Abstract
SMRT (silencing mediator for retinoid and thyroid hormone receptors) and N-CoR (nuclear receptor copressor) mediate transcriptional repression of important regulators that are involved in many signaling pathways. SMRT and N-CoR are related proteins that form complexes with mSin3A/B and histone deacetylases to induce local chromatin condensation and transcriptional repression. However, SMRT is substantially smaller than N-CoR, lacking an N-terminal domain of approximately 1,000 aa that are present in N-CoR. Here, we report the identification of SMRT-extended (SMRTe), which contains an N-terminal sequence that shows striking similarity with N-CoR. As in N-CoR, this SMRTe-N-terminal domain also represses basal transcription. We find that SMRTe expression is regulated during cell cycle progression and SMRTe transcripts are present in many embryonic tissues. These data redefine a structurally and functionally more related nuclear receptor corepressor family and suggest an additional role for SMRTe in the regulation of cycle-specific gene expression in diverse signaling pathways.
Transcriptional repression plays an important role in the proper regulation of cell growth, differentiation, and development (1–3). In one mechanism of transcriptional inhibition, a repressor competes with an activator for DNA binding. Alternatively, transcriptional repressors also can inhibit basal transcription through direct interaction with general transcription factors, or indirectly by promoting chromatin condensation, thereby preventing the loading of general transcription factors to the promoter (1–3).
Transcriptional repression by unliganded nuclear receptors such as TR (thyroid hormone receptor) and RAR (retinoic acid receptor) provide an excellent system for dissecting the pathways that lead to gene repression. TR and RAR play important roles in the regulation of cell growth, differentiation, and homeostasis. In the absence of hormone, TR and RAR actively repress target gene expression (4). Unliganded TR and RAR interact with the corepressors SMRT (silencing mediator for retinoid and thyroid hormone receptors) and N-CoR (nuclear receptor corepressor) (5, 6), which are components of corepressor complexes that also contain mSin3A/B and histone deacetylases (7–9). The binding of hormone to these receptors induces active conformations (10–12), causing dissociation of the corepressor complex. A coactivator complex then is recruited, leading to transcriptional activation (13).
In addition to TR and RAR, SMRT and N-CoR interact with other transcriptional regulators involved in various signaling pathways. These regulators include the orphan nuclear receptors COUP-TF1 (14), Rev-Erb, RVR (15), and DAX-1 (16), the latter of which is involved in congenital X-linked adrenal hypoplasia (17). SMRT and N-CoR also interact with antagonist-bound progesterone and estrogen receptors (18, 19), suggesting that the corepressors may play a role in determining the antagonistic activities of antihormones. Functional complexes containing SMRT and N-CoR also have been observed with several non-nuclear receptor proteins such as the homeodomain proteins Rpx2 and Pit-1 (20), as well as the mammalian homologue of Drosophila Suppressor of Hairless CBF1/RBP-Jkappa, which is involved in Notch signaling (21). Other corepressor-interacting proteins include the promyelocyte zinc finger protein PLZF, which is found in t(11:17) translocated acute promyelocytic leukemia (22–25), and the acute myeloid leukemia fusion partner ETO, which is involved in t(8;21) translocation (26–28). Together, these studies indicate that SMRT and N-CoR are involved in a wide array of biological processes and signaling pathways.
Several isoforms of SMRT and N-CoR have been reported. These include the SMRT dominant negative form TRAC1 (29), which contains only the C-terminal nuclear receptor-interacting domain, and the N-CoR/RIP13 form (30) that is similar in size and structure to SMRT. The full-length N-CoR encodes a 2,453-aa protein, whereas SMRT encodes a 1,495-aa protein that is related to the C-terminal half of N-CoR. In the present study, we identify SMRTe, which contains an additional N-terminal domain when compared with the previously identified SMRT (5). Surprisingly, this N-terminal extended sequence exhibits striking similarity with the N-terminal 1,000 aa of N-CoR, suggesting that SMRTe and N-CoR share more related structure and function. Furthermore, we show that SMRTe expression is regulated during cell cycle progression and that SMRTe transcripts are present in many tissues during early mouse embryogenesis.
MATERIALS AND METHODS
Library Screening.
A 5′-stretched λgt11 HeLa cDNA library was screened for human SMRTe according to the manufacturer’s protocol (CLONTECH). Mouse SMRTe was isolated from a λACT mouse embryonic cDNA library. The inserts were resubcloned into the pBluescript vector, and the nucleotide sequences were determined by chain termination reactions using the sequenase kit (Amersham Pharmacia). Sequence analyses were assisted by programs of the GCG package (University of Wisconsin).
