Abstract
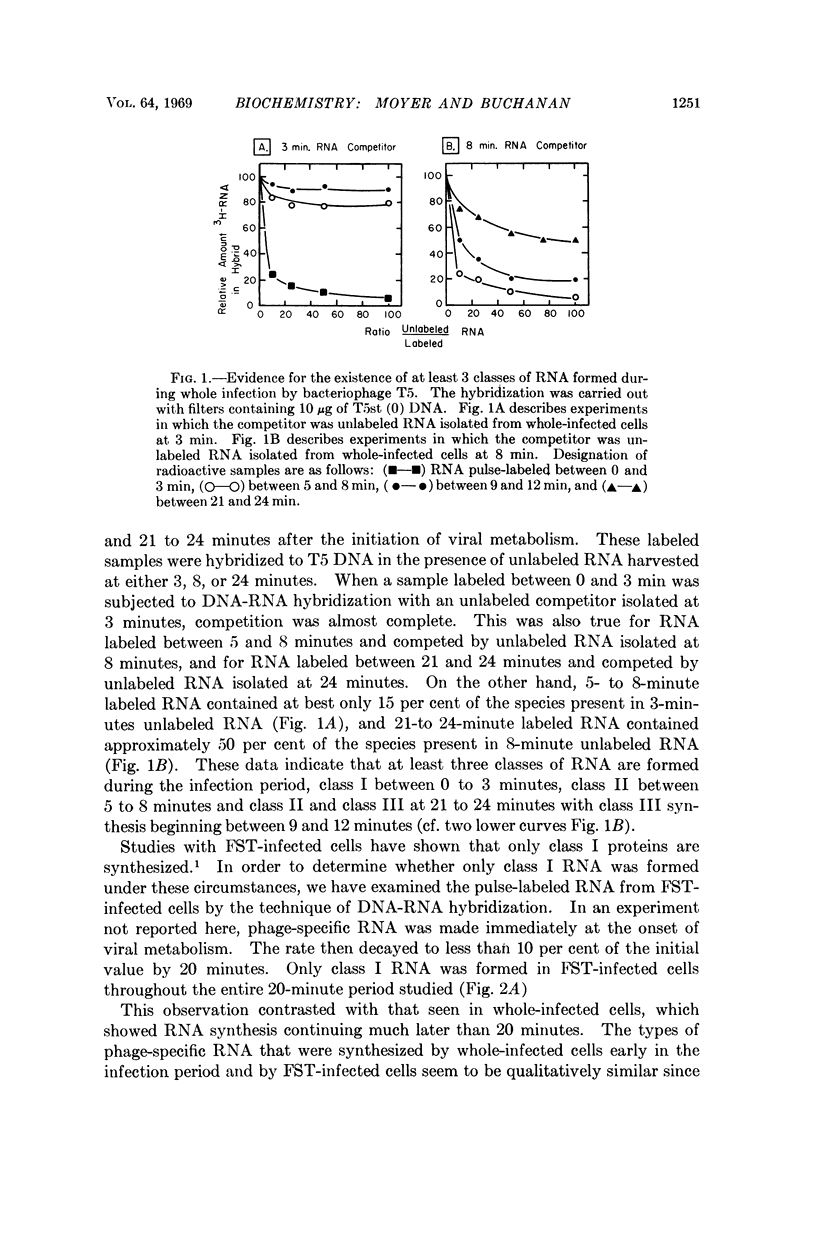
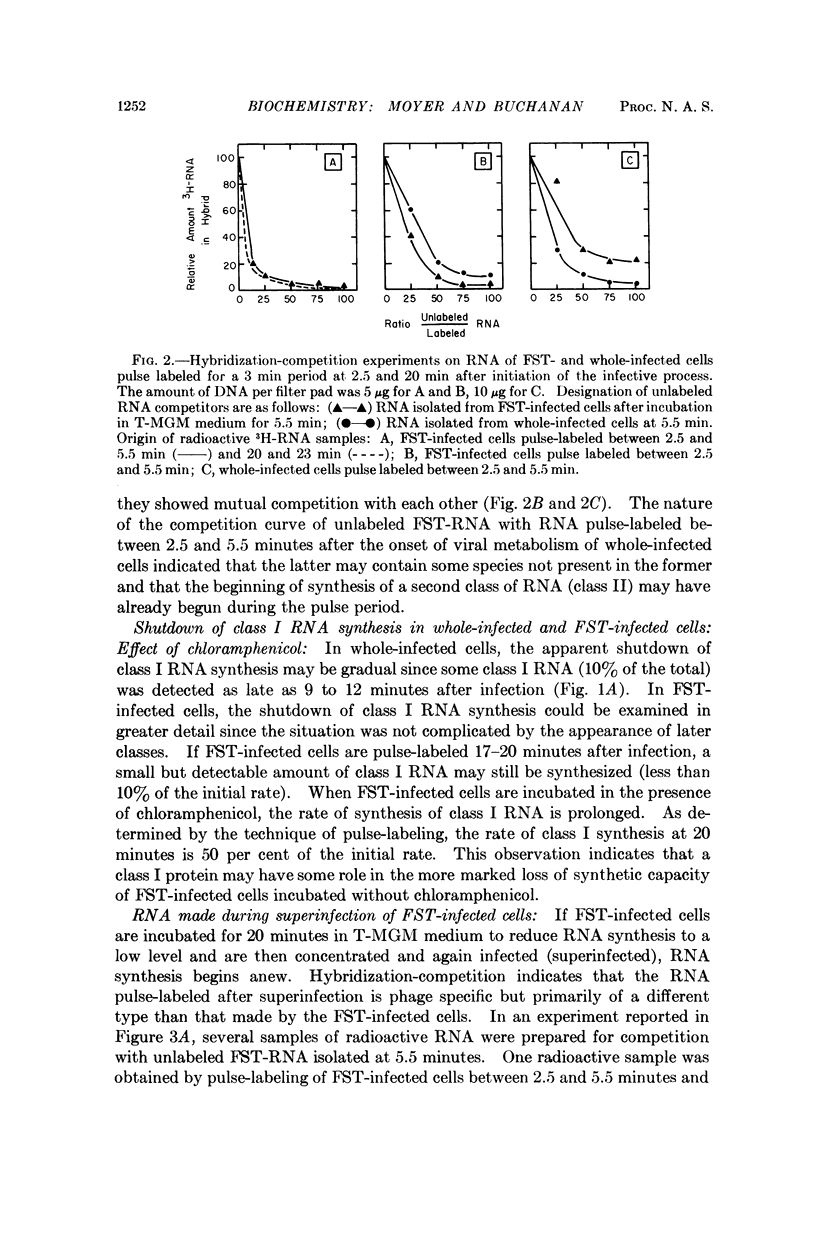
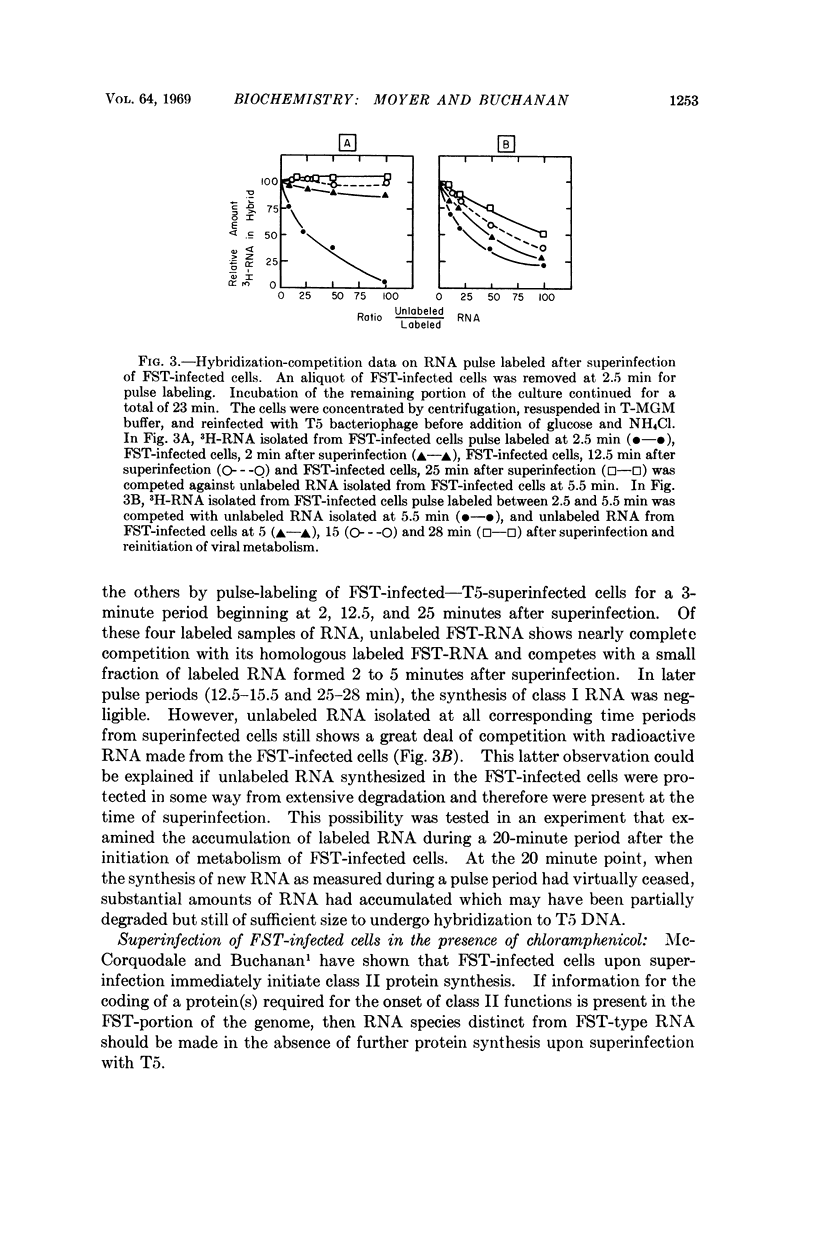
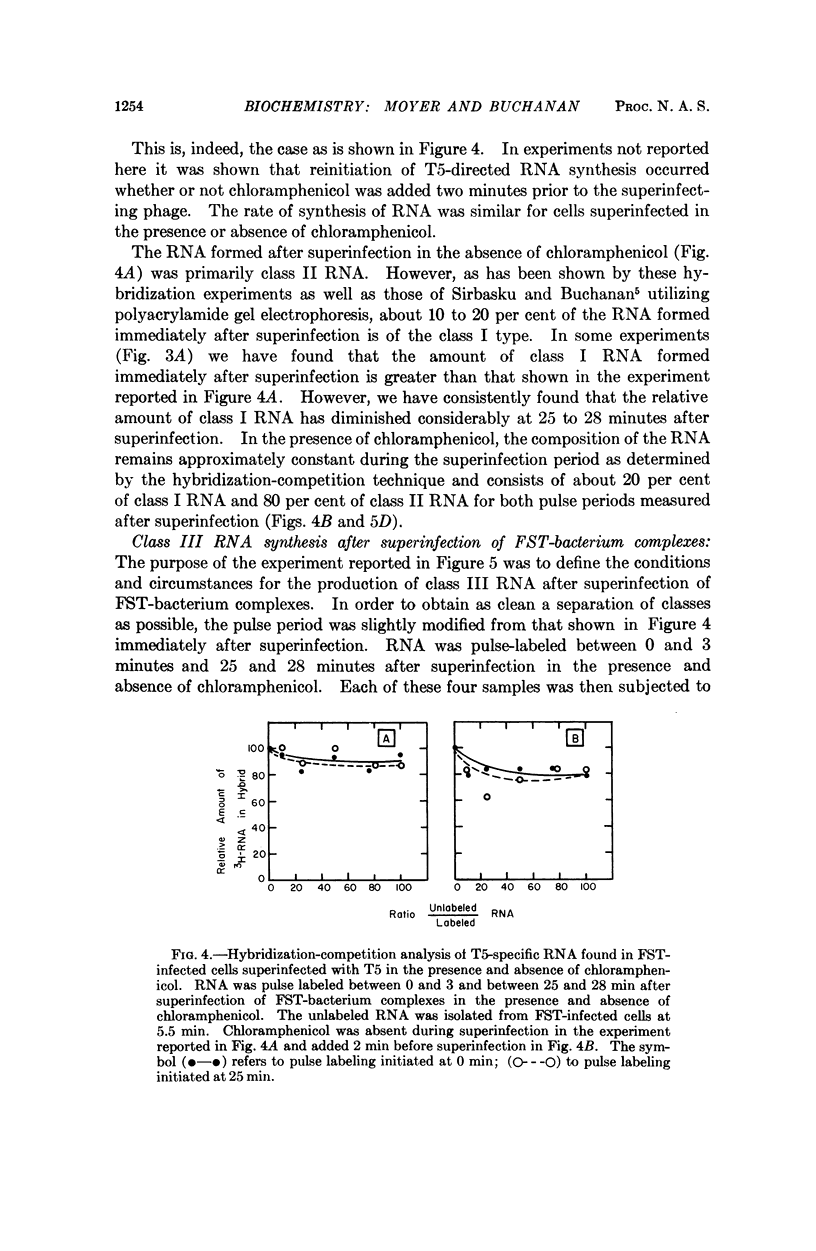
The RNA-labeling patterns obtained after T5 infection of Escherichia coli F agree with the patterns of protein labeling published by McCorquodale and Buchanan.1 Three distinct classes of RNA formed sequentially during the period of viral development can be recognized by the DNA-RNA hybridization-competition technique. Class I RNA is formed within 5 minutes after the beginning of viral metabolism and corresponds to the RNA synthesized in response to infection with the 8 per cent segment of T5 DNA. Protein synthesis directed by this 8 per cent segment is required in some capacity for the cessation of class I synthesis and the beginning of the synthesis of class II at 4 to 5 min after infection. Class III RNA synthesis begins between 9 and 12 minutes. Its appearance is prevented when chloramphenicol is added immediately after complete expression of class I functions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRAWFORD L. V. Nucleic acid metabolism in Escherichia coli infected with phage T5. Virology. 1959 Apr;7(4):359–374. doi: 10.1016/0042-6822(59)90065-0. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- KAISER A. D., HOGNESS D. S. The transformation of Escherichia coli with deoxyribonucleic acid isolated from bacteriophage lambda-dg. J Mol Biol. 1960 Dec;2:392–415. doi: 10.1016/s0022-2836(60)80050-2. [DOI] [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. III. Stages revealed by changes in susceptibility of early complexes to abortive infection. Virology. 1961 Oct;15:127–135. doi: 10.1016/0042-6822(61)90229-x. [DOI] [PubMed] [Google Scholar]
- LANNI Y. T., MCCORQUODALE D. J., WILSON C. M. MOLECULAR ASPECTS OF DNA TRANSFER FROM PHAGE T5 TO HOST CELLS. II. ORIGIN OF FIRST-STEP-TRANSFER DNA FRAGMENTS. J Mol Biol. 1964 Oct;10:19–27. doi: 10.1016/s0022-2836(64)80024-3. [DOI] [PubMed] [Google Scholar]
- Lanni Y. T. DNA transfer from phage T5 to host cells: dependence on intercurrent protein synthesis. Proc Natl Acad Sci U S A. 1965 May;53(5):969–973. doi: 10.1073/pnas.53.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembach K. J., Kuninaka A., Buchanan J. M. The relationship of DNA replication to the control of protein synthesis in protoplasts of T4-infected Escherichia coli B. Proc Natl Acad Sci U S A. 1969 Feb;62(2):446–453. doi: 10.1073/pnas.62.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCORQUODALE D. J., LANNI Y. T. MOLECULAR ASPECTS OF DNA TRANSFER FROM PHAGE T5 TO HOST CELLS. I. CHARACTERIZATION OF FIRST-STEP-TRANSFER MATERIAL. J Mol Biol. 1964 Oct;10:10–18. doi: 10.1016/s0022-2836(64)80023-1. [DOI] [PubMed] [Google Scholar]
- McCorquodale D. J., Buchanan J. M. Patterns of protein synthesis in T5-infected Escherichia coli. J Biol Chem. 1968 May 25;243(10):2550–2559. [PubMed] [Google Scholar]
- Salser W., Gesteland R. F., Bolle A. In vitro synthesis of bacteriophage lysozyme. Nature. 1967 Aug 5;215(5101):588–591. doi: 10.1038/215588a0. [DOI] [PubMed] [Google Scholar]



