Abstract
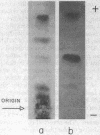
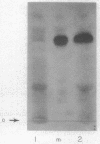
The administration of glucagon to rats causes a marked increase in the phosphorylation of a specific serine residue in lysine-rich (f1) histone of liver during a one-hour period following the administration of the hormone. It is proposed that histone phosphorylation is the mechanism by which glucagon, and perhaps other hormones whose actions are mediated by adenosine 3′,5′-cyclic phosphate (cyclic AMP), induce RNA synthesis in target tissues. The incorporation of 32P-phosphate into lysine-rich histone is determined by isolation of a tryptic peptide which contains the phosphorylated serine residue. This peptide is identical to the major tryptic phosphopeptide obtained from lysine-rich histone after phosphorylation in vitro by a purified cyclic AMP-dependent liver histone kinase preparation; the partial sequence Lys-Ala-SerPO4(Thr,Ser,Glu,Pro2,Gly,Val,Ile,Leu)Lys has been determined for the peptide. Hydrocortisone and adrenocorticotrophic hormone do not cause a detectable increase in histone phosphorylation in liver. However, insulin, which like glucagon induces an actinomycin sensitive synthesis of liver enzymes, also causes increased histone phosphorylation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., LITTAU V. C., MIRSKY A. E. On the role of of histones in regulation ribonucleic acid synthesis in the cell nucleus. Proc Natl Acad Sci U S A. 1963 Mar 15;49:414–421. doi: 10.1073/pnas.49.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhor I., Kung G. M., Bonner J. Sequence-specific interaction of DNA and chromosomal protein. J Mol Biol. 1969 Jan;39(2):351–364. doi: 10.1016/0022-2836(69)90322-2. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., LIPMANN F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953 Mar;201(1):235–243. [PubMed] [Google Scholar]
- Exton J. H., Park C. R. The stimulation of gluconeogenesis from lactate by epinephrine, glucagon, cyclic 3',5'-adenylate in the perfused rat liver. Pharmacol Rev. 1966 Mar;18(1):181–188. [PubMed] [Google Scholar]
- FERGUSON J. J., Jr PROTEIN SYNTHESIS AND ADRENOCORTICOTROPIN RESPONSIVENESS. J Biol Chem. 1963 Aug;238:2754–2759. [PubMed] [Google Scholar]
- Farese R. V. Effects of actinomycin D on ACTH-induced corticosteroidogenesis. Endocrinology. 1966 May;78(5):929–936. doi: 10.1210/endo-78-5-929. [DOI] [PubMed] [Google Scholar]
- Garren L. D., Ney R. L., Davis W. W. Studies on the role of protein synthesis in the regulation of corticosterone production by adrenocorticotropic hormone in vivo. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1443–1450. doi: 10.1073/pnas.53.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- Granner D., Chase L. R., Aurbach G. D., Tomkins G. M. Tyrosine aminotransferase: enzyme induction independent of adenosine 3', 5'-monophosphate. Science. 1968 Nov 29;162(3857):1018–1020. doi: 10.1126/science.162.3857.1018. [DOI] [PubMed] [Google Scholar]
- HUANG R. C., BONNER J. Histone, a suppressor of chromosomal RNA synthesis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1216–1222. doi: 10.1073/pnas.48.7.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager C. B., Kenney F. T. Regulation of tyrosine-alpha-ketoglutarate transaminase in rat liver. VII. Hormonal effects of synthesis in the isolated, perfused liver. J Biol Chem. 1968 Jun 25;243(12):3296–3300. [PubMed] [Google Scholar]
- Holten D., Kenney F. T. Regulation of tyrosine alpha-ketoglutarate transaminase in rat liver. VI. Induction by pancreatic hormones. J Biol Chem. 1967 Oct 10;242(19):4372–4377. [PubMed] [Google Scholar]
- Jefferson L. S., Exton J. H., Butcher R. W., Sutherland E. W., Park C. R. Role of adenosine 3',5'-monophosphate in the effects of insulin and anti-insulin serum on liver metabolism. J Biol Chem. 1968 Mar 10;243(5):1031–1038. [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost J. P., Hsie A. W., Rickenberg H. V. Regulation of the synthesis of rat liver serine dehydratase by adenosine 3', 5'- cyclic monophosphate. Biochem Biophys Res Commun. 1969 Mar 31;34(6):748–754. doi: 10.1016/0006-291x(69)90242-3. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Khairallah E. A., Pitot H. C. Studies on the induction and repression of enzymes in rat liver. V. Regulation of the rate of synthesis and degradation of serine dehydratase by dietary amino acids and glucose. J Biol Chem. 1968 Jun 10;243(11):3057–3066. [PubMed] [Google Scholar]
- Langan T. A. Action of adenosine 3',5'-monophosphate-dependent histone kinase in vivo. J Biol Chem. 1969 Oct 25;244(20):5763–5765. [PubMed] [Google Scholar]
- Langan T. A. Histone phosphorylation: stimulation by adenosine 3',5'-monophosphate. Science. 1968 Nov 1;162(3853):579–580. doi: 10.1126/science.162.3853.579. [DOI] [PubMed] [Google Scholar]
- Marsh J. M., Butcher R. W., Savard K., Sutherland E. W. The stimulatory effect of luteinizing hormone on adenosine 3',5'-monophosphate accumulation in corpus luteum slices. J Biol Chem. 1966 Nov 25;241(22):5436–5440. [PubMed] [Google Scholar]
- Meisler M. H., Langan T. A. Characterization of a phosphatase specific for phosphorylated histones and protamine. J Biol Chem. 1969 Sep 25;244(18):4961–4968. [PubMed] [Google Scholar]
- Ney R. L., Dexter R. N., Davis W. W., Garren L. D. A study of mechanisms by which adrenocorticotropic hormone maintains adrenal steroidogenic responsiveness. J Clin Invest. 1967 Dec;46(12):1916–1924. doi: 10.1172/JCI105681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Variations in the phosphate content and thiołdisulphide ratio of histones during the cell cycle. Studies with regenerating rat liver and sea urchins. Biochem J. 1968 Apr;107(3):403–410. doi: 10.1042/bj1070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Regulation of beta-galactosidase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1968 Oct 25;243(20):5420–5427. [PubMed] [Google Scholar]
- Pogo B. G., Pogo A. O., Allfrey V. G., Mirsky A. E. Changing patterns of histone acetylation and RNA synthesis in regeneration of the liver. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1337–1344. doi: 10.1073/pnas.59.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVARD K., MARSH J. M., RICE B. F. GONADOTROPINS AND OVARIAN STEROIDOGENESIS. Recent Prog Horm Res. 1965;21:285–365. [PubMed] [Google Scholar]
- STEDMAN E. Cell specificity of histones. Nature. 1950 Nov 4;166(4227):780–781. doi: 10.1038/166780a0. [DOI] [PubMed] [Google Scholar]
- Voytovich A. E., Owens I. S., Topper Y. J. A novel action of insulin on phosphoprotein formation by mammary gland explants. Proc Natl Acad Sci U S A. 1969 May;63(1):213–217. doi: 10.1073/pnas.63.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks W. D. Tyrosine-alpha-ketoglutarate transaminase: induction by epinephrine and adenosine-3',5'-cyclic phosphate. Science. 1968 May 31;160(3831):997–998. doi: 10.1126/science.160.3831.997. [DOI] [PubMed] [Google Scholar]
- Yeung D., Oliver I. T. Induction of phosphopyruvate carboxylase in neonatal rat liver by adenosine 3',5'-cyclic monophosphate. Biochemistry. 1968 Sep;7(9):3231–3239. doi: 10.1021/bi00849a028. [DOI] [PubMed] [Google Scholar]
- de NOOIJ E., WESTENBRINK H. G. Isolation of a homogeneous lysine-rich histone from calf thymus. Biochim Biophys Acta. 1962 Aug 27;62:608–609. doi: 10.1016/0006-3002(62)90254-8. [DOI] [PubMed] [Google Scholar]