Abstract
The cGMP sensitivity of cyclic nucleotide–gated (CNG) channels can be modulated by changes in phosphorylation catalyzed by protein tyrosine kinases (PTKs) and protein tyrosine phosphatases. Previously, we used genistein, a PTK inhibitor, to probe the interaction between PTKs and homomeric channels comprised of α subunits (RETα) of rod photoreceptor CNG channels expressed in Xenopus oocytes. We showed that in addition to inhibiting phosphorylation, genistein triggers a noncatalytic interaction between PTKs and homomeric RETα channels that allosterically inhibits channel gating. Here, we show that native CNG channels from rods, cones, and olfactory receptor neurons also exhibit noncatalytic inhibition induced by genistein, suggesting that in each of these sensory cells, CNG channels are part of a regulatory complex that contains PTKs. Native CNG channels are heteromers, containing β as well as α subunits. To determine the contributions of α and β subunits to genistein inhibition, we compared the effect of genistein on native, homomeric (RETα and OLFα), and heteromeric (RETα+β, OLFα+β, and OLFα+RETβ) CNG channels. We found that genistein only inhibits channels that contain either the RETα or the OLFβ subunits. This finding, along with other observations about the maximal effect of genistein and the Hill coefficient of genistein inhibition, suggests that the RETα and OLFβ subunits contain binding sites for the PTK, whereas RETβ and OLFα subunits do not.
Keywords: cyclic guanosine monophosphate, protein tyrosine kinase, photoreceptor, olfactory receptor neuron
INTRODUCTION
Cyclic nucleotide–gated (CNG) channels are crucial for sensory transduction in rod and cone photoreceptors and olfactory sensory neurons. The cyclic nucleotide sensitivity of CNG channels can be modulated by various intracellular signals including Ca2+/calmodulin (Chen et al. 1994; Grunwald et al. 1998) and phosphorylation (Gordon et al. 1992; Molokanova et al. 1997; Muller et al. 1998). We recently showed that the cyclic GMP sensitivity of CNG channels expressed in Xenopus oocytes, consisting of homomers of bovine rod α subunits (RETα) can be modulated by protein tyrosine kinases (PTKs) and phosphatases (PTPs) intrinsic to the oocyte (Molokanova et al. 1997, Molokanova et al. 1999b). These enzymes are constitutively active and remain associated with inside-out membrane patches for many minutes after excision, consistent with a relatively stable multimolecular complex including CNG channels, PTKs, and PTPs. Similar complexes between ion channels, protein kinases, and protein phosphatases have been shown for several other channels (Levitan 1999) and may be the rule rather than the exception.
We have probed the interaction between the rod CNG channel and the oocyte PTK using the tyrosine kinase inhibitor genistein (Molokanova et al. 1999a). In addition to preventing PTK-catalyzed changes in cGMP sensitivity when ATP is present, genistein also inhibits channel gating in the absence of ATP. Thus, genistein can inhibit CNG channels even when the PTK is catalytically inactive. The simplest possible explanation would involve direct binding of genistein to the channel. However, genistein inhibition can be suppressed by agents that have no direct effect on CNG channels, but that do inhibit PTKs. Erbstatin, a noncompetitive inhibitor of the ATP binding site on PTKs (Imoto et al. 1987), noncompetitively prevents genistein inhibition. Adenylylimidodiphosphate (AMP-PNP), a nonhydrolyzable ATP analogue that competitively inhibits PTKs (Akiyama et al. 1987), competitively inhibits genistein inhibition. These results and other observations lead to the conclusion that genistein indirectly influences RETα channels by triggering a noncatalytic interaction between oocyte PTKs and the channel that allosterically inhibits channel gating.