Transient Transfection.
HeLa cells were maintained in DMEM supplemented with 10% FBS. About 12 hr before transfection, 104 cells were seeded in 12-well plates. Transient transfection was conducted by using a standard calcium phosphate precipitate method (42). Transfection was continued for 12 hr, and the precipitates were removed with PBS. Transfected cells were refed fresh media and harvested 48 hr afterward. Cells were harvested and processed for luciferase and β-galactosidase assays as described (42).
Western Blot Analysis.
The SDS protein gel was electroblotted onto a nitrocellulose filter by using the Trans-Blot semidry transfer system (Bio-Rad). The filter then was processed for Western blotting by 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium color reaction (Vector Laboratories) or the ECL kit (Amersham Pharmacia). The anti-SMRT rabbit polyclonal antibody (Upstate Biotechnology, Lake Placid, NY) was affinity-purified by chromatography using a glutathione S-transferase-C-SMRT protein column as described (43).
Cell Cycle Synchronization.
Mitotic cells were collected every 2 hr by mitotic shake-off and were seeded into tissue culture plates. The cells were harvested by trypsinization, and the cell numbers were determined by a hemaecytometer. The cells then were lysed in SDS sample buffer, and cellular proteins were separated by SDS/PAGE and processed for Western blotting as described above.
Indirect Immunofluorescence.
HeLa and A549 cells were grown on coverglasses in 12-well plate at least 24 hr before the analysis. Immunocytochemistry was performed as described (44). Briefly, cells were washed twice with PBS and fixed in methanol/acetone (1:1) for 1 min on dry ice. The affinity-purified anti-SMRT antibody was used at 1:100 dilution. After extensive washing, a fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibody was added, and the cells were later counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride hydrate (Sigma). The coverglasses were mounted on microscope slides with Fluoromount G (Southern Biotechnology Associates). The samples were imaged on an epi-fluorescent microscope (Olympus IX-70) with a back-illuminated charge-coupled device camera (Princeton Instruments, Trenton, NJ, 1,000 × 800) and metamorph software (Universal Imaging, Media, PA).
In Situ Hybridization.
Embryos at different developmental stages were fixed for 2 hr in 4% paraformaldehyde, serially dehydrated, cleared in xylene, and embedded in paraffin. Sections (7 mm) were cut and mounted on ProbeOn Plus slide (Fisher Scientific). The sections were deparaffinized and processed for in situ hybridization according to a standard protocol (45, 46). Briefly, the sections were washed in PBS 3× and treated with 0.2 M HCl for 20 min followed by proteinase K (5 mg/ml in 0.1 M Tris⋅HCl, 50 mM EDTA, pH 8.0) treatment for 30 min at 37°C. The sections were washed again in PBS containing 2 mg/ml of glycine and PBS and then fixed in 4% formaldehyde for 20 min and washed in PBS with glycine again. The sections were immersed in a freshly prepared mixture of 0.25% acetic anhydride in 0.1 M triethanolamine buffer for 10 min with occasional shaking, washed in 2× standard saline citrate (SSC) before prehybridization. Prehybridization was performed in a humid chamber at 55°C for 1 hr with hybridization buffer [50% formamide/2× SSC/5 mM EDTA/50 mg/ml yeast RNA/0.2% Tween-20/0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)/100 mg/ml heparin]. The sections then were hybridized in a fresh hybridization buffer containing 0.1–1 ng/ml digoxigenin (DIG)-labeled RNA probe at 65°C overnight. After hybridization, the sections were washed in 2× SSC, 0.1% CHAPS, and 50% formamide at 60°C and 1× SSC once at room temperature. The section was washed again with buffer I (0.1 M maleic acid, pH 7.5/0.15 M NaCl/0.3% Tween-20) and blocked with 2% Boehringer Blocking Reagent (BBR) and 5% heat-treated sheep serum in buffer I for 1 hr. The section was incubated with buffer I containing 2% BBR, 5% heat-treated serum, and 1/1,000 dilution of alkaline phosphatase-anti-DIG antibody at room temperature for 4 hr. The sections then were washed in buffer I before being equilibrated with buffer III (0.1 M Tris⋅HCl, pH 9.5/0.1 M NaCl/50 mM MgCl2/1 mM levamisole) and developed by using 75 mg/ml nitroblue tetrazolium/50 mg/ml 5-bromo-4-chloro-3-indolyl phosphate color reaction in buffer III. Riboprobes were synthesized by using the DIG RNA labeling system (Boehringer Mannheim). The riboprobe was dissolved in 0.1× Tris-EDTA/90% formamide solution and checked by agarose gel electrophoresis.
RESULTS
Identification of Endogenous SMRT Proteins.
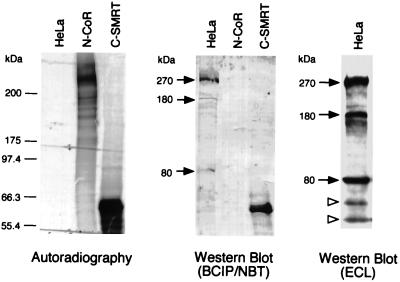
We conducted Western blotting on cell extract by using an affinity-purified anti-SMRT antibody to detect the natural SMRT proteins. HeLa cell nuclear extract, together with the in vitro-translated N-CoR (6) and C-SMRT (5), were separated by SDS/PAGE. The N-CoR protein migrates as a 270-kDa polypeptide and the C-SMRT as a 60-kDa protein as detected by autoradiography (Fig. 1, Left). On the Western blot, we find that the anti-SMRT antibody reacts strongly with C-SMRT and does not crossreact with N-CoR (Fig. 1, Center). In HeLa nuclear extract, the anti-SMRT antibody detects a major polypeptide of 270 kDa that migrates at a position similar to that of N-CoR and recognizes two weak polypeptides of approximately 180 and 80 kDa (Fig. 1, Center). The 180- and 80-kDa bands were more evident when the Western blot was developed with the ECL+ reagents (Fig. 1, Right). Preincubating the antibody with purified SMRT antigen eliminates all three SMRT signals except nonspecific bands. In contrast, preincubating with purified N-CoR antigen does not reduce the SMRT signals (data not shown). We also detected the same 270-kDa SMRT protein in many different cell lines, including CV-1, 293, NB4, MCF7, T47D, and HBL100 (data not shown). These results suggest that SMRT is expressed primarily as a 270-kDa protein, in addition to two shorter proteins.
Figure 1.
Detection of endogenous SMRT proteins. HeLa nuclear extract, together with in vitro-translated [35S]methionine-labeled N-CoR and C-SMRT, were separated on a SDS/PAGE. The N-CoR and C-SMRT polypeptides were detected by autoradiography (Left). An identical gel was processed for Western blotting using an affinity-purified rabbit anti-C-SMRT polyclonal antibody and detected by 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitroblue tetrazolium (NBT) color reaction (Center). One major polypeptide similar to the size of N-CoR (270 kDa) was detected in the HeLa nuclear extract, in addition to two minor bands of 180 and 80 kDa, respectively (arrows). The anti-SMRT antibody does not crossreact with N-CoR. The same HeLa nuclear extract also was processed for Western blotting using anti-C-SMRT antibody but developed by ECL+ reaction (Right). The three specific SMRT polypeptides and two nonspecific bands (open arrowheads) below 80 kDa were indicated.
Cloning and Characterization of the 270-kDa Form of SMRT.
To isolate cDNA that encodes the 270-kDa protein, we screened a HeLa cDNA library by using a DNA probe corresponding to the first transcriptional repression domain between amino acids 137 and 475 of SMRT (5). Initially, two positive clones were identified that both contain sequences identical to SMRT downstream from the ninth amino acid, but have distinct upstream sequences. Further sequencing analyses revealed that the upstream sequences of both clones contain a continuous ORF, suggesting that they are fragments of a longer SMRT isoform. Three further screenings were conducted, resulting in the isolation of 11 overlapping clones that together span an additional 3,190 nt upstream from the ninth amino acid of SMRT. We termed this extended SMRT transcript SMRTe (SMRT-extended) to distinguish it from SMRT (5). Subsequently, the mouse SMRTe cDNA also was isolated by using human SMRTe as probe, indicating that the SMRTe isoform is present in both human and mouse.
Human SMRTe contains 2,507 aa with a calculated molecular mass of 273,234 Da, whereas mouse SMRTe contains 2,462 aa (Fig. 2A). The human and mouse proteins share 87% identity, indicating that the SMRTe gene is highly conserved. We also identified a mouse clone that lacks a large internal fragment and contains only the N-terminal 609-aa and an unrelated 64-aa tail (Fig. 2A, ΔC). The human SMRTe shares 44% identity with human N-CoR (31), whereas the mouse SMRTe shares 42% identity with mouse N-CoR, indicating that SMRTe and N-CoR are partially related. Interestingly, an N-terminal domain between amino acids 166 and 429 is strikingly conserved between SMRTe and N-CoR (86% identity and 91% similarity) (Fig. 2 B and C). We refer to this domain as SMRTe and N-CoR conserved (SNC) domain. At the N terminus of the SNC domain, we find an amphipathic α-helix containing five hydrophobic heptad repeats (Fig. 2C). The SNC domain is followed by two conserved repeats known as the SANT (SWI3, ADA2, N-CoR, and TFIIIB B") domains (32). The two SANT motifs are only marginally related to one another within the same protein (30% identity), whereas individual motif is highly conserved between SMRTe and N-CoR in both the human and mouse origins (>75% identity) (Fig. 2D). Therefore, we refer to the N-terminal SANT motif as SANT-A and the C-terminal motif as SANT-B (Fig. 2 A and D). The SANT-A and SANT-B motifs are separated by an intervening sequence of approximately 120 aa, which contains a polyglutamine track and a charged acidic-basic region followed by a short segment that also is highly conserved between SMRTe and N-CoR (Fig. 2A).
