt evoked by triphosphate nucleotides likely reflects changes in the electrical diffusion potential dominated by transcellular Cl− secretion as previously reported (Lazarowski et al. 1997). However, we cannot rule out other anions secreted during purinergic receptor activation in human airway epithelia. For example, HCO3 − has been implicated as a relevant anion during transepithelial ion transport (Illek et al. 1997; Clarke and Harline 1998). Moreover, CFTR has a finite, but small, permselectivity for HCO3 − (Linsdell et al. 1999), and CaCC has been reported to conduct HCO3 − in CFTR knockout gallbladder epithelium (Clarke et al. 2000). Therefore, the more appropriate designation for the secretory pattern induced by P2Y2-R activation, in our present study, is anion secretion.
Asymmetric Responses of Ca2+ i and Anion Secretion to Serosal and Mucosal ATP
In normal airway epithelia (Fig. 1 A), serosal ATP induces an initial rapid (6–8 s) increase in Ca2+ i (spike), followed by a relaxation of Ca2+ i levels to a sustained plateau over the next 2–3 min. We have previously shown in normal nasal monolayers that the initial spike in Ca2+ i, in response to serosal ATP, was entirely due to an internal Ca2+ release that was functionally confined to the basolateral domain of cells, whereas the sustained plateau phase results from influx of Ca2+ solely across the basolateral membrane of cells (Paradiso et al. 1995). Concomitant with these changes of Ca2+ i, serosal ATP elicited changes in Vt, reaching peak hyperpolarizing values more slowly (i.e., ΔVt in which the luminal side becomes more negative with respect to the serosal bath) than the Ca2+ i peak (30–60 s), then relaxing to a sustained plateau level over the next 2–4 min.
As with serosal ATP additions, mucosal ATP elicited a similar, but moderately smaller, biphasic change in Ca2+ i in normal airway epithelia (Fig. 1 A; also see Fig. 2). We have previously demonstrated (Paradiso et al. 1995) that the initial spike of Ca2+ i in response to a mucosal ATP challenge results from internal Ca2+ release from stores in the apical domain of cells, and the sustained plateau results from Ca2+ influx solely across the apical membrane of these cells. However, in contrast to the pattern of Vt changes noted with serosal ATP, mucosally applied ATP induced a larger hyperpolarizing change in Vt, a more rapid rise to peak values (15–30 s), and sustained levels that were higher (see Fig. 2) than those detected for serosal application of the triphosphate nucleotide.
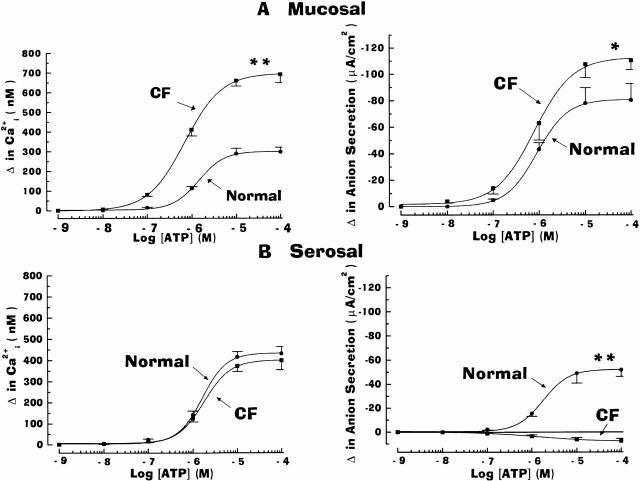
Figure 2.
Dose–response relationship for the effects of ATP on the change in anion secretion and intracellular Ca2+ (Ca2+ i) in normal and cystic fibrosis (CF) nasal epithelia. In monolayers perfused in serosal Kreb's bicarbonate Ringer and luminal Na+-free/low Cl− Ringer, ATP (10−9–10−4 M) was added to either mucosal (A) or serosal (B) bathing solution. The change in anion transport and Ca2+ i was calculated as the absolute difference between maximal ATP-stimulated anion transport and Ca2+ i and steady-state equivalent current and Ca2+ i before ATP addition. Each data point represents mean ± SEM for at least six independent experiments (six or more different individuals). Normal and CF values are significantly different (*P < 0.05 and **P < 0.01) from each other. For basolateral changes in Ca2+ i, values were not significantly different (P > 0.05; symbol not shown) between the normal and CF epithelia. For normal airway, the change in anion secretion and Ca2+ i in response to apically applied ATP was significantly different (P < 0.05; symbol not shown) from basolaterally applied ATP.
For CF nasal epithelium, ATP added to the basolateral surface of cells elicited an identical pattern of change of Ca2+ i, as was noted with serosal application of ATP in the normal airway epithelium (Fig. 1 B; also see Fig. 2). However, rather than eliciting a hyperpolarization of Vt, the serosal addition of ATP resulted in a small depolarization of Vt (i.e., ΔVt in which the luminal side becomes more positive with respect to the serosal bath) in CF (Fig. 1 B). Mucosal administration of ATP resulted in a markedly larger (Fig. 1 and Fig. 2), biphasic change of Ca2+ i in CF compared with normal nasal cells. These changes in Ca2+ i were associated with a very rapid (8–10 s) hyperpolarization of Vt, reaching higher initial (relative to normal nasal) peak values within 10–15 s, before relaxing to sustained levels over the next 1–2 min.
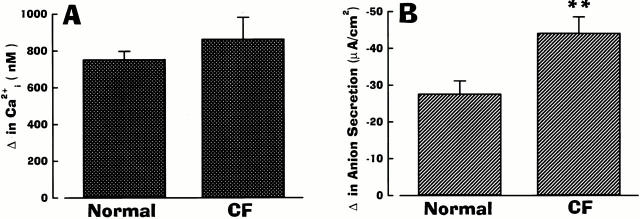
Fig. 2 summarizes the dose–response relationships between Ca2+ i and anion transport induced by mucosal (Fig. 2 A) or serosal (Fig. 2 B) ATP in normal and CF human airway epithelia. Mucosal addition of ATP (Fig. 2 A) was more efficacious in eliciting both changes in Ca2+ i and anion secretion in CF as compared with normal airway epithelium. For both normal and CF, maximal responses of Ca2+ i and anion secretion were obtained at 10−5–10−4 M. In both tissues, ATP was equipotent for ΔCa2+ i and Δ anion secretion: for the normal airway, the ED50 for Ca2+ i mobilization and anion transport were 1.04 × 10−6 M and 1.02 × 10−6 M, respectively; for CF preparations, the ED50 for Ca2+ i and anion transport were 0.69 × 10−6 M and 0.78 × 10−6 M, respectively.
Several key observations in normal and CF tissues were revealed by serosal administration of ATP (Fig. 1 and Fig. 2). First, in contrast to mucosal ATP, serosally applied ATP was equally effective in mobilizing Ca2+ i in normal and CF tissues, with the maximal efficacy obtained at 10−5–10−4 M. In terms of potencies, the ED50 for Ca2+ i in normal and CF were 1.44 × 10−6 M and 1.58 ×10−6 M, respectively. These potency values of nucleotide-mobilized Ca2+ i were not distinguishable from values obtained for mucosal administration of ATP determined in normal and CF airway epithelia. Second, in normal airway epithelia, the anion secretory response to mucosal ATP was greater than after serosal ATP addition, despite the larger ΔCa2+ i for serosal compared with mucosal ATP administration. Finally, in stark contrast to normal nasal tissue, serosally applied ATP failed to induce anion secretion in CF airway tissue.
Effects of UTP on Changes in Ca2+ i and Anion Secretion in Airway Epithelia
Because ATP can be hydrolyzed to other agonists (e.g., adenosine) that modulate anion secretion via cAMP-dependent regulation of CFTR (Lazarowski et al. 1992), we next examined changes in Ca2+ i and anion transport to UTP. Note that UTP is a potent P2Y2-R agonist (Mason et al. 1991) and its hydrolysis product, uridine, does not activate adenosine receptors present in airway epithelia (Lazarowski et al. 1992). Because of the limited availability of CF tissues, only a single dose of UTP (100 μM) was tested.