Are native CNG channels in sensory cells, like CNG channels expressed in oocytes, associated with PTKs? Our recent work shows that CNG channels in rod outer segments are subject to modulation by changes in tyrosine phosphorylation, the final step in a biochemical cascade in which neuromodulators can alter the rod light response (Savchenko, Kraft, Molokanova, Kramer, manuscript in preparation). Since genistein has been useful in probing the interaction between the cloned RETα channel and oocyte PTKs, we asked whether genistein would reveal a similar interaction between native CNG channels and PTKs in sensory cells, namely rods, cones, and olfactory receptor neurons.
Both the native rod and olfactory CNG channels are heteromers containing α and β subunits (Bradley et al. 1994; Chen et al. 1994, Liman and Buck 1994; Koerschen et al. 1995), named RETα and RETβ in rods and OLFα and OLFβ in olfactory neurons. Exogenous expression of α subunits alone generates functional CNG channels in oocytes. In contrast, β subunits do not form functional channels when expressed alone. However, coexpression of α and β subunits yields channels with properties that more closely resemble native rod and olfactory channels. To better understand how α and β subunits of CNG channels interact with PTKs, we compared the effects of genistein on native, cloned homomeric, and cloned heteromeric channels. Our results suggest that all three native CNG channels (rod, cone, and olfactory) are indeed associated with PTKs. However, for the rod channel, the α subunit is crucial for allowing interaction with the PTK, whereas in the olfactory CNG channel, the β subunit is crucial.
MATERIALS AND METHODS
Recording from Native CNG Channels
Water-phase tiger salamanders (Ambystoma tigrinum) maintained in a temperature-controlled aquarium (4°C) on a 12/12 light/dark cycle were used in experiments at the same time of day. Animals were dark-adapted for 1 h and anesthetized in an ice-cold solution containing 1 g/liter of 2-amino benzoic acid for 20 min before decapitation and removal of eyes under dim red light. Eyes were placed in physiological saline containing (mM): 155 NaCl, 2.5 KCl, 1 CaCl2, 2 MgCl2, 10 glucose, and 10 HEPES, pH 7.5. Retinas were removed and gently triturated to yield isolated rods, cones, or rod outer segments. Borosilicate glass pipettes (3–5 MΩ) were filled with standard patch solution containing (mM): 115 NaCl, 5 EGTA, 1 EDTA, and 5 HEPES, pH 7.5 with NaOH, and were used to obtain excised inside-out patches from outer segments of rods and cones.
Olfactory receptor neurons were acutely dissociated from the olfactory epithelium of land-phase tiger salamanders. After dissection, the epithelium was placed for 18–22 min at 21°C in a tube containing 3 ml of physiological solution [(mM)148 NaCl, 4 KCl, 1.5 CaCl2, 2 MgCl2, 10 glucose, 10 HEPES, pH 7.4] containing 48 μl of cysteine-activated papain (Worthington Pharmaceuticals). The tissue was then continuously washed for 10 min in ice cold papain-free solution and gently triturated to isolate individual olfactory receptor neurons. Patch pipettes with resistances of 10–20 MΩ were used for obtaining excised patches. Pipettes were filled with a solution containing (mM): 150 NaCl, 4 KCl, 5 EGTA, 0.2 MgCl2, 10 glucose, 10 HEPES, pH 7.4. This also served as the bath solution and cGMP perfusion solution for experiments on olfactory neurons.
Expression and Recording from CNG Channels Expressed in Xenopus Oocytes
To generate homomeric channels, a cDNA clone encoding the α subunits of the bovine rod photoreceptor CNG channel (Kaupp et al. 1989) or the rat olfactory CNG channels (Dhallan et al. 1992) were used for in vitro transcription of mRNA, which was injected into Xenopus oocytes (50 nl per oocyte at 1 ng/nl). After 2–7 d, the vitelline membrane was removed from injected oocytes, which were then placed in a chamber for patch-clamp recording at 21–24°C. Glass-patch pipettes (2–3 MΩ) were filled with standard patch solution, which also served as the standard bath solution and cGMP perfusion solution. After formation of a gigaohm seal, inside-out patches were excised and the patch pipette was quickly (<30 s) placed in the outlet of a 1-mm diameter tube for application of cGMP and other agents. We used a perfusion manifold containing up to eight different solutions capable of solution changes within 50 ms. Most patches contained 100–200 channels.
