Abstract
Light polypeptide chains from both normal human γG immunoglobulin and Bence-Jones proteins can be cleaved into halves by limited proteolysis with trypsin, pepsin, or papain. The fragments were obtained in yields of up to 22 per cent, had molecular weights of 10,000 to 11,000, and were shown by amino acid analysis and antigenic analysis to correspond to variable or constant regions of light chains. Starch gel electrophoresis in urea suggested that each half consisted of a single polypeptide chain. A fragment from the urine of a myeloma patient corresponded almost exactly to the constant half of a γ-chain and had a compact shape (Stokes radius of 16 Å; frictional ratio of 1.1). Similar Stokes radii were estimated both on fragments from normal urine and fragments produced by proteolysis of normal light chains.
The results are consistent with the view that most urinary fragments have a catabolic origin and suggest that there is a small stretch of polypeptide chain between the compact V and C regions of light chains which is particularly susceptible to proteolysis. This lends support to the hypothesis that immunoglobulin chains consist of globular domains connected by more extended stretches of polypeptide chain.
Full text
PDF


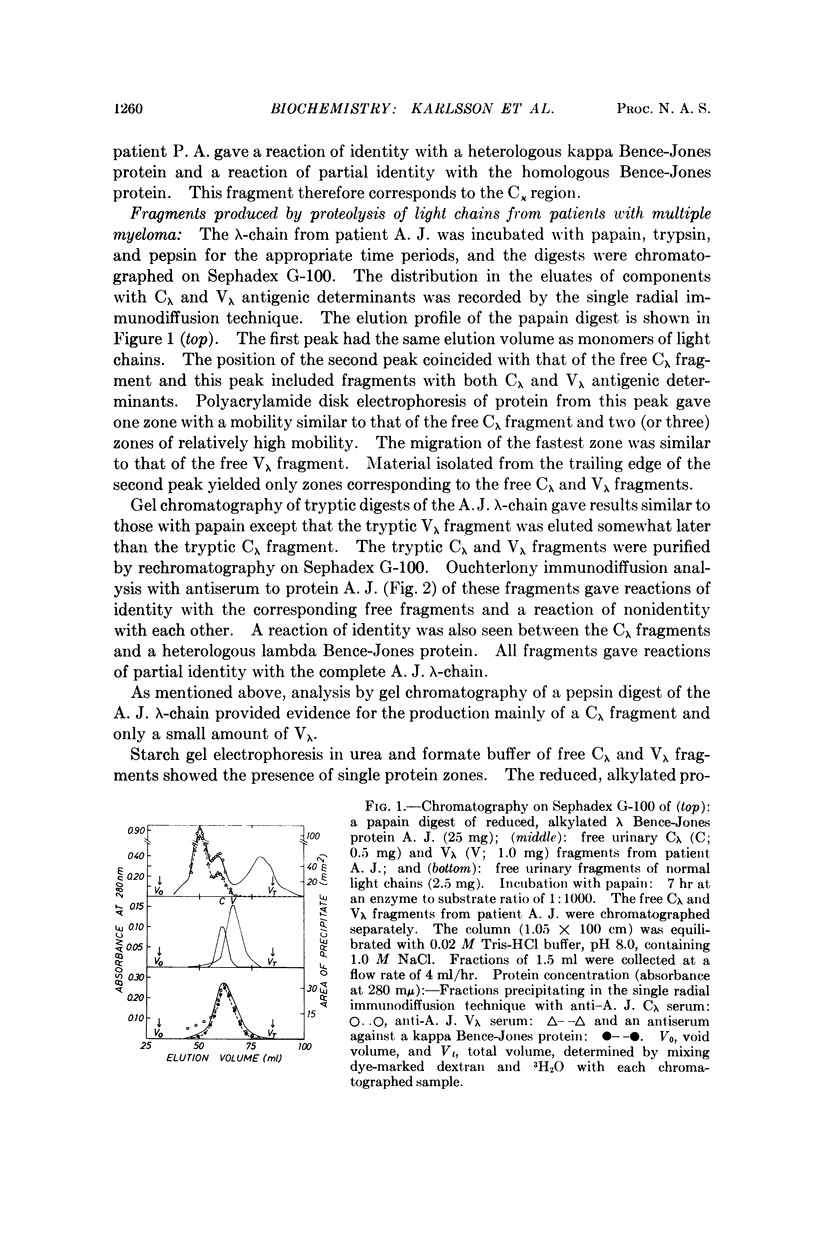
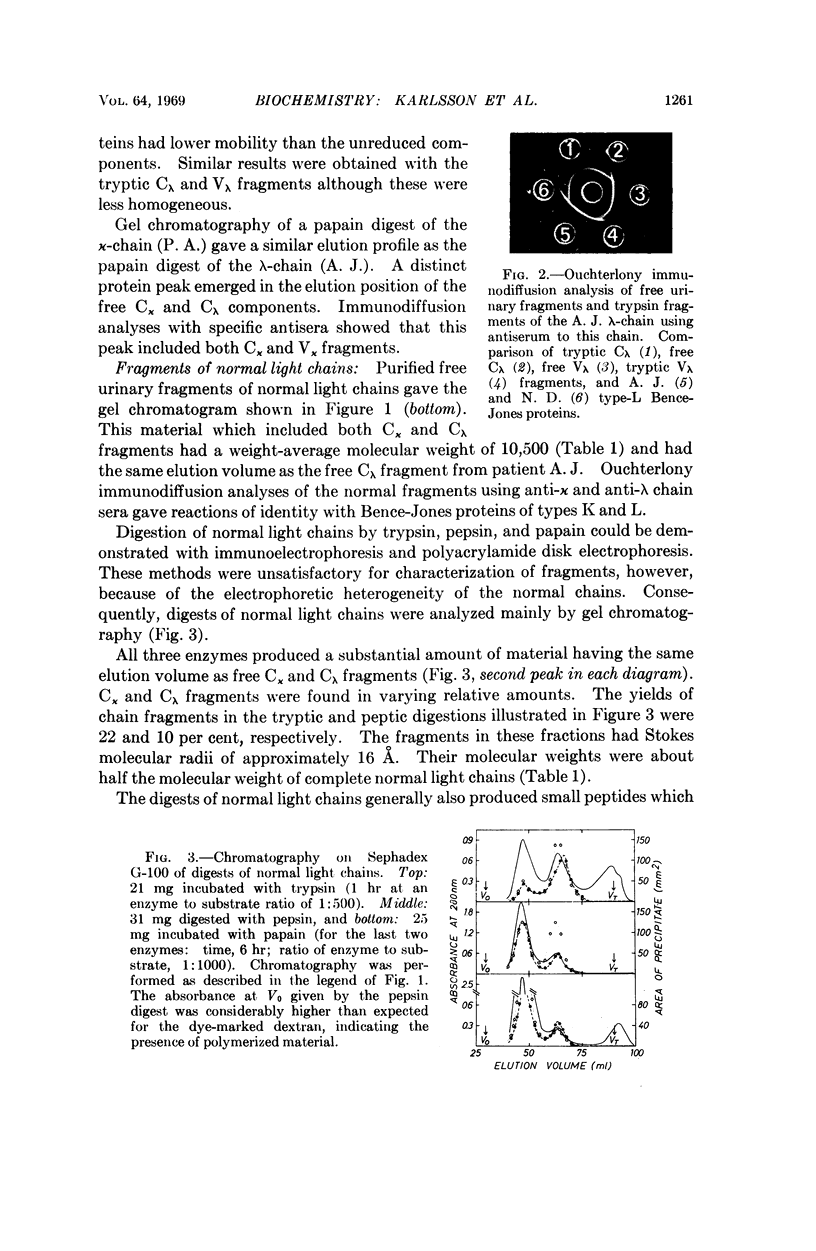


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Berggård I., Peterson P. A. Polymeric forms of free normal kappa and lambda chains of human immunoglobulin. J Biol Chem. 1969 Aug 25;244(16):4299–4307. [PubMed] [Google Scholar]
- Cioli D., Baglioni C. Catabolic origin of a Bence Jones protein fragment. J Exp Med. 1968 Sep 1;128(3):517–532. doi: 10.1084/jem.128.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioli D., Baglioni C. Origin of structural variation in Bence Jones proteins. J Mol Biol. 1966 Jan;15(1):385–388. doi: 10.1016/s0022-2836(66)80237-1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DEUTSCH H. F. Crystalline low molecular weight gamma-globulin from a human urine. Science. 1963 Aug 2;141(3579):435–436. doi: 10.1126/science.141.3579.435. [DOI] [PubMed] [Google Scholar]
- EDELMAN G. M., POULIK M. D. Studies on structural units of the gamma-globulins. J Exp Med. 1961 May 1;113:861–884. doi: 10.1084/jem.113.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Gally J. A. Antibody structure, diversity, and specificity. Brookhaven Symp Biol. 1968 Jun;21(2):328–344. [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- Hood L., Ein D. Immunologlobulin lambda chain structure: two genes, one polypeptide chain. Nature. 1968 Nov 23;220(5169):764–767. doi: 10.1038/220764a0. [DOI] [PubMed] [Google Scholar]
- Irimajiri S., Franklin E. C., Woods K. R. C-terminal sequence of the F'c fragment of human gamma G globulin. Nature. 1968 Nov 9;220(5167):612–614. doi: 10.1038/220612a0. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Milstein C., Milstein C. P., Feinstein A. Non-allelic nature of the basic sequences of normal immunoglobulin K chains. Nature. 1969 Jan 11;221(5176):151–154. doi: 10.1038/221151a0. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson D. M., Foster J. F. Subtilisin cleavage of bovine plasma albumin. Reversible association of the two primary fragments and their relation to the structure of the parent protein. Biochemistry. 1969 Jun;8(6):2357–2365. doi: 10.1021/bi00834a016. [DOI] [PubMed] [Google Scholar]
- Ponstingl H., Hess M., Hilschmann N. Die vollständige Aminosäure-Sequenz des Bence-Jones-Proteins Kern Eine neue Untergruppe der Immunglobulin-L-Ketten vom lambda-typ. Hoppe Seylers Z Physiol Chem. 1968 Jun;349(6):867–871. [PubMed] [Google Scholar]
- Prahl J. W. Enzymic degradation of the Fc fragment of rabbit immunoglobulin IgG. Biochem J. 1967 Aug;104(2):647–655. doi: 10.1042/bj1040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A., Killander J., Grey H. M., Kunkel H. G. Low-molecular-weight proteins related to Bence Jones proteins in multiple myeloma. Science. 1966 Mar 11;151(3715):1237–1239. doi: 10.1126/science.151.3715.1237. [DOI] [PubMed] [Google Scholar]
- Solomon A., McLaughlin C. L. Bence-Jones proteins and light chains of immunoglobulins. I. Formation and characterization of amino-terminal (variant) and carboxyl-terminal (constant) halves. J Biol Chem. 1969 Jun 25;244(12):3393–3404. [PubMed] [Google Scholar]
- Tan M., Epstein W. Antigenic analysis of an N-terminal fragment of a type k Bence Jones protein. J Immunol. 1967 Mar;98(3):568–575. [PubMed] [Google Scholar]
- Turner M. W., Bennich H. Subfragments from the Fc fragment of human immunoglobulin G. Isolation and physicochemical charaterization. Biochem J. 1968 Mar;107(2):171–178. doi: 10.1042/bj1070171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Pinnell S. R., Bratt G. T. Low molecular weight L-chain components related to Bence-Jones proteins. J Lab Clin Med. 1966 Jul;68(1):81–89. [PubMed] [Google Scholar]
- van Eyk H. G., Myszkowska K. On the localisation of antigenic determinants in a Bence Jones protein. Clin Chim Acta. 1967 Nov;18(2):101–106. doi: 10.1016/0009-8981(67)90141-6. [DOI] [PubMed] [Google Scholar]