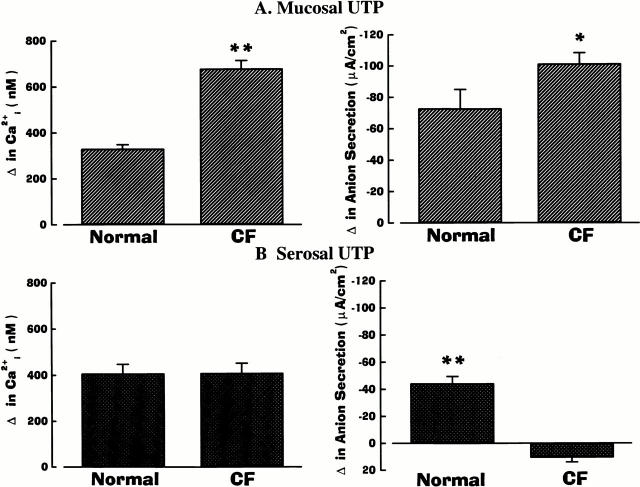
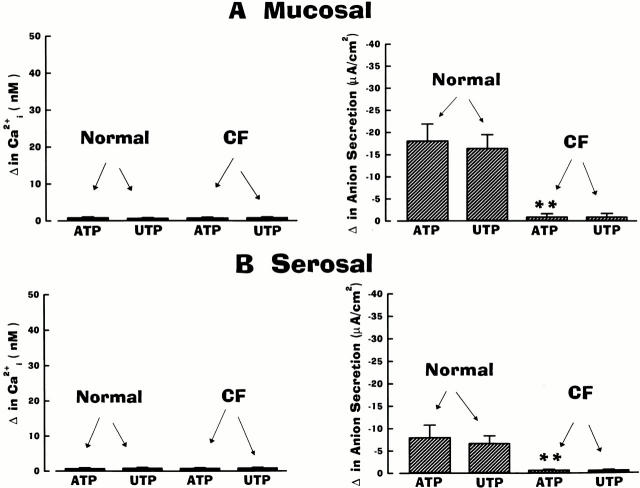
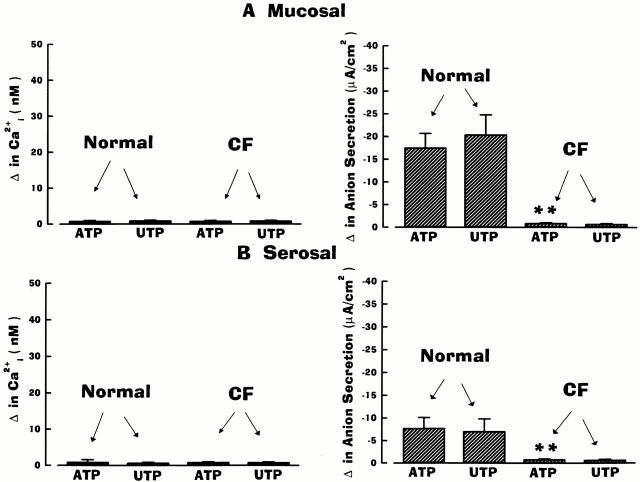
As presented in Fig. 3, mucosal/serosal administration of UTP elicited the same asymmetric pattern of responses in cell Ca2+ and anion secretion as ATP in normal and CF airway epithelia. Mucosal additions of UTP (Fig. 3 A) were effective in mobilizing Ca2+ i and increasing anion transport in normal and CF airway preparations, but both responses were again of greater magnitude in CF. Serosal administration of UTP was again effective in raising Ca2+ i to the same extent in normal and CF airways (Fig. 3 B). Like the ATP-induced anion responses in normal airway epithelium, the anion secretory response upon serosal UTP administration was smaller than after mucosal addition, despite the larger change in Ca2+ i (Fig. 3). Again, in contrast to normal nasal tissues, serosal UTP failed to evoke anion secretion in CF tissues (Fig. 3 B).
Figure 3.
Summary data on anion secretion and intracellular Ca2+ (Ca2+ i) in normal and cystic fibrosis (CF) nasal epithelia exposed to 100 μM of mucosal (A) or serosal (B) UTP. Normal and CF values are significantly different (*P < 0.05 and **P < 0.01) from each other. For basolateral changes in Ca2+ i, values were not significantly different (P > 0.05; symbol not shown) between normal and CF epithelia. Each bar represents the mean ± SEM for eight independent experiments (five different individuals).
As noted above, because of the limited availability of tissues, only a single dose of UTP was tested on CF airway epithelia. However, in experiments performed in normal airway, mucosal/serosal UTP (10−9–10−4 M; n = 5 per dose; three or more individuals) was equipotent with ATP for ΔCa2+ i and Δ anion secretion. For mucosal addition of UTP, the ED50 for Ca2+ i mobilization and anion transport was 0.98 × 10−6 M and 1.06 × 10−6 M, respectively; for serosal application of UTP, the ED50 for Ca2+ i and anion transport was 1.12 × 10−6 M and 1.08 × 10−6 M, respectively.
Protocols Using H2DIDS to Block the Ca2+-activated Anion Conductance
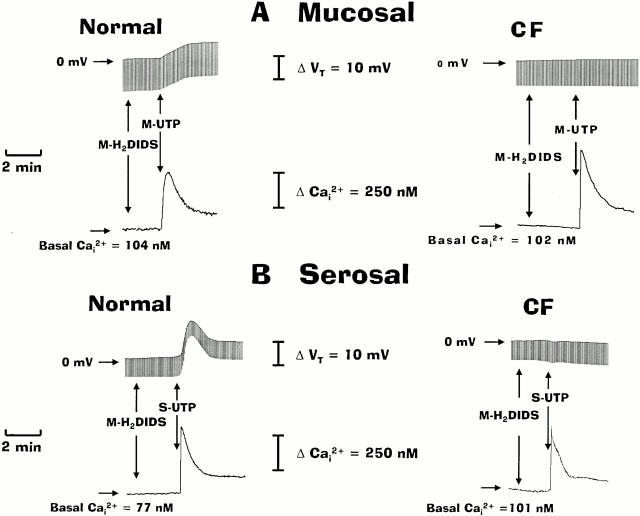
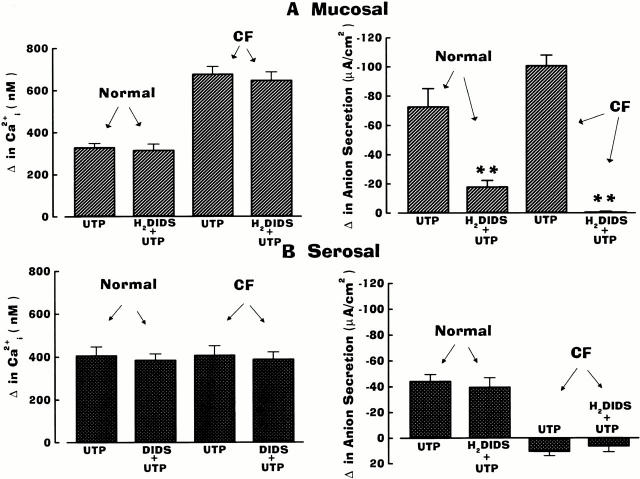
Because CFTR-mediated Cl− secretion in normal airway is resistant to inhibition by disulfonic stilbene derivatives (e.g., DIDS), whereas Ca2+-mediated Cl− transport is blocked by these drugs (Stutts et al. 1994), we next examined the contribution of CFTR to UTP-induced anion secretion when CaCC was inhibited by H2DIDS. Representative traces shown in Fig. 4 depict the responses of Ca2+ i and anion transport activities in normal and CF nasal epithelial monolayers treated first with mucosal H2DIDS (1.5 mM) before UTP (100 μM) additions. In the normal human airway, H2DIDS markedly inhibited anion secretion (∼75%) in response to mucosal UTP, and H2DIDS abolished anion transport activity in the CF airway tissue exposed to mucosal UTP (Fig. 4 A and 5). In contrast to mucosal UTP addition, pretreatment of normal nasal tissues with H2DIDS had no inhibitory effects on anion secretory responses to serosal UTP (Fig. 4 B and 5). In CF airway epithelium, serosal UTP elicited a depolarization in Vt in the presence of H2DIDS, similar to that detected in H2DIDS-free experiments (Fig. 4 B). Importantly, mucosal H2DIDS did not block changes in Ca2+ i in response to mucosal or serosal UTP in either normal or CF nasal tissues (Fig. 4), suggesting that the inhibition of anion secretion with mucosal UTP in both normal and CF nasal tissues was not due to inhibition of P2Y2-R–dependent Ca2+ i signals. The data derived from these studies are summarized and compared with the data generated in tissues that were not pretreated with H2DIDS (Fig. 5).
Figure 4.