To generate heteromeric RETα+β channels, mRNA encoding RETα and RETβ (Koerschen et al. 1995) were coinjected into oocytes. The presence of heteromers in excised patches was confirmed by application of saturating (2 mM) cGMP in conjunction with 10 μM l-cis diltiazem, which selectively blocks channels containing β subunits, but is ineffective at blocking homomeric RETα channels (Chen et al. 1993). Injection of a 1:8 (n = 6) or 1:10 (n = 14) ratio of RETα to RETβ mRNA produced channels that were inhibited 78 and 80%, respectively, by 10 μM l-cis diltiazem. In contrast, a 1:5 ratio generated channels inhibited by 50%, suggesting that a RETα:RETβ mRNA ratio of 1:10 is sufficient to saturate the RETβ contribution to heteromeric channels, consistent with previous results (Shammat and Gordon 1999). For expression of OLFα+β heteromeric channels, OLFα and OLFβ (Liman and Buck 1994) mRNAs were injected at a ratio of 4:1, previously reported to saturate the contribution of OLFβ to olfactory heteromeric channels (Shapiro and Zagotta 1998). In agreement with these earlier results, we found that injection of this ratio of mRNAs generated channels with increased sensitivity to cAMP as compared with homomeric OLFα channels [K 1/2 of 9.9 ± 0.8 μM (n = 11) and 130 ± 9 μM (n = 12), respectively].
For expression of hybrid OLFα+RETβ channels, mRNAs encoding the two subunits were co-injected into oocytes. The presence of RETβ in hybrid channels was confirmed by applying 10 μM l-cis diltiazem (Finn et al. 1998). This inhibitor blocked channels resulting from OLFα to RETβ mRNA-injection ratios of 1:1.5 and 1:3 by 36.8 ± 2.9% (n = 3) and 32.8 ± 1.4% (n = 6), respectively. These results suggest that a OLFα to RETβ injection ratio of 1:3 is sufficient to saturate the contribution of RETβ to hybrid channels.
Analysis of Genistein Inhibition
Genistein and erbstatin (stable analogue) were purchased from Alexis Corp. and were prepared as concentrated stock solutions in DMSO. Aqueous solutions containing the final concentrations were prepared for use as needed. The final concentration of DMSO did not exceed 0.1%, which had no effect on CNG channels or their modulation. Cyclic GMP and AMP-PNP were purchased from Sigma Chemical Co. Current responses through CNG channels were obtained with an Axopatch 200A patch clamp (Axon Instruments, Inc.), digitized, stored, and later analyzed using pClamp 6.0 software. Membrane potential was held at −75 mV in all experiments. Current responses were normalized to the maximal CNG current (Imax), elicited by saturating (2 mM) cGMP. To estimate the K i for genistein, we used a modified Hill equation: Ib/Imax = [1 − (Ib(max)/Imax)]/[1 + (K i/B)n] + Ib(max)/Imax, where B is the concentration of blocker, Ib and Ib(max) are the currents activated by saturating cGMP in the presence of a given blocker concentration, and a saturating blocker concentration, respectively. Genistein inhibition of closed and fully activated channels was analyzed as described previously (Molokanova et al. 1999a). Variability among measurements is expressed as mean ± SEM.
RESULTS
Genistein Inhibition of Native CNG Channels
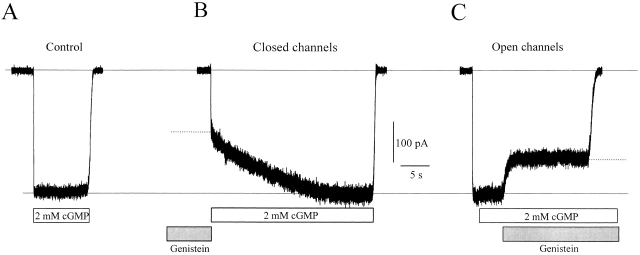
Application of genistein on expressed homomeric rod channels (RETα) stabilizes the closed state of the channel, such that preapplication of genistein slows activation upon exposure to cGMP (Molokanova et al. 1999a). Fig. 1 shows that genistein has a similar effect on native channels in patches excised from salamander rod outer segments. Without genistein, saturating cGMP (2 mM) rapidly activates CNG current (Fig. 1 A). However, if the channels are pretreated with genistein (Fig. 1 B), a fraction of the CNG current activates very slowly (τ = 8.5 s), while the residual current activates over the normal rapid time course (τ = 20 ms). By examining these two components with different concentrations of genistein, we previously showed that the slowly activating current reflects channels inhibited by genistein, whereas the residual current reflects channels that are unaffected by genistein. Genistein also inhibits channels after they have been maximally activated by saturating cGMP (Fig. 1 C), but the extent of inhibition is less than for closed channels. Hence, genistein more effectively inhibits closed than open rod channels, as was observed previously for the expressed RETα channel.
Figure 1.
Effect of genistein on native rod CNG channels. (A) Activation of CNG channels by saturating cGMP without genistein. (B) Effect of genistein on closed channels. Response to saturating cGMP after pre-exposure to 100 μM genistein for 1 min. The broken line distinguishes the slowly activating current component inhibited by genistein and the rapidly activating residual current. (C) Effect of 100 μM genistein on fully activated channels, indicated by the broken line.
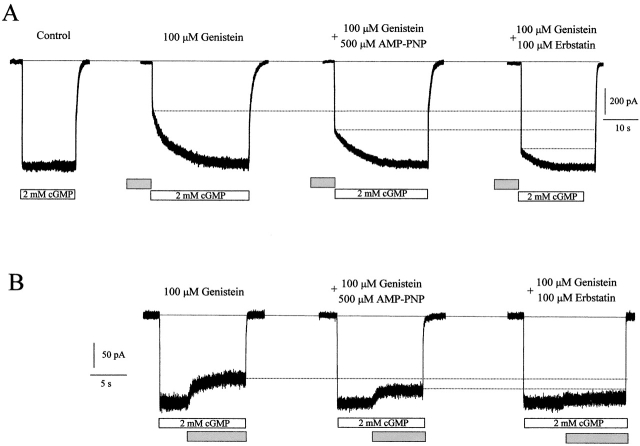
Genistein inhibition of the expressed RETα channel is suppressed by coapplication of PTK inhibitors that have no direct effects on CNG channels (Molokanova et al. 1999a). These include AMP-PNP, a nonhydrolyzable ATP analogue that competes with genistein for the ATP binding site on PTKs, and erbstatin, a noncompetitive inhibitor of PTKs with respect to genistein. Fig. 2 shows that both AMP-PNP and erbstatin also reduce genistein inhibition of the native rod channels. Thus, genistein inhibition of both closed (Fig. 2 A) and fully activated (B) native rod CNG channels is markedly reduced by coapplication of AMP-PNP or erbstatin. AMP-PNP and erbstatin have no effect on the kinetics, cGMP sensitivity, or maximal current in the absence of genistein (n = 4). The observation that genistein inhibition is suppressed by two known PTK inhibitors that have no direct effect on the rod channel strongly suggests that, as in oocytes, genistein inhibits the native rod channel indirectly, by first binding to a PTK.
Figure 2.
PTK inhibitors reduce the genistein inhibition. (A) Inhibition of closed rod photoreceptor CNG channels by 1 min pre-exposure to 100 μM genistein alone or together with PTK inhibitors (500 μM AMP-PNP or 100 μM erbstatin). Closed channels were activated by application of 2 mM cGMP (control). (B) Inhibition of fully activated channels by genistein alone or together with PTK inhibitors.
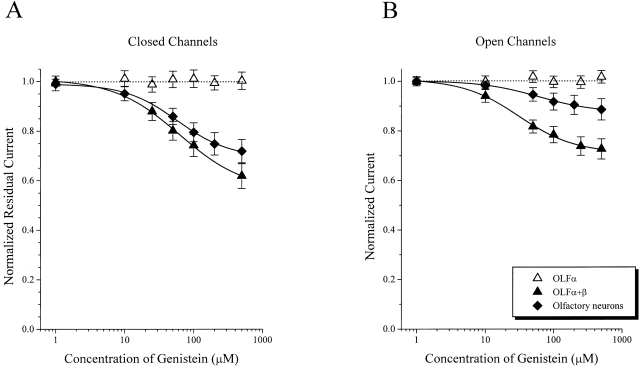
Native CNG channels differ in their sensitivity to genistein inhibition (Fig. 3). Genistein has a much larger effect on CNG channels from rods and cones than on channels from olfactory receptor neurons. Maximal inhibition of closed channels by saturating concentrations of genistein (500 μM) was 61 ± 8% (n = 9) for rod, 50 ± 3% (n = 8) for cone, and 28 ± 4% (n = 5) for olfactory channels (Fig. 3 A). As observed in homomeric RETα channels, genistein was less effective at inhibiting fully activated channels (Fig. 3 B) [inhibition of 31 ± 4% (n = 5) for rod, 37 ± 3% (n = 4) for cone, and 11 ± 4% (n = 3) for olfactory channels]. Moreover, for rod and cone CNG channels, fully activated channels had a higher apparent K i for genistein than did closed channels (Fig. 3 C), again consistent with previous observations. For olfactory CNG channels, K i values for genistein inhibition of closed and open channels were not significantly different. Not only did genistein have a smaller maximal effect on olfactory channels, but the apparent K i for genistein was also higher than values for channels from rods or cones (Fig. 3 C).
Figure 3.
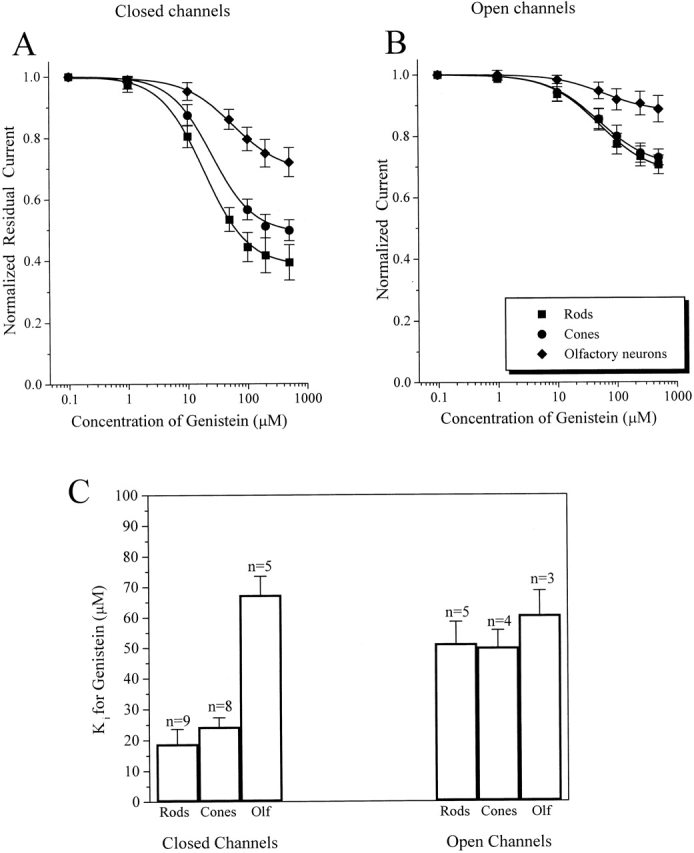
Comparison of genistein inhibition of native CNG channels. Dose-inhibition curves of the effect of genistein on native closed (A) and fully activated (B) CNG channels from rod and cone