Abstract
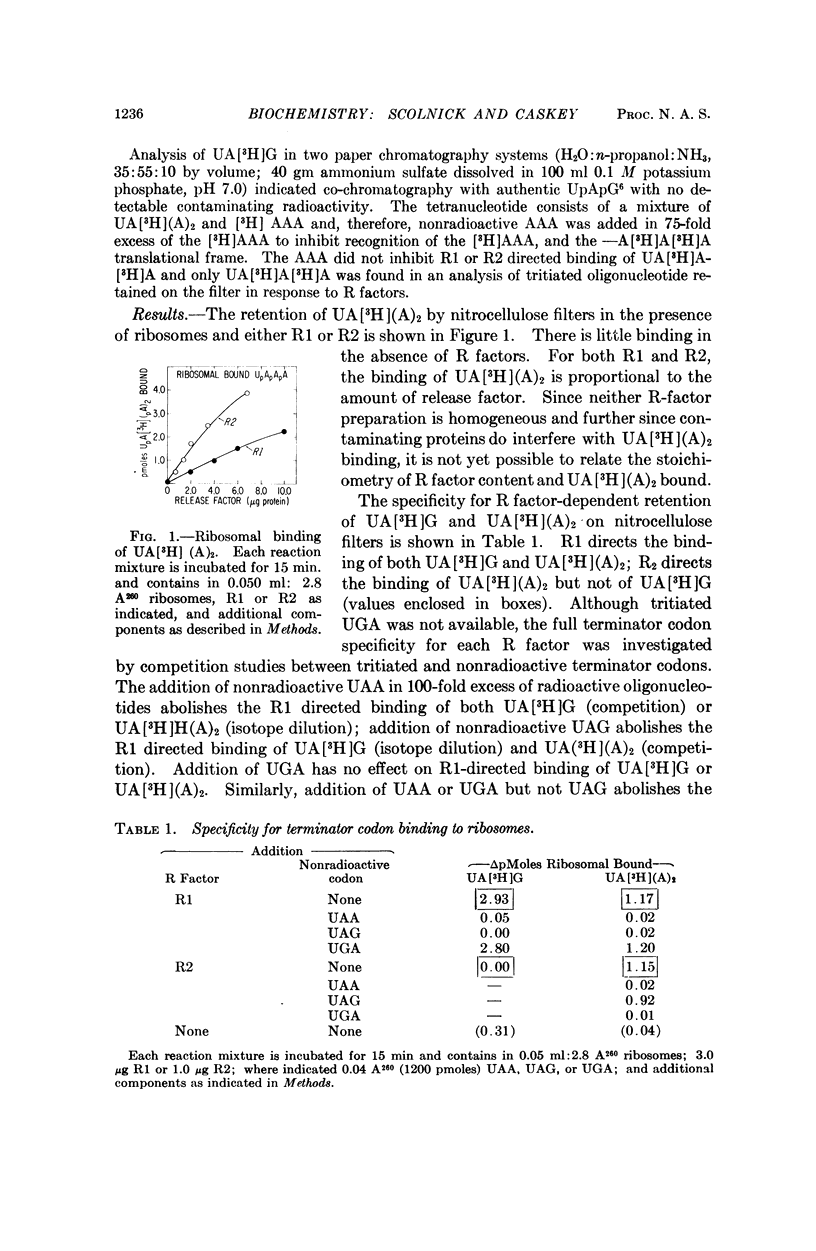
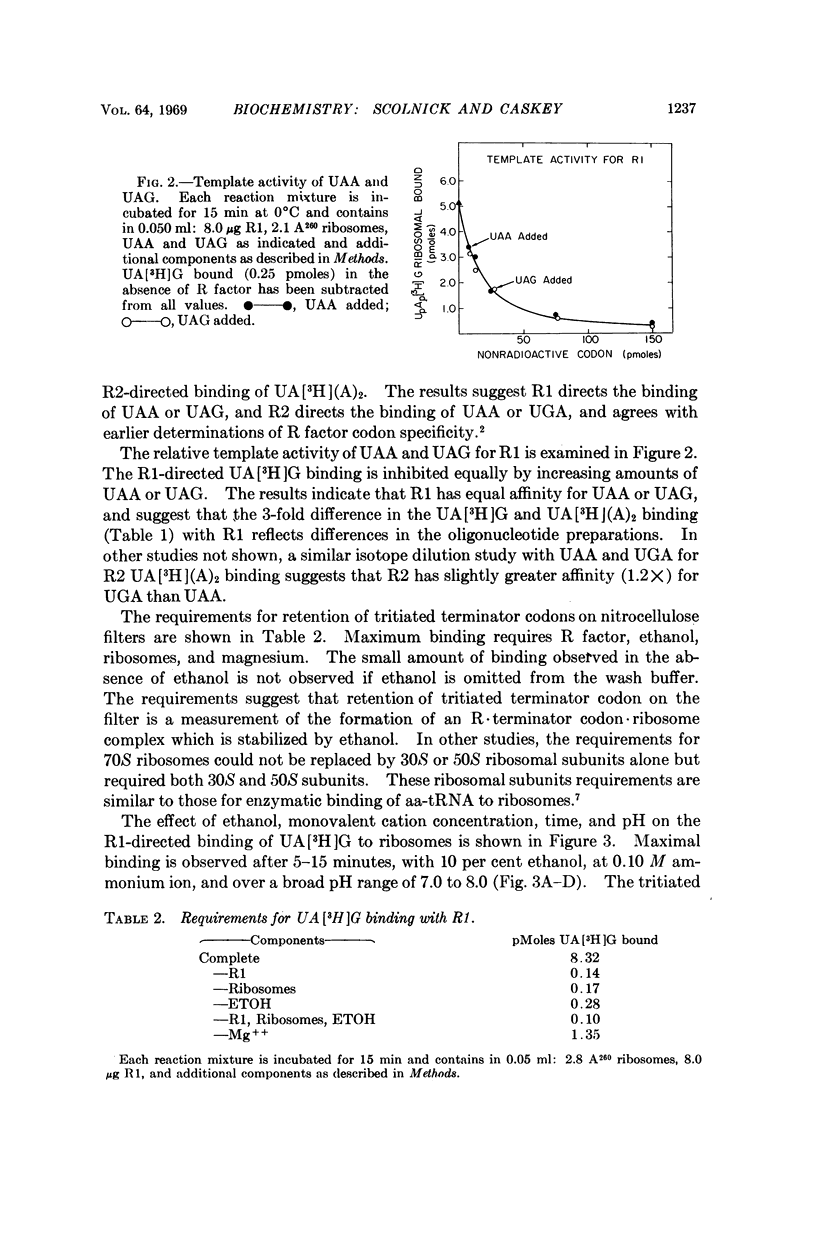
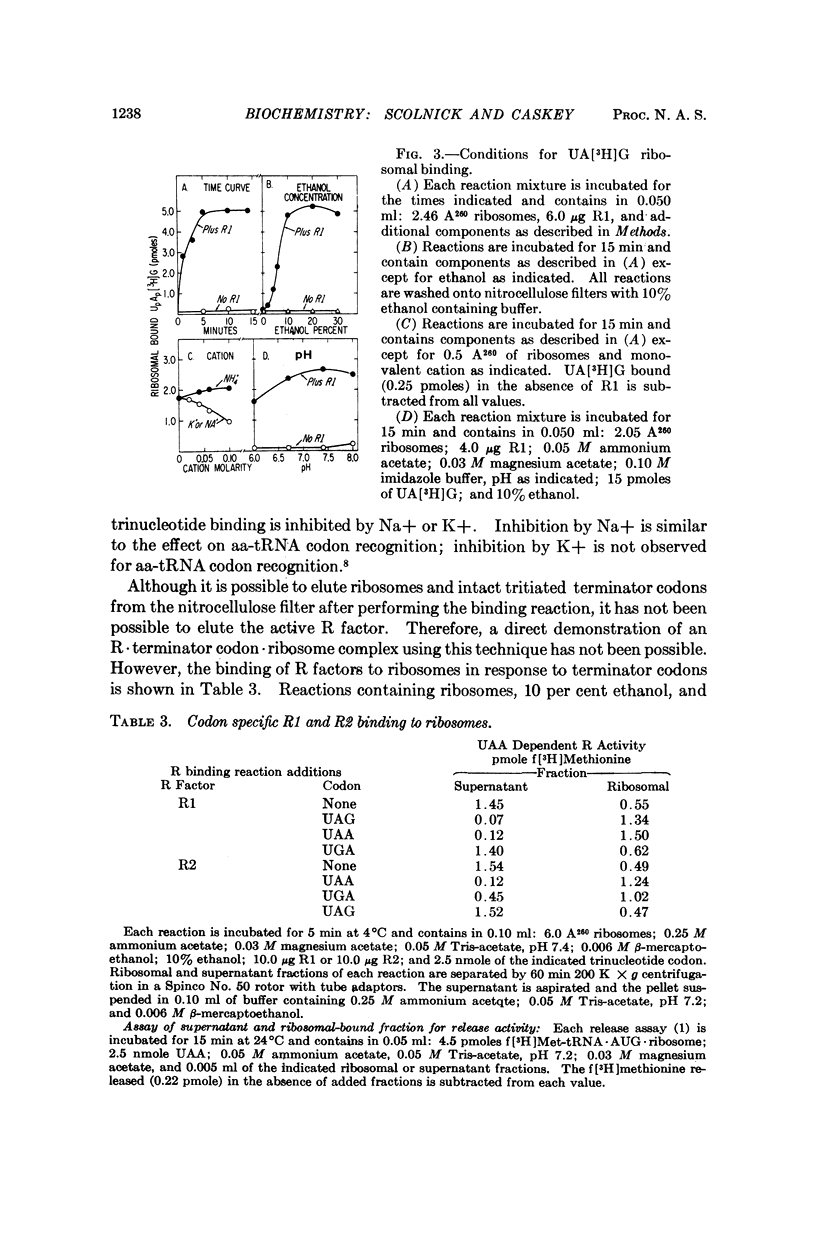
The protein release factors, R1 and R2, bind to ribosomes in response to specific terminator codons (R1 to UAA or UAG, R2 to UAA or UGA). In reactions containing ribosomes, the tritiated oligonucleotide UA[3H](A)2 is retained on nitrocellulose filters in response to either R1 or R2, and UA[3H]G in response to R1. These results indicate that an R·terminator codon·70S ribosome intermediate occurs during terminator codon recognition and suggest that protein release factors R1 and R2 recognize terminator codons.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capecchi M. R. Polypeptide chain termination in vitro: isolation of a release factor. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1144–1151. doi: 10.1073/pnas.58.3.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. T., Tompkins R., Scolnick E., Caryk T., Nirenberg M. Sequential translation of trinucleotide codons for the initiation and termination of protein synthesis. Science. 1968 Oct 4;162(3849):135–138. doi: 10.1126/science.162.3849.135. [DOI] [PubMed] [Google Scholar]
- Hatfield D. Oligonucleotide-ribosome-AA-sRNA interactions. Cold Spring Harb Symp Quant Biol. 1966;31:619–622. doi: 10.1101/sqb.1966.031.01.080. [DOI] [PubMed] [Google Scholar]
- Leder P., Singer M. F., Brimacombe R. L. Synthesis of trinucleoside diphosphates with polynucleotide phosphorylase. Biochemistry. 1965 Aug;4(8):1561–1567. doi: 10.1021/bi00884a015. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J., Haenni A. L. Requirement of granosine 5'-triphosphate for ribosomal binding of aminoacyl-SRNA. Proc Natl Acad Sci U S A. 1968 Feb;59(2):554–560. doi: 10.1073/pnas.59.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G., Goldstein J., Scolnick E., Caskey T. Peptide chain termination. 3. Stimulation of in vitro termination. Proc Natl Acad Sci U S A. 1969 May;63(1):183–190. doi: 10.1073/pnas.63.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monro R. E., Vazquez D. Ribosome-catalysed peptidyl transfer: effects of some inhibitors of protein synthesis. J Mol Biol. 1967 Aug 28;28(1):161–165. doi: 10.1016/s0022-2836(67)80085-8. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Nirenberg M., Caskey T., Marshall R., Brimacombe R., Kellogg D., Doctor B., Hatfield D., Levin J., Rottman F., Pestka S. The RNA code and protein synthesis. Cold Spring Harb Symp Quant Biol. 1966;31:11–24. doi: 10.1101/sqb.1966.031.01.008. [DOI] [PubMed] [Google Scholar]
- Pestka S., Nirenberg M. Regulatory mechanisms and protein synthesis. X. Codon recognition on 30 S ribosomes. J Mol Biol. 1966 Oct 28;21(1):145–171. doi: 10.1016/0022-2836(66)90085-4. [DOI] [PubMed] [Google Scholar]
- Scolnick E., Tompkins R., Caskey T., Nirenberg M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci U S A. 1968 Oct;61(2):768–774. doi: 10.1073/pnas.61.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M. Recognition of nucleotide sequences. Annu Rev Biochem. 1969;38:841–880. doi: 10.1146/annurev.bi.38.070169.004205. [DOI] [PubMed] [Google Scholar]