Effects of H2DIDS and UTP applied to the mucosal (A) or serosal (B) compartments on the transepithelial potential difference (Vt) and intracellular Ca2+ (Ca2+ i) in normal and cystic fibrosis (CF) nasal monolayers. For these protocols, monolayers were perfused serosally with Kreb's bicarbonate Ringer and mucosally with Na+-free/low Cl− Ringer before adding H2DIDS (1.5 mM) to the mucosal perfusate as indicated in the traces. Serosal (S) or mucosal (M) UTP (100 μM) was applied to the apical or basolateral membrane as shown. Each trace is representative of four or more independent experiments (three different individuals).
Figure 5.
Summary data on anion secretion and intracellular Ca2+ (Ca2+ i) in normal and cystic fibrosis (CF) nasal epithelia pretreated with luminal H2DIDS (1.5 mM) and exposed to a single concentration (100 μM) of mucosal (A) or serosal (B) UTP. Data are included (from Fig. 3) for tissues not treated with H2DIDS for comparison with tissues treated with the disulfonic stilbene. Values for H2DIDS-treated tissues and tissues not treated with H2DIDS are significantly different (**P < 0.01) or otherwise not different (P > 0.05; symbol not shown) from each other. For H2DIDS-treated tissue, each bar is representative of four or more independent experiments (three different individuals).
Effects of ATP/UTP on Anion Transport in Ca2+ i-clamped Airway Epithelial Cells
The residual anion secretory response in normal airway epithelia resulting from mucosal UTP addition after H2DIDS pretreatment suggested that triphosphate nucleotides can modulate CFTR via a signal transduction pathway that may or may not involve changes in Ca2+ i. Thus, an important aspect of this study was to evaluate whether regulation of anion secretion by extracellular ATP/UTP is tightly coupled to Ca2+ i in normal and CF human airway epithelia. To address this issue, two different maneuvers were used in an attempt to test whether ATP/UTP-regulated Ca2+ i could be dissociated from anion secretion.
As illustrated in Fig. 6, the first approach involved maximal elevation of Ca2+ i by exposing cells to ionomycin and the endoplasmic reticulum Ca2+ pump blocker thapsigargin, followed by ATP. In these studies, normal (Fig. 6A and Fig. B) and CF (Fig. 6 C) nasal monolayers were first exposed to mucosal ionomycin (300 nM), which, in the presence of symmetrical extracellular Ca2+ (1.3 mM), resulted in a maximal increase of Ca2+ i; subsequent addition of serosal ionomycin (300 nM; not shown) or mucosal thapsigargin (500 nM; Fig. 6) caused no additional change in Vt and cell Ca2+. We next tested the effects of unilaterally applied triphosphate nucleotides on changes of Ca2+ i and Vt. In the normal airway, serosal (Fig. 6 A) and mucosal (Fig. 6 B) ATP (100 μM) induced an additional increase in Vt without an apparent additional change in Ca2+ i. In contrast to normal nasal tissues, neither serosal nor mucosal administration of ATP elicited changes in Vt in CF airway epithelium (Fig. 6 C). The summary data derived from these protocols using ATP or UTP as the nucleotide agonist were analyzed sequentially, first, for the effects of ionomycin alone on Ca2+ i and anion secretion (see Fig. 7) and, second, for the effects of mucosal and serosal ATP/UTP on Ca2+ i and anion transport (see Fig. 8) after pretreatment with ionomycin/thapsigargin in normal and CF airway preparations.
Figure 6.
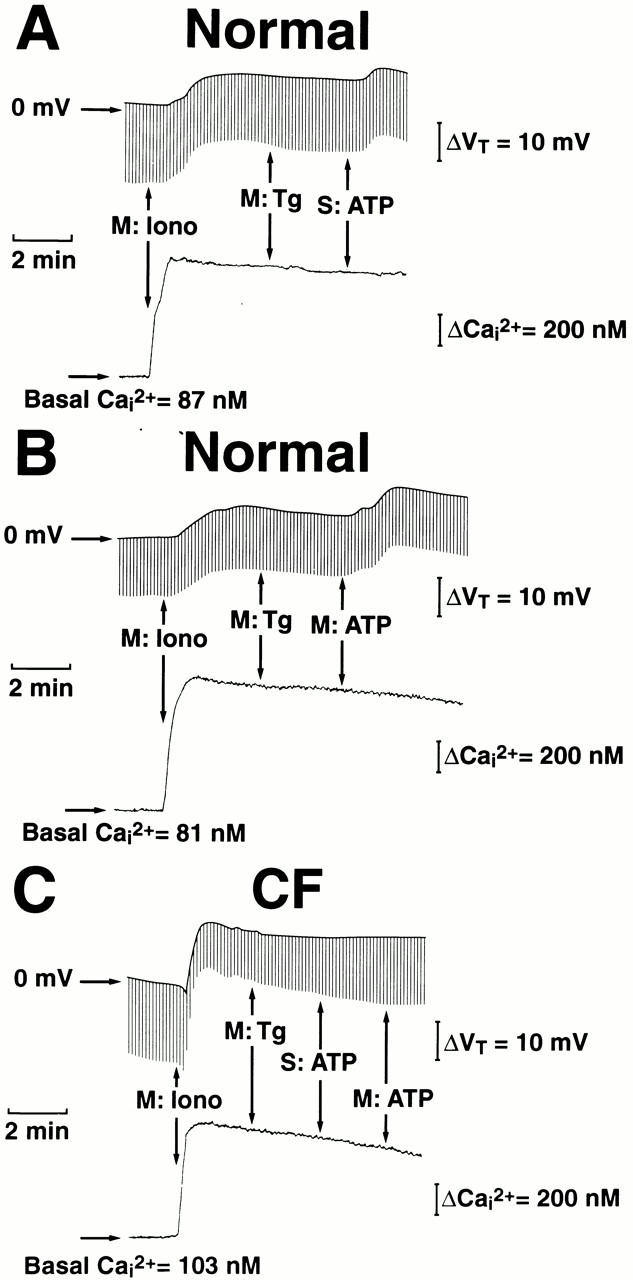
Separation between intracellular Ca2+ (Ca2+ i) and the transepithelial potential difference (Vt) in response to ATP in normal (A and B) and cystic fibrosis (CF; C) nasal epithelia. For these protocols, monolayers were perfused serosally with Kreb's bicarbonate Ringer and mucosally with Na+-free/low Cl− Ringer before adding 300 nM ionomycin (Iono) and 500 nM thapsigargin (Tg) to the mucosal perfusate as indicated in the traces. Serosal (S) or mucosal (M) ATP (100 μM) was applied to the basolateral or apical membrane as shown. Each trace is representative of four or more independent experiments (three different individuals).
Figure 7.
Summary data showing changes in intracellular Ca2+ (Ca2+ i; A) and anion secretion (B) in normal and cystic fibrosis (CF) monolayers exposed to mucosal ionomycin (300 nM). Each bar represents mean ± SEM for 40 or more independent experiments (three different individuals). For Ca2+ i, normal and CF values are not significantly different (P > 0.05; symbol not shown) from each other; for anion secretion, normal and CF values are significantly different (**P < 0.01) from each other.
Figure 8.
Summary data showing changes in anion secretion and intracellular Ca2+ (Ca2+ i) in normal and cystic fibrosis (CF) nasal epithelia exposed to mucosal (A) or serosal (B) ATP (100 μM) or UTP (100 μM) under high Ca2+ i conditions. Monolayers were pretreated with 300 nM ionomycin + 500 nM thapsigargin before ATP/UTP additions (see Fig. 6). Each bar represents mean ± SEM for four or more independent experiments (three different individuals). Mucosal ATP/UTP-stimulated anion transport activities were significantly (**P < 0.01) larger than serosal ATP/UTP equivalent currents. No significant change (P > 0.05; symbol not shown) in Ca2+ i was detected with mucosal/serosal addition of agonists.
As shown in Fig. 7, Ca2+ i increased to the same extent in normal and CF airway epithelia in response to ionomycin (Fig. 7 A). However, in contrast to ionomycin-induced changes in Ca2+ i, anion secretion in response to ionomycin was of greater magnitude in CF as compared with normal nasal tissues (Fig. 7 B). The differences in ionomycin-induced changes in anion secretion between normal and CF nasal epithelia, as reported here, are consistent with previous reports of upregulation of ionomycin-stimulated anion secretion in cultured human CF nasal epithelium (Johnson et al. 1995).
The ATP/UTP summary data are shown in Fig. 8. Under conditions in which the cell Ca2+ was clamped at high levels with ionomycin/thapsigargin pretreatment, ATP and UTP were equally effective in inducing anion secretion without affecting Ca2+ i when applied to either the mucosal (Fig. 8 A) or serosal (Fig. 8 B) compartment in normal nasal monolayers. However, the anion secretory response was of a greater magnitude to mucosal additions of triphosphate nucleotides as compared with serosal additions of the agonists. Under these same conditions, neither ATP nor UTP applied to either membrane (apical/basolateral) induced an anion secretory or Ca2+ i response in CF airway cells (Fig. 8).
In the second approach, normal and CF airway epithelia were exposed to BAPTA-AM to clamp Ca2+ i to low levels. To reduce Ca2+ influx that would result from P2Y2-R activation (Paradiso et al. 1995), nasal monolayers were exposed to bilateral low Ca2+ (300 μM) solutions. As illustrated in Fig. 9, the addition of serosal (Fig. 9 A) or mucosal (Fig. 9 B) ATP (100 μM) failed to raise Ca2+ i, but increased Vt, in normal nasal epithelium. The responsiveness of preparations in the presence of BAPTA was tested by the response to the cAMP-mediated agonist forskolin. Mucosally applied forskolin (10 μM) evoked an additional increase in Vt in normal airway tissue (Fig. 9A and Fig. B). In marked contrast to the normal nasal preparation, neither serosal nor mucosal ATP, nor mucosal forskolin, elicited changes in Vt in CF (Fig. 9 C). However, when monolayers were exposed bilaterally to high extracellular Ca2+ (1.3 mM) plus mucosal ionomycin (300 nM), Ca2+ i slowly increased, followed by a small anion secretory response in CF, verifying the responsiveness of the preparation. It should be noted that BAPTA is a very effective buffer of Ca2+. To detect a change in cell Ca2+ in BAPTA-loaded cells, sufficient Ca2+ influx must occur to overwhelm the buffering capacity of BAPTA, thus, giving rise to the slow increase in Ca2+ i (Fig. 9 C). The small magnitude of change in Vt shown in Fig. 9 C may be related not only to the magnitude change in Ca2+ i, but also to the rate of change of cell Ca2+ (which is not a focus of our study). Data for the mucosal and serosal ATP/UTP on changes in anion transport rates and Ca2+ i levels in BAPTA-treated normal and CF airway epithelia are summarized in Fig. 10 (A and B).
Figure 9.
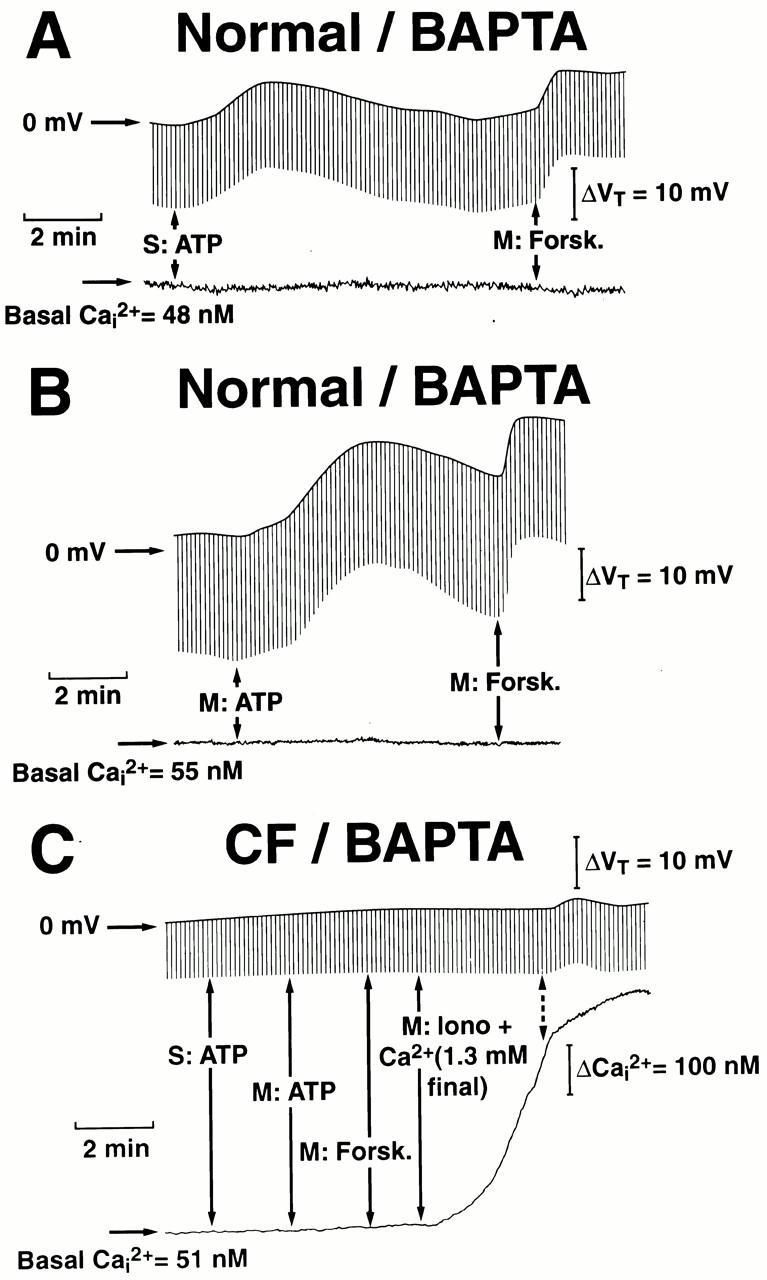
Changes in the transepithelial potential difference (Vt) and intracellular Ca2+ (Ca2+ i) activities in response to serosal (S) or mucosal (M) ATP (100 μM) in normal (A and B) or cystic fibrosis (CF; C) BAPTA-treated nasal epithelial preparations. Monolayers were initially perfused with serosal Kreb's bicarbonate Ringer/mucosal Na+-free/low Cl− Ringer and low bilateral extracellular Ca2+ (300 μM) before the addition of ATP, as indicated in the traces. For normal and CF nasal preparations, forskolin (Forsk; 10 μM) was added to the mucosal perfusate at the times indicated. In CF preparations, ionomycin (Iono; 300 nM) + extracellular Ca2+ (1.3 mM final concentration) was added to the mucosal bath as shown in the trace. Each trace is representative of at least five independent experiments (four or more different individuals).
Figure 10.
Summary data showing changes in anion transport and intracellular Ca2+ (Ca2+ i) activities in normal and cystic fibrosis (CF) nasal epithelium exposed to mucosal (A) or serosal (B) ATP (100 μM) or UTP (100 μM) in BAPTA pretreated cells. Monolayers were perfused with serosal Kreb's bicarbonate Ringer/mucosal Na+-free/low Cl− Ringer and low bilateral extracellular Ca2+ (300 μM) before triphosphate nucleotide addition. Each bar represents mean ± SEM for at least five independent experiments (four or more different individuals). Mucosal ATP/UTP-stimulated equivalent currents were significantly (**P < 0.01) larger than serosal ATP/UTP transport activities. No significant change (P > 0.05; symbol not shown) in Ca2+ i was detected with mucosal/serosal addition of agonists.
There are several relevant observations arising from the Ca2+ clamp experiments that should be of note. First, in normal nasal monolayers, the magnitude of the anion secretory response to apically applied ATP/UTP was identical under experimental conditions when cell Ca2+ was clamped at high levels with ionomycin/thapsigargin treatment or low levels with BAPTA treatment (Fig. 8 and Fig. 10, respectively), strongly indicating that the signaling pathway underlying triphosphate nucleotide stimulation of anion secretion has a molecular component that is independent of Ca2+ i, likely via CFTR, during P2Y2-R activation. Second, in normal airway cells, serosal ATP/UTP-stimulated anion transport rates under both Ca2+ i-clamped conditions were smaller than those observed for mucosal additions of ATP/UTP. These data suggest that molecular mechanism(s) underlying the signaling pathway for the stimulation of anion transport by ATP/UTP under Ca2+ i-clamped conditions is less efficient for serosal compared with mucosal addition of these triphosphate nucleotides. Finally, in CF nasal monolayers, mucosal addition of ATP/UTP failed to elicit an anion secretory response in Ca2+ i-clamped cells, suggesting that CaCC is the sole Cl− channel mediating anion transport activity in CF nasal epithelia.
Effects of PKC Activation and Inhibition on UTP-stimulated Anion Secretion in Ca2+i-clamped Normal Nasal Tissue
We have previously reported (Boucher et al. 1989) no detectable difference in total PKC activity in normal and CF airway, despite the fact that the PKC activator PMA had the capacity to stimulate anion transport in normal, but not CF, nasal epithelia, via PKC-dependent phosphorylation of CFTR. Based on this earlier work, and our data showing that ATP/UTP applied to either membrane (apical/basolateral) of normal airway epithelial cells induce anion secretion independently from changes in Ca2+ i (Fig. 8 and Fig. 10), we tested whether a Ca2+-independent PKC was a functional regulator of CFTR-mediated anion secretion after P2Y2-R activation. In these studies, monolayers of normal nasal epithelia were Ca2+ i-clamped by BAPTA, and the effects of PKC activation or inhibition by PMA or chelerythrine, respectively, on UTP-elicited anion secretion were examined.
As shown in Fig. 11, mucosal addition of PMA (100 nM) to normal airway epithelia caused an increase in Vt with no change in Ca2+ i (Fig. 11 A). Importantly, subsequent exposure of nasal cells to serosal and mucosal UTP (100 μM) failed to cause an additional increase in Vt, despite the fact that mucosal addition of forskolin (10 μM) markedly stimulated anion transport activity (Fig. 11 A).
Figure 11.
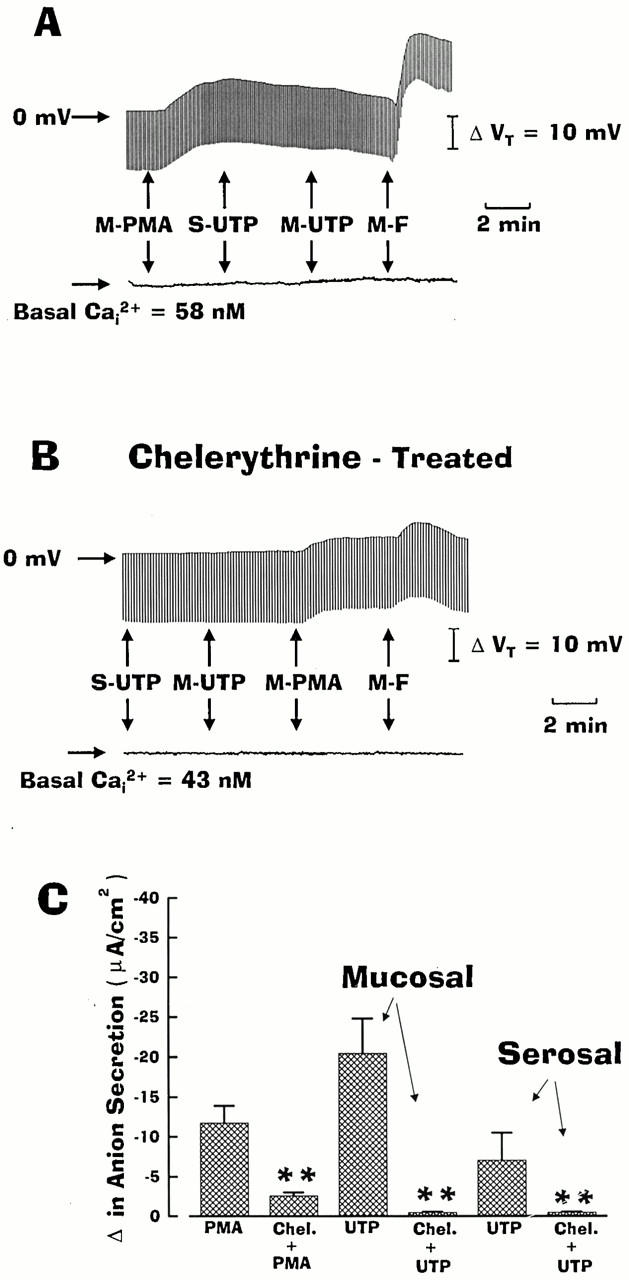
Effect of PMA and chelerythrine chloride on UTP-stimulated anion secretion in intracellular Ca2+ (Ca2+ i)-clamped normal nasal monolayers. In the absence (A) or presence (B) of chelerythrine (1 μM), monolayers were perfused serosally with Kreb's bicarbonate Ringer and mucosally with Na+-free/low Cl− Ringer before adding serosal (S) or mucosal (M) PMA (100 nM), UTP (100 μM), or forskolin (F; 10 μM) as indicated. C shows summary data generated from four or more independent experiments (three different individuals). Chelerythrine significantly (**P < 0.01) blocked PMA and UTP-induced anion transport activities.
At the concentration used in the study, chelerythrine chloride is a specific but broad spectrum inhibitor of PKC isozymes (Herbert et al. 1990). Therefore, we next examined whether this agent could block both the UTP- and PMA-stimulated anion transport in Ca2+ i-clamped normal nasal monolayers. As shown in Fig. 11 B, pretreating nasal monolayers with chelerythrine (1 μM) completely blocked serosal and mucosal UTP-stimulated increase in anion secretion, and markedly inhibited both PMA- and forskolin-stimulated anion transport. The data generated from these studies are summarized in Fig. 11 C.
DISCUSSION
Purinoceptors Involved in Airway Epithelial Ion Transport Regulation
Extracellular nucleotides regulate cellular processes via interactions with cell-surface ion-gated (P2X) and G protein–coupled (P2Y) receptors. Previous studies in human airways (Paradiso et al. 1995) have identified a major role for P2Y-Rs and a little role for P2X receptors in the lumen facing (columnar) cells of the superficial epithelium of airways. Moreover, the observation that ATP and UTP were equipotent in mediating ΔCa2+ i (Fig. 2 and associated text of Fig. 3) argues that the P2Y2-R, and not P2X-Rs, mediated the nucleotide responses on both the apical and basolateral surfaces of our airway preparations.
P2Y2 purinoceptors activate PLCβ in a heterotrimeric G protein–dependent manner (Boeynaems et al. 1998) and increase Ca2+ i through two distinct, but related pathways. Initially, IP3 is formed from PLC-induced breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) and activates IP3 channel receptors in the endoplasmic reticulum, resulting in channel opening and release of stored calcium into the cytoplasm. Subsequently, depletion of intracellular Ca2+ stores activates a pathway for Ca2+ influx across the plasma membrane, which was originally termed “capacitative Ca2+ entry” or, more recently, “store-operated calcium entry” (Clapham 1995; Putney 1986, Putney 1990). Both phases of Ca2+ mobilization can act in concert for the subsequent modulation of multiple plasma membrane transport processes, including the Ca2+-activated Cl− channel (i.e., CaCC) in airway epithelia.
Besides PLC-mediated changes of Ca2+ i, another consequence of PLC-dependent PIP2 breakdown is the formation of diacylglycerol (DAG) and its activation of PKC. PKC activation may principally regulate human airway secretion via interactions with CFTR. For example, regulation of the CFTR Cl− channel by PKC has been reported in previous studies performed in a variety of epithelial cell lines expressing CFTR (Dechecchi et al. 1992, Dechecchi et al. 1993; Bajnath et al. 1993; Winpenny et al. 1995; Jia et al. 1997). Moreover, studies by Picciotto and co-workers (Picciotto et al. 1992) showed that PKC phosphorylated CFTR in a Ca2+-independent manner, and their findings agree with more recent studies by Liedtke and Cole 1998, who reported that Ca2+-independent PKC-ɛ regulates cAMP-dependent stimulation of the CFTR Cl− channel in Calu-3 cells, an airway epithelial cell line.
Compelling evidence has been reported that the regulation of Cl− secretion in normal airway epithelia may reflect more than a simple change in Ca2+ i mediated by extracellular triphosphate nucleotides. For example, Hwang et al. 1996 reported that triphosphate nucleotides activated multiple types of Ca2+-sensitive and Ca2+-insensitive Cl− conductances in rat tracheal epithelial cells. A dissociation between the regulation of Cl− conductance and Ca2+ i activity by extracellular ATP was also seen in voltage clamp studies in CFT1 cells by Stutts and co-workers (Stutts et al. 1994), suggesting multiple modes of regulation of Cl− transport rates by extracellular ATP. However, these earlier studies used separate experimental systems for monitoring ion transport activities and for measurements of Ca2+ i (either directly or indirectly) in airway epithelial preparations. To better quantitate the role of intracellular Ca2+ in modulating anion transport activity in polarized tissues, we used a unique experimental technique that allowed us to measure simultaneous changes of Ca2+ i and anion secretion induced by P2Y2-R activation in polarized normal and CF human airway epithelia (Fig. 1). Because the results of these studies were extensive and complex, we have elected to present them with respect to nucleotide addition selectively at each barrier, starting with the simplest system (i.e., CF) in which CFTR function is absent. We then present data in the more complex normal airway epithelium.
Apical P2Y2-R Regulation of Anion Secretion in CF Airway Epithelia
The large anion secretory responses of CF tissues to mucosal ATP/UTP (Fig. 2 and Fig. 3) are consistent with previous results seen in CF airway epithelia (Clarke and Boucher 1992). The Cl− secretory response in cultured CF airway epithelia in response to ionomycin or ATP was shown directly by microelectrode studies to arise principally from activation of a Cl− conductance (Fig. 2 and Fig. 7) in the apical membrane (Willumsen and Boucher 1989; Clarke and Boucher 1992).
A comprehensive set of protocols was developed to directly test the linkage between apical (versus basolateral) nucleotide–induced Ca2+ i and anion secretion. Contrasting maneuvers were used to clamp Ca2+ i at different levels in CF nasal tissues. The first approach involved maximally elevating Ca2+ i by the addition of ionomycin, followed by thapsigargin. CF nasal tissue pretreated with these agents exhibited no additional change in Ca2+ i in response to apically or basolaterally applied ATP/UTP, and ATP/UTP failed to increase the anion transport rate (Fig. 6 and Fig. 8). In the second approach, nasal monolayers were pretreated with BAPTA-AM to clamp Ca2+ i to low levels. These experiments revealed that the addition of mucosal ATP/UTP to BAPTA-treated CF cells caused no change in Ca2+ i, and again failed to increase anion secretion in CF epithelium (Fig. 9 and Fig. 10). The data showing that Ca2+ i-clamped CF epithelium failed to elicit an anion secretory response to mucosal ATP/UTP, coupled with data that showed that anion transport induced by mucosal UTP in CF epithelium was completely inhibited by H2DIDS (Fig. 4 and Fig. 5), argue that anion secretion in CF epithelia in response to ATP/UTP is mediated exclusively via Ca2+ i regulation of CaCC.
A key observation of this study was the markedly larger Ca2+ i response of CF as compared with normal tissues to mucosal ATP/UTP (Fig. 2 and Fig. 3), raising the possibility that regulation of Ca2+ i metabolism is different in CF. Based on our functional data alone, it is not apparent how mucosal ATP/UTP activated a larger Ca2+ i signal in CF compared with normal cells. However, in human proximal airway epithelia, we have recently performed preliminary studies using immunofluorescent confocal imaging of the endoplasmic reticulum (the site of IP3-releasable Ca2+ stores) markers, calreticulin, and IP3 receptors, which show that endoplasmic reticulum Ca2+ stores are preferentially distributed to the apical domain, and that CF cells exhibit a greater expression of apical ER Ca2+ stores (Ribeiro et al. 1999).
Basolateral P2Y2-R Regulation of Anion Secretion in CF Airway Epithelia
A central observation of this study was that basolateral activation of P2Y2-R with ATP or UTP raised Ca2+ i to the same extent in both CF and normal airway epithelia, but failed to induce anion secretion in CF. This observation strongly indicates that Ca2+, mobilized by activation of the basolateral P2Y2-R, is functionally confined to that barrier, i.e., the Ca2+ i released from basolateral stores cannot activate apical Ca2+-sensitive Cl− channels. Our data in normal tissues imply that this compartmentalized release/regulation of Ca2+ i in response to basolateral ATP/UTP is also a feature of normal airway epithelia (Fig. 4 and Fig. 5). Previous microelectrode studies demonstrated that apical nucleotide administration, unlike basolateral nucleotide administration, did not activate basolateral Ca2+-activated K+ channels (Clarke and Boucher 1992), suggesting that this compartmentalization is symmetric. The mechanism for compartmentalization of Ca2+ signaling in airway epithelia is unknown, but it may involve the actions of mitochondria acting as a “Ca2+ fence” (Tinel et al. 1999) and/or Ca2+ binding proteins (Nomiya et al. 1998).
Apical Membrane P2Y2-R Regulation of Anion Secretion in Normal Airway Epithelia
For apical P2Y2-R–regulated anion secretion in normal airway tissue, we propose that two distinct Cl− channels (e.g., CaCC and CFTR) mediate anion efflux across the apical membrane, and that the linkage coupling activation of apical P2Y2-R to the induction of anion transport involves both Ca2+ i-regulated CaCC and Ca2+-independent PKC-regulated CFTR. We make these speculations based on the following observations. In normal human airway epithelial cells, the majority of anion secretion mediated by apical P2Y2-Rs reflects Ca2+ i-regulated activation of CaCC. In support of this notion, anion transport activity was substantially blocked (∼75%) by luminal H2DIDS in response to mucosal UTP (Fig. 4 and Fig. 5). Additional support for CaCC as the major efflux pathway during ATP/UTP-regulated anion secretion came from experiments that showed markedly reduced anion transport activity in Ca2+ i-clamped normal airway monolayers in response to apically applied ATP/UTP (Fig. 8 and Fig. 10).
However, the observation that mucosally applied ATP/UTP-stimulated anion secretion was reduced, but not abolished, in normal airway epithelium by H2DIDS or Ca2+ i-clamping agents indicates that apical P2Y2-R activation modulates multiple Cl− channels via Ca2+-dependent (i.e., CaCC) and Ca2+-independent signal transduction pathways. We propose that the Ca2+-independent signaling pathway linking apical P2Y2-R activation to anion secretion reflects Ca2+-independent PKC regulation of CFTR-mediated anion secretion for several reasons. First, as noted above, activation of the apical P2Y2-Rs by ATP/UTP increased anion secretory activity in a Ca2+-independent manner (Fig. 8 and Fig. 10). Second, the PMA-stimulated anion secretion abolished UTP-regulated anion secretion (Fig. 11) in Ca2+ i-clamped monolayers, suggesting that the PMA- and UTP-regulated signaling pathways occur via the same cellular mechanism (i.e., Ca2+-independent PKC). Finally, PKC inhibition by chelerythrine completely abolished apically applied UTP-regulated anion secretion under Ca2+ i-clamped conditions (Fig. 11), which is consistent with the notion that a Ca2+-independent PKC is the intracellular second messenger relevant to CFTR-mediated anion secretion in normal airway epithelia.
Basolateral Membrane P2Y2-R Activation of Anion Transport in Normal Airway Epithelia
For basolateral P2Y2-R–regulated secretion, we propose that (1) only a single Cl− channel (i.e., CFTR) mediates anion efflux across the apical membrane, and (2) that the linkage between P2Y2-R activation and CFTR involves regulation via PKC rather than Ca2+ i. Several observations support these hypotheses. In normal nasal epithelium, the addition of basolateral ATP/UTP consistently increased the anion secretory rate, but no changes in anion secretion were noted in CF. These data clearly point to the requirement for functional CFTR to mediate anion secretion after basolateral addition of ATP/UTP. The identification of CFTR and not CaCC as the apical membrane anion efflux pathway during basolateral P2Y2-R activation is further supported by data that show that mucosal H2DIDS failed to block anion secretion in normal nasal tissues induced by serosal UTP, but abolished anion secretion in response to mucosal UTP in CF (Fig. 4 and Fig. 5).
The notion that the apical membrane CFTR-mediated anion conductance is regulated by a Ca2+-independent PKC is based on several interrelated observations derived from the Ca2+ i-clamped studies. First, as noted above, activation of the basolateral P2Y2-Rs by ATP/UTP increased anion transport activity in a Ca2+-independent manner (Fig. 8 and Fig. 10). Second, no additivity was observed between the PMA-stimulated anion secretion and basolateral UTP-regulated anion transport (Fig. 11) in Ca2+ i-clamped monolayers, again suggesting that the PMA- and UTP-regulated signaling pathways occur via the same cellular mechanism, i.e., Ca2+-independent PKC. Finally, PKC inhibition by chelerythrine completely abolished serosally applied UTP-regulated anion secretion under Ca2+ i clamp conditions (Fig. 11), which is again consistent with the notion that a Ca2+-independent PKC is the intracellular messenger relevant to CFTR-mediated anion secretion in normal human airway epithelia.
Ion Transport Model
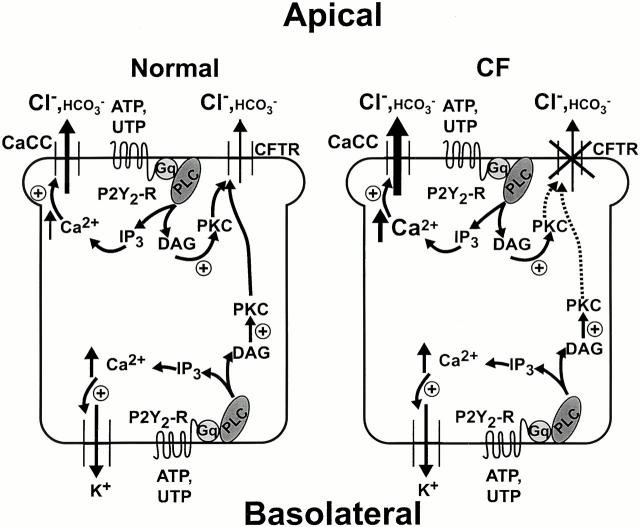
In summary, the model depicted in Fig. 12 shows that, in normal epithelium, basolateral P2Y2-R activation couples to apical anion secretion through two related pathways. First, the Ca2+ i signal resulting from PLC-generated IP3 activates Ca2+-dependent K+ channels on the basolateral membrane, promoting membrane hyperpolarization and generation of a loop current responsible for CFTR-mediated anion secretion. Second, PLC-generated DAG activates a Ca2+-independent PKC which, directly or indirectly, activates CFTR-dependent anion transport. In this model, PKC (or one of its targets) translocates from the basolateral to the apical domain where it can modulate CFTR function, whereas the Ca2+ i signal generated by basolateral nucleotide application is restricted to the basolateral compartment. In contrast, apical P2Y2-R activation in normal epithelium increases anion secretion as a result of Ca2+-dependent activation of CaCC as well as CFTR regulation by a Ca2+-independent PKC.
Figure 12.
Model for basolateral and apical P2Y2 receptor (P2Y2-R) activation of anion secretion in normal and cystic fibrosis (CF) human airway epithelia. ATP; UTP; P2Y2-R (P2Y2 purinergic receptor); Gq (heterotrimeric GTP-binding protein); PLC (phospholipase C); IP3 (inositol 1,4,5-triphosphate); Ca2+ i (intracellular Ca2+); DAG (diacylglycerol); PKC; CFTR (cystic fibrosis transmembrane conductance regulator); and CaCC (Ca2+-activated Cl− channel).
In CF epithelium, although basolateral P2Y2-R activation stimulates PLC to the same extent as in normal epithelium, Ca2+-activated K+ channel–dependent membrane hyperpolarization and DAG-activated PKC do not generate anion secretion because of the lack of functional CFTR expression at the apical barrier. However, apical P2Y2-R activation in CF results in a large Ca2+ i mobilization, accounting for the large CaCC-mediated anion secretion compared with normal epithelium. Similar to basolateral P2Y2-R–dependent signal transduction, apical receptor stimulation–activated PKC does not couple to anion transport in CF because of the absence of functional CFTR.
Role of Apical and Basolateral P2Y2-R in Airway Epithelial Function
The protocols using Na+-free/low Cl− luminal solutions were designed to allow us to use anion secretion as a sensitive read-out of P2Y2-R–mediated signal transduction at apical and basolateral barriers. This strategy allowed us to discover that airway epithelia appear to be able to functionally confine Ca2+-regulated signaling to the barrier ipsilateral to receptor stimulation, whereas other pathways (e.g., PKC) are not. Thus, both normal and CF airways may be able to respond selectively to nucleotide (and perhaps other agonists) stimulation at the apical or basolateral barriers.
These data also could have implications for regulation of net transepithelial ion transport rates. Although not explored in this study, extracellular nucleotides inhibit epithelial Na+ channel (Devor and Pilewski 1999) and limit Na+ absorption in airway epithelia. Under these conditions, driving forces for Cl− secretion across epithelia exist. In normal airways, anion transport resulting from P2Y2-R stimulation at either barrier, via regulation of CFTR and/or CaCC, could play a vital role in the composition, pH (e.g., secreted HCO3 −), and depth of airway surface liquids, facilitating the maintenance of the mucociliary clearance mechanism and, thus, airway homeostasis. On the other hand, in CF airways, there is a predicted failure to respond to nucleotides released in the vicinity of the basolateral barrier, e.g., from inflammatory cells or autocrine release. The failure to respond could limit the ability of the CF epithelium to respond to submucosal stresses. Conversely, although CaCC, under resting circumstances, appears to be inactive, the large Ca2+-releasable stores and apparent upregulation of CaCC make activation of the pathway to initiate anion secretion to restore volume on airway surfaces by luminal nucleotides an attractive therapeutic strategy.
Acknowledgments
We thank L. Brown for typing the manuscript and for editorial comments and Dr. J.R. Yankaskas for technical assistance in developing human nasal epithelial cell monolayers. We especially thank Dr. E.H. Larsen and his laboratory (Zoophysiological Laboratory A, August Kroh Institute, University of Copenhagen, Denmark) for constructing the miniature Ussing chamber that made these studies possible.
This work was supported by the National Institutes of Health grants HL-44173, HL34322, and HL42384, and Cystic Fibrosis Foundation grant R026 (to R.C. Boucher and A.M. Paradiso).
Footnotes
Abbreviations used in this paper: CaCC, Ca2+-activated Cl− channel; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; Ca2+ i, intracellular free Ca2+; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphate; P2Y2-R, P2Y2 purinergic receptor.
References
- Al-Bazzaz F.J., Jayaram T. Ion transport by canine tracheal mucosaeffect of elevation of cellular calcium. Exp. Lung Res. 1981;2:121–130. doi: 10.3109/01902148109052308. [DOI] [PubMed] [Google Scholar]
- Bajnath R.B., Groot J.A., de Jonge H.R., Kansen M., Bijman J. Synergistic activation of non-rectifying small-conductance chloride channels by forskolin and phorbol esters in cell-attached patches of the human colon carcinoma cell line HT-29cl.19A. Pflügers Arch. 1993;425:100–108. doi: 10.1007/BF00374509. [DOI] [PubMed] [Google Scholar]
- Barthelson R.A., Jacoby D.B., Widdicombe J.H. Regulation of chloride secretion in dog tracheal epithelium by protein kinase C. Am. J. Physiol. 1987;253:C802–C808. doi: 10.1152/ajpcell.1987.253.6.C802. [DOI] [PubMed] [Google Scholar]
- Boeynaems J.M., Communi D., Janssens R., Motte S., Robaye B., Pirotton S. Nucleotide receptors coupling to the phospholipase C signaling pathway. In: Turner J.T., Weisman G.A., Fedan J.S., editors. The P2 Nucleotide Receptors. Humana Press; Totowa: 1998. pp. 169–183. [Google Scholar]
- Boucher R.C., Cheng E.H.C., Paradiso A.M., Stutts M.J., Knowles M.R., Earp H.S. Chloride secretory response of cystic fibrosis human airway epitheliapreservation of calcium but not protein kinase C– and A–dependent mechanisms. J. Clin. Invest. 1989;84:1424–1431. doi: 10.1172/JCI114316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H.A., Lazarowski E.R., Boucher R.C., Harden T.K. Evidence that UTP and ATP regulate phospholipase C through a common extracellular 5′-nucleotide receptor in human airway epithelial cells. Mol. Pharmacol. 1991;40:648–655. [PubMed] [Google Scholar]
- Clapham D.E. Intracellular calcium. Replenishing the stores. Nature. 1995;375:634–635. doi: 10.1038/375634a0. [DOI] [PubMed] [Google Scholar]
- Clarke L.L., Boucher R.C. Chloride secretory response to extracellular ATP in normal and cystic fibrosis nasal epithelia. Am. J. Physiol. 1992;263:C348–C356. doi: 10.1152/ajpcell.1992.263.2.C348. [DOI] [PubMed] [Google Scholar]
- Clarke L.L., Harline M.C. Dual role of CFTR in cAMP-stimulated HCO3 − secretion across murine duodenum. Am. J. Physiol. 1998;274:G718–G726. doi: 10.1152/ajpgi.1998.274.4.G718. [DOI] [PubMed] [Google Scholar]
- Clarke L.L., Harline M.C., Gawenis L.R., Walker N.M., Turner J.T., Weisman G.A. Extracellular UTP stimulates electrogenic bicarbonate secretion across CFTR knockout gallbladder epithelium. Am. J. Physiol. 2000;279:G132–G138. doi: 10.1152/ajpgi.2000.279.1.G132. [DOI] [PubMed] [Google Scholar]
- Dechecchi M.C., Rolfini R., Tamanini A., Gamberi C., Berton G., Cabrini G. Effect of modulation of protein kinase C on the cAMP-dependent chloride conductance in T84 cells. FEBS Lett. 1992;311:25–28. doi: 10.1016/0014-5793(92)81358-s. [DOI] [PubMed] [Google Scholar]
- Dechecchi M.C., Tamanini A., Berton G., Cabrini G. Protein kinase C activates chloride conductance in C127 cells stably expressing the cystic fibrosis gene. J. Biol. Chem. 1993;268:11321–11325. [PubMed] [Google Scholar]
- Devor D.C., Pilewski J.M. UTP inhibits Na+ absorption in wild-type and ΔF508 CFTR-expressing human bronchial epithelia. Am. J. Physiol. 1999;276:C827–C837. doi: 10.1152/ajpcell.1999.276.4.C827. [DOI] [PubMed] [Google Scholar]
- Grubb B.R., Boucher R.C. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol. Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- Hartmann T., Kondo M., Mochizuki H., Verkman A.S., Widdicombe J.H. Calcium-dependent regulation of Cl secretion in tracheal epithelium. Am. J. Physiol. 1992;262:L163–L168. doi: 10.1152/ajplung.1992.262.2.L163. [DOI] [PubMed] [Google Scholar]
- Herbert J.M., Augereau J.M., Gleye J., Maffrand J.P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Homolya L., Steinberg A.D., Boucher R.C. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J. Cell Biol. 2000;150:1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T.H., Schwiebert E.M., Guggino W.B. Apical and basolateral ATP stimulates tracheal epithelial chloride secretion via multiple purinergic receptors. Am. J. Physiol. 1996;270:C1611–C1623. doi: 10.1152/ajpcell.1996.270.6.C1611. [DOI] [PubMed] [Google Scholar]
- Illek B., Yankaskas J.R., Machen T.E. cAMP and genistein stimulate HCO3 − conductance through CFTR in human airway epithelia. Am. J. Physiol. 1997;272:L752–L761. doi: 10.1152/ajplung.1997.272.4.L752. [DOI] [PubMed] [Google Scholar]
- Jia Y., Mathews C.J., Hanrahan J.W. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J. Biol. Chem. 1997;272:4978–4984. doi: 10.1074/jbc.272.8.4978. [DOI] [PubMed] [Google Scholar]
- Johnson L.G., Boyles S.E., Wilson J., Boucher R.C. Normalization of raised sodium absorption and raised calcium-mediated chloride secretion by adenovirus-mediated expression of cystic fibrosis transmembrane conductance regulator in primary human cystic fibrosis airway epithelial cells. J. Clin. Invest. 1995;95:1377–1382. doi: 10.1172/JCI117789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M.R., Clarke L.L., Boucher R.C. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N. Engl. J. Med. 1991;325:533–538. doi: 10.1056/NEJM199108223250802. [DOI] [PubMed] [Google Scholar]
- Larsen E.H., Novak I., Pedersen P.S. Cation transport by sweat ducts in primary culture. Ionic mechanism of cholinergically evoked current oscillations. J. Physiol. 1990;424:109–131. doi: 10.1113/jphysiol.1990.sp018058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski E.R., Mason S.J., Clarke L.L., Harden T.K., Boucher R.C. Adenosine receptors on human airway epithelia and their relationship to chloride secretion. Br. J. Pharmacol. 1992;106:774–782. doi: 10.1111/j.1476-5381.1992.tb14412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski E.R., Paradiso A.M., Watt W.C., Harden T.K., Boucher R.C. UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y2 receptor. Proc. Natl. Acad. Sci. USA. 1997;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuba D., De Ribaupierre Y., Kucera P. Ion transport, ciliary activity, and mechanosensitivity of sinusal mucosaan in vitro study. Am. J. Physiol. 1996;271:L349–L358. doi: 10.1152/ajplung.1996.271.3.L349. [DOI] [PubMed] [Google Scholar]
- Liedtke C.M., Cole T.S. Antisense oligonucleotide to PKC-epsilon alters cAMP-dependent stimulation of CFTR in Calu-3 cells. Am. J. Physiol. 1998;275:C1357–C1364. doi: 10.1152/ajpcell.1998.275.5.C1357. [DOI] [PubMed] [Google Scholar]
- Linsdell P., Tabcharani J.A., Rommens J.M., Hou Y.X., Chang X.B., Tsui L.C., Riordan J.R., Hanrahan J.W. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J. Gen. Physiol. 1999;110:355–364. doi: 10.1085/jgp.110.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason S.J., Paradiso A.M., Boucher R.C. Regulation of transepithelial ion transport and intracellular calcium by extracellular adenosine triphosphate in human normal and cystic fibrosis airway epithelium. Br. J. Pharmacol. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiya S., Nishizaki K., Anniko M., Karita K., Ogawa T., Masuda Y. Appearance and distribution of two Ca2+-binding proteins during development of the cochlea in the musk shrew. Dev. Brain Res. 1998;110:7–19. doi: 10.1016/s0165-3806(98)00087-x. [DOI] [PubMed] [Google Scholar]
- Paradiso A.M. ATP-activated basolateral Na+/H+ exchange in human normal and cystic fibrosis airway epithelium. Am. J. Physiol. 1997;273:L148–L158. doi: 10.1152/ajplung.1997.273.1.L148. [DOI] [PubMed] [Google Scholar]
- Paradiso A.M., Mason S.J., Lazarowski E.R., Boucher R.C. Membrane-restricted regulation of Ca2+ release and influx in polarized epithelia. Nature. 1995;377:643–646. doi: 10.1038/377643a0. [DOI] [PubMed] [Google Scholar]
- Parr C.E., Sullivan D.M., Paradiso A.M., Lazarowski E.R., Burch L.H., Olsen J.C., Erb L., Weisman G.A., Boucher R.C., Turner J.T. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc. Natl. Acad. Sci. USA. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto M.R., Cohn J.A., Bertuzzi G., Greengard P., Nairn A.C. Phosphorylation of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 1992;267:12742–12752. [PubMed] [Google Scholar]
- Putney J.W., Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J.W., Jr. Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Ribeiro C.P., Paradiso A.M., Boucher R.C. Apical domains of inositol 1,4,5-triphosphate (IP3)-sensitive Ca2+ stores in normal and cystic fibrosis (CF) human airway epithelia Pediatr. Pulmonol. Suppl 19 1999. 253(Abstr.) [Google Scholar]
- Snouwaert J.N., Brigman K.K., Latour A.M., Malouf N.N., Boucher R.C., Smithies O., Koller B.H. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Stutts M.J., Fitz J.G., Paradiso A.M., Boucher R.C. Multiple modes of regulation of airway epithelial chloride secretion by extracellular ATP. Am. J. Physiol. 1994;267:C1442–C1451. doi: 10.1152/ajpcell.1994.267.5.C1442. [DOI] [PubMed] [Google Scholar]
- Taylor A.L., Kudlow B.A., Marrs K.L., Gruenert D.C., W.B.Guggino, Schwiebert E.M. Bioluminescence detection of ATP release mechanisms in epithelia. Am. J. Physiol. 1998;275:C1391–C1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- Tinel H., Cancela J.M., Mogami H., Gerasimenko J.V., Gerasimenko P.V., Tepikin A.V., Petersen O.H. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:4999–5008. doi: 10.1093/emboj/18.18.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M.J. Effect of phorbol ester and calcium ionophore on chloride secretion in dog tracheal epithelium by protein kinase C. Am. J. Physiol. 1987;253:C828–C834. doi: 10.1152/ajpcell.1987.253.6.C828. [DOI] [PubMed] [Google Scholar]
- Willard H.H., Merritt L.L., Jr., Dean J.A. Instrumental Methods of Analysis Fifth edition 1974. D. Van Nostrand Company; New York: pp. 209 pp [Google Scholar]
- Willumsen N.J., Boucher R.C. Activation of an apical Cl− conductance by Ca2+ ionophores in cystic fibrosis airway epithelia. Am. J. Physiol. 1989;256:C226–C233. doi: 10.1152/ajpcell.1989.256.2.C226. [DOI] [PubMed] [Google Scholar]
- Winpenny J.P., McAlroy H.L., Gray M.A., Argent B.E. Protein kinase C regulates the magnitude and stability of CFTR currents in pancreatic duct cells. Am. J. Physiol. 1995;268:C823–C828. doi: 10.1152/ajpcell.1995.268.4.C823. [DOI] [PubMed] [Google Scholar]
- Wu R., Yankaskas J., Cheng E., Knowles M.R., Boucher R.C. Growth and differentiation of human nasal epithelial cells in culture. Serum-free, hormone-supplemented medium and proteoglycan synthesis. Am. Rev. Resp. Dis. 1985;132:311–320. doi: 10.1164/arrd.1985.132.2.311. [DOI] [PubMed] [Google Scholar]