Figure 2.
Sequences and domain comparison of mouse and human SMRTe and N-CoR. (A) The amino acid sequence of human (h) SMRTe is shown in the upper strand, and the mouse (m) SMRTe sequence is in the bottom strand. Identical amino acids between human and mouse SMRTe are indicated by hyphens. Dots are gaps introduced during the alignment. The COOH-terminal tail of the mSMRTeΔC (ΔC), the starting amino acids of the previously identified SMRT and TRAC1 also are indicated. (B) Domain comparison between SMRTe and N-CoR. The black bars indicate areas of high homology. Special domains are indicated in gray with labels (AB, acidic-basic domain; S1–4, the SIT repeated motifs, ref. 47; KGH, the KGH repeated motifs, ref. 47; SG, the serine/glycine-rich region; and SNC). The SMRTe repression domains (SRD), the N-CoR repression domains (NRD), and the nuclear receptor interacting domains (RID) are also shown. Domains involved in interactions with other proteins are also indicated. The numbering of residues is based on mouse N-CoR and human SMRTe sequence. (C) Sequence comparison of the SNC domains of human (h) and mouse (m) SMRTe (S) and N-CoR (N). The identical residues are shown in black and the conserved residues are shown in gray. The amphipathic helix and the hydrophobic heptad repeats are indicated by a black line and stars, respectively. The amino acid residues are shown on the left. (D) Comparison of SANT-A and SANT-B domains. Identical amino acids are shown in black background and the conserved residues are in gray. The Myb DNA binding domain signature sequences and the three helices (h) are also indicated in between the SANT-A and SANT-B motifs.
Transcriptional Repression by SMRTe N-Terminal Domain.
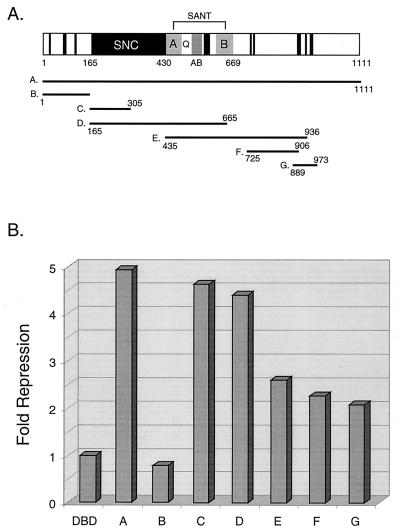
To shed light on the function of the N-terminal sequence of SMRTe, we tested its ability to repress basal transcription in reporter gene assays in mammalian cells. As expected, when linked with a Gal4 DNA binding domain (DBD), SMRTe (1–1111) efficiently represses basal transcription from a luciferase reporter containing four copies of Gal4 binding sites (Fig. 3 A and B). The N-terminal sequence of SMRTe then was divided into overlapping fragments (Fig. 3A), which were individually linked to Gal4 DBD and assayed for their transcriptional repression activities. We find that the N-terminal 140 aa of the SNC domain contains strong transcriptional repression activity (Fig. 3B), suggesting that one role for this SNC domain is to repress basal transcription. Regions outside of the SNC domain, except the N-terminal 165 aa, also exhibit weak repression activities in this assay (Fig. 3 A and B). These results indicate that, like N-CoR, the N-terminal domain of SMRTe is involved in transcription repression and that the SNC domain may be crucial for this function.
Figure 3.
Transcriptional repression by the N-terminal domain of SMRTe. (A) Schematic of the Gal4 DBD fusion constructs used in this experiment. The domains are as described in Fig. 2B. The numbers indicate amino acid residues. The seven different SMRTe N-terminal fragments (A to G) are fused into Gal4 DBD and their effects on reporter gene expression are shown in B. (B) Transcriptional repression by Gal4 DBD-SMRTe fusions. The fold repression of each construct was determined by average relative luciferase activity using Gal4 DBD as a standard in a triplicate experiment.
Cell Cycle-Dependent Expression of SMRTe.
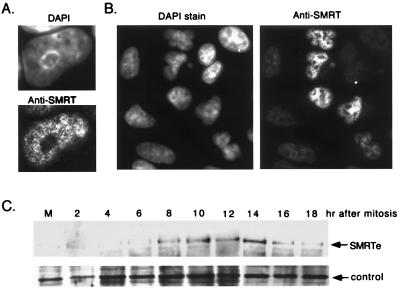
By using the affinity purified anti-SMRT antibody, we also examined the subcellular distribution of endogenous SMRTe protein by immunofluorescence staining. Consistent with a previous study (33), in HeLa cell nuclei we observe fine granules that are excluded from nucleoli (Fig. 4A). Because A549 cells fail to express detectable SMRTe message in Northern blotting, we used these cells as controls in the immunofluorescence study. As expected, the overall intensity of SMRT staining in A549 cells is weaker than in the HeLa cells (Fig. 4B, Right). However, we find that there is a subset of A549 cells expressing relatively higher levels of SMRTe (Fig. 4B, Right). We estimate that approximately 20% of the A549 cells display clearly detectable levels of SMRTe in this assay.
Figure 4.
Cell cycle-dependent expression of SMRTe. (A) Immunofluorescence staining of endogenous SMRTe in HeLa cells. The nucleus stained by 4′,6-diamidino-2-phenylindole (DAPI) dihydrochloride hydrate (Upper) and the SMRTe signal (Lower) is shown. Note that SMRTe is distributed as fine granules in the nucleus and is excluded from the nucleoli. (B) Immunostaining of SMRTe in an unsynchronized population of A549 cells. Strong SMRTe signals were detected in a subset of A549 cells. (C) Western blotting for SMRTe in A549 cells at different time points after release from mitosis. The 270-kDa SMRTe signal (Upper) and a nonspecific band (Lower) are shown.
This fluctuation in immunostaining suggests that SMRTe expression may be regulated in a cell cycle-dependent manner. Therefore, we synchronized A549 cells and analyzed the endogenous SMRTe protein by Western blotting at different time points after release from mitosis. We find that the 270-kDa SMRTe protein level increases at a time when cells normally would enter S phase between 8 and 14 hr after mitosis (Fig. 4C, Upper). A nonspecific band shows approximately equal intensity in all samples that have been preadjusted by cell number (Fig. 4C, Lower). These results indicate that SMRTe expression is cell cycle regulated, suggesting that SMRTe may play a role in cell cycle progression.
Expression of SMRT in Mouse Embryo.
Previously, we have detected the SMRT message in all stages of mouse embryos in Northern blotting (33). To provide further insight into the expression of SMRTe during embryogenesis, we analyzed the distribution of SMRTe transcripts in early mouse embryos by in situ hybridization. By using a DIG-labeled antisense mouse SMRTe riboprobe, we detected SMRTe transcripts in thin sections of mouse embryos at embryonic day (E) 9.0, E11.5, and E13.5 postconception (Fig. 5). SMRTe transcripts are found at E9.0–E13.5 in nearly all tissues with low levels of expression in the heart and liver. The expression in the frontal section of E9.0 is most prominent in the neural tube and undetectable in the heart. In the saggital section of E11.5, the SMRTe transcripts are high in the condensation of sclerotome, lung, the first bronchial arch, and cerebellar plate (metencephalon). However, it is expressed at low levels in the liver and the atrium and ventricle of heart. In the saggital section of E13.5 embryo, the SMRTe transcripts are expressed in the lung, brain, and the perichondrium of the head, neck, and the ribs. Little or no expression was observed in the developed vertebrate body, liver, or heart. These results indicate that SMRTe transcripts are widely expressed in early mouse embryos, supporting a role for SMRTe in multiple biological processes during embryogenesis.
Figure 5.
SMRTe transcripts in mouse embryos. SMRTe transcripts were detected by in situ hybridization to thin sections of (A) E9.0 days postconception, (B) E11.5, and (C) E13.5 using DIG-labeled antisense riboprobe. c1 and c2 show enlargement of areas in the cartilage and lung at E13.5 indicated by rectangles in C. (D) DIG-labeled sense probe shows the background signal. b, brain; ba, bronchial arch; br, bronchus; c, cartilage; cp, cerebellar plate; h, heart; lm, limb; lu, lung; lv, liver; nt, neural tube; pc, perichondrium; sc, sclerotome; vb, vertebra body.
DISCUSSION
In the present study, we have identified a full-length isoform of SMRT. Several lines of evidence strongly indicate that the SMRTe form is the primary product of the SMRT gene. First, the major endogenous SMRT protein is 270 kDa, similar in size to N-CoR and consistent with the estimated molecular mass of SMRTe. Second, the SMRTe N-terminal domain is highly conserved with that of N-CoR, suggesting that SMRTe is more related to N-CoR. Third, the previously identified 9.5-kb SMRT message represents SMRTe because it hybridizes to SMRTe-specific probes (data not shown).
The identification of the N-terminal extended domain of SMRTe reveals several interesting relationships with N-CoR. First, this region contains a 300-aa domain that shares more than 90% similarity with N-CoR. Because this region of N-CoR is involved in both transcriptional repression and protein–protein interactions, the high homology suggests that this domain of SMRTe may have similar function. Accordingly, we find that the highly conserved SNC domain is crucial for transcriptional repression. Second, SMRTe contains a unique polyglutamine track that is absent in N-CoR. Polyglutamine tracks are found in a number of transcriptional regulators, and the expansion of glutamines relates to several human diseases (34–36). The unique polyglutamine track in SMRTe might reflect a differential functional property between SMRTe and N-CoR. Third, the two SANT motifs previously found in N-CoR and other transcriptional regulators (32) also are present in SMRTe, indicating that SMRTe is a SANT-containing protein.
Presently, the function of the SANT motifs in SMRTe and N-CoR is unclear, but in Myb oncoproteins, they mediate DNA binding by resembling the homeodomain-like, helix–turn–helix motifs (37). The minimal Myb DBD contains two tandem repeats (38), and thus the two SANT repeats in SMRTe and N-CoR might contribute to DNA binding. In this case, SMRTe and N-CoR might act as sequence-specific transcription repressors or might contribute to DNA binding while associating with DNA binding proteins. Alternatively, the SANT domains might play a role in protein–protein interaction required for assembly of nuclear corepressor complexes. Although the two SANT motifs appear to differ from each other, they might functionally complement one another. In SMRTe, the SANT-A and SANT-B domains are separated by a polyglutamine track, a highly charged motif, and a conserved segment. We propose that these intervening sequences may regulate a functional interaction between the SANT-A and SANT-B motifs.
The N-terminal 160-aa of N-CoR interacts with mSiah2, which targets N-CoR for proteosome-mediated degradation in a cell-dependent manner (39). This region of N-CoR is not conserved within SMRTe, suggesting that SMRTe might not interact with mSiah2 and that the mechanism of SMRTe turnover may differ from that of N-CoR. On the other hand, a component of the HDAC-containing corepressor complex, SAP30, interacts with the N-terminal 312 aa of N-CoR (40). This region contains a significant portion of the highly conserved domain, suggesting that SAP30 also might interact with SMRTe. Furthermore, amino acids 254–312 of N-CoR have been shown to interact with both Pit1 (20) and mSin3A/B (9). Within this 59-aa region, only five residues differ between SMRTe and N-CoR, suggesting that this region of SMRTe might interact with Pit1 and mSin3.
Immunofluorescence studies suggest that the endogenous SMRTe protein resides in fine granules in the nucleus, contrasting the distribution of overexpressed SMRT (25). Possibly, overexpressed SMRT may form inclusion bodies in the nucleus. We also find that SMRTe protein level is regulated during cell-cycle progression as indicated by both immunofluorescence staining and Western blotting. Accordingly, a cell cycle-dependent modification of the coactivator CBP has been reported recently (41). Further experiments will be required to determine the exact stage of the cell cycle where SMRTe expression is maximal and the function of SMRTe in these cells. These data suggest that the activities of nuclear receptor corepressors may be regulated during cell cycle progression. For instance, the corepressor may repress expression of cell cycle-specific genes, and thus contribute to regulation of cell cycle progression. Alternatively, the corepressor may be involved in other cellular processes occurring at specific stages of the cell cycle, such as DNA replication. It remains to be determined whether N-CoR expression is similarly regulated during cell cycle progression and whether SMRTe and N-CoR may function together.
In conclusion, we have identified the SMRTe isoform that is very similar in size and structure to N-CoR, thereby redefining a more conserved nuclear receptor corepressor family. Characterization of the SMRTe N terminus reveals striking similarity with that of N-CoR, suggesting that SMRTe and N-CoR may share highly related functions. The physiological function of SMRTe in nuclear receptor and other signaling pathways remains to be determined. The finding that SMRTe is differentially expressed during cell cycle progression and widely distributed in embryonic tissues provides additional insights for understanding the function of nuclear receptor corepressors in biological processes.
Acknowledgments
We are grateful to Dr. Kai Lin and Christopher Leo for critical reading of this manuscript. We thank other members of the Chen laboratory for discussion and suggestions. We also thank Robyn Greener for help with initial library screening and subcloning of plasmid constructs. This work was made possible by Grant DK52542 from the National Institutes of Health (to J.D.C.). E.-J.P. was supported by a postdoctoral fellowship from the Korea Research Foundation. D.J.S. was supported by a postdoctoral fellowship from the Arthritis Foundation. J.D.C. was supported partly from a junior scholar award of the American Society of Hematology.
ABBREVIATIONS
- SMRT
silencing mediator for retinoid and thyroid hormone receptors
- SMRTe
SMRT-extended
- N-CoR
nuclear receptor corepressor
- RAR
retinoic acid receptor
- TR
thyroid hormone receptor
- DIG
digoxigenin
- SNC
SMRTe and N-CoR conserved domain
- SANT
SWI3/ADA2/N-CoR/TFIIIB B"
- DBD
DNA binding domain
- E(n)
embryonic day n
Footnotes
References
- 1.Johnson A D. Cell. 1995;81:655–658. doi: 10.1016/0092-8674(95)90524-3. [DOI] [PubMed] [Google Scholar]
- 2.Hanna-Rose W, Hansen U. Trends Genet. 1996;12:229–234. doi: 10.1016/0168-9525(96)10022-6. [DOI] [PubMed] [Google Scholar]
- 3.DePinho R A. Nature (London) 1998;391:533. doi: 10.1038/35257. , 535–536. [DOI] [PubMed] [Google Scholar]
- 4.Baniahmad A, Kohne A C, Renkawitz R. Cell. 1992;11:1015–1023. doi: 10.1002/j.1460-2075.1992.tb05140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J D, Evans R M. Nature (London) 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 6.Horlein A J, Naar A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass C K, Rosenfeld M G. Nature (London) 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 7.Nagy L, Kao H Y, Chakravarti D, Lin R J, Hassig C A, Ayer D E, Schreiber S L, Evans R M. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 8.Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho R A. Nature (London) 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 9.Heinzel T, Lavinsky R M, Mullen T M, Soderstrom M, Laherty C D, Torchia J, Yang W M, Brard G, Ngo S D, Davie J R, et al. Nature (London) 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 10.Renaud J P, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D. Nature (London) 1995;378:681–689. doi: 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- 11.Rastinejad F, Perlmann T, Evans R M, Sigler P B. Nature (London) 1995;375:203–211. doi: 10.1038/375203a0. [DOI] [PubMed] [Google Scholar]
- 12.Bourguet W, Ruff M, Chambon P, Gronemeyer H, Moras D. Nature (London) 1995;375:377–382. doi: 10.1038/375377a0. [DOI] [PubMed] [Google Scholar]
- 13.Chen J D, Li H. Crit Rev Eukaryot Gene Exp. 1998;8:169–190. doi: 10.1615/critreveukargeneexpr.v8.i2.40. [DOI] [PubMed] [Google Scholar]
- 14.Shibata H, Nawaz Z, Tsai S Y, O’Malley B W, Tsai M J. Mol Endocrinol. 1997;11:714–724. doi: 10.1210/mend.11.6.0002. [DOI] [PubMed] [Google Scholar]
- 15.Zamir I, Harding H P, Atkins G B, Horlein A, Glass C K, Rosenfeld M G, Lazar M A. Mol Cell Biol. 1996;16:5458–5465. doi: 10.1128/mcb.16.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford P A, Dorn C, Sadovsky Y, Milbrandt J. Mol Cell Biol. 1998;18:2949–2956. doi: 10.1128/mcb.18.5.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muscatelli F, Strom T M, Walker A P, Zanaria E, Recan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, et al. Nature (London) 1994;372:672–676. doi: 10.1038/372672a0. [DOI] [PubMed] [Google Scholar]
- 18.Wagner B L, Norris J D, Knotts T A, Weigel N L, McDonnell D P. Mol Cell Biol. 1998;18:1369–1378. doi: 10.1128/mcb.18.3.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Jeyakumar M, Petukhov S, Bagchi M K. Mol Endocrinol. 1998;12:513–524. doi: 10.1210/mend.12.4.0089. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Lavinsky R M, Dasen J S, Flynn S E, McInerney E M, Mullen T M, Heinzel T, Szeto D, Korzus E, Kurokawa R, et al. Nature (London) 1998;395:301–306. doi: 10.1038/26270. [DOI] [PubMed] [Google Scholar]
- 21.Kao H Y, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner C R, Evans R M, Kadesch T. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He L Z, Guidez F, Tribioli C, Peruzzi D, Ruthardt M, Zelent A, Pandolfi P P. Nat Genet. 1998;18:126–135. doi: 10.1038/ng0298-126. [DOI] [PubMed] [Google Scholar]
- 23.Hong S H, David G, Wong C W, Dejean A, Privalsky M L. Proc Natl Acad Sci USA. 1997;94:9028–9033. doi: 10.1073/pnas.94.17.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong C W, Privalsky M L. J Biol Chem. 1998;273:27695–27702. doi: 10.1074/jbc.273.42.27695. [DOI] [PubMed] [Google Scholar]
- 25.Lin R J, Nagy L, Inoue S, Shao W, Miller W H, Jr, Evans R M. Nature (London) 1998;391:811–814. doi: 10.1038/35895. [DOI] [PubMed] [Google Scholar]
- 26.Westendorf J J, Yamamoto C M, Lenny N, Downing J R, Selsted M E, Hiebert S W. Mol Cell Biol. 1998;18:322–333. doi: 10.1128/mcb.18.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutterbach B, Westendorf J J, Linggi B, Patten A, Moniwa M, Davie J R, Huynh K D, Bardwell V J, Lavinsky R M, Rosenfeld M G, et al. Mol Cell Biol. 1998;18:7176–7184. doi: 10.1128/mcb.18.12.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelmetti V, Zhang J, Fanelli M, Minucci S, Pelicci P G, Lazar M A. Mol Cell Biol. 1998;18:7185–7191. doi: 10.1128/mcb.18.12.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sande S, Privalsky M L. Mol Endocrinol. 1996;10:813–825. doi: 10.1210/mend.10.7.8813722. [DOI] [PubMed] [Google Scholar]
- 30.Seol W, Choi H S, Moore D D. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Hoshino T, Redner R L, Kajigaya S, Liu J M. Proc Natl Acad Sci USA. 1998;95:10860–10865. doi: 10.1073/pnas.95.18.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aasland R, Stewart A F, Gibson T. Trends Biochem Sci. 1996;21:87–88. [PubMed] [Google Scholar]
- 33.Chen J D, Umesono K, Evans R M. Proc Natl Acad Sci USA. 1996;93:7567–7571. doi: 10.1073/pnas.93.15.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischbeck K H. J Inherit Metab Dis. 1997;20:152–158. doi: 10.1023/a:1005344403603. [DOI] [PubMed] [Google Scholar]
- 35.Reddy P S, Housman D E. Curr Opin Cell Biol. 1997;9:364–372. doi: 10.1016/s0955-0674(97)80009-9. [DOI] [PubMed] [Google Scholar]
- 36.Davies S W, Beardsall K, Turmaine M, DiFiglia M, Aronin N, Bates G P. Lancet. 1998;351:131–133. doi: 10.1016/S0140-6736(97)08360-8. [DOI] [PubMed] [Google Scholar]
- 37.Frampton J, Gibson T J, Ness S A, Doderlein G, Graf T. Protein Eng. 1991;4:891–901. doi: 10.1093/protein/4.8.891. [DOI] [PubMed] [Google Scholar]
- 38.Ogata K, Morikawa S, Nakamura H, Sekikawa A, Inoue T, Kanai H, Sarai A, Ishii S, Nishimura Y. Cell. 1994;79:639–648. doi: 10.1016/0092-8674(94)90549-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Guenther M G, Carthew R W, Lazar M A. Genes Dev. 1998;12:1775–1780. doi: 10.1101/gad.12.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laherty C D, Billin A N, Lavinsky R M, Yochum G S, Bush A C, Sun J M, Mullen T M, Davie J R, Rose D W, Glass C K, et al. Mol Cell. 1998;2:33–42. doi: 10.1016/s1097-2765(00)80111-2. [DOI] [PubMed] [Google Scholar]
- 41.Ait-Si-Ali S, Ramirez S, Barre F X, Dkhissi F, Magnaghi-Jaulin L, Girault J A, Robin P, Knibiehler M, Pritchard L L, Ducommun B, et al. Nature (London) 1998;396:184–186. doi: 10.1038/24190. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Gomes P J, Chen J D. Proc Natl Acad Sci USA. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 44.Dyck J A, Maul G G, Miller W H, Jr, Chen J D, Kakizuka A, Evans R M. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 45.Harland R M. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- 46.Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Nature (London) 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Leo C, Schroen D J, Chen J D. Mol Endocrinol. 1997;11:2025–2037. doi: 10.1210/mend.11.13.0028. [DOI] [PubMed] [Google Scholar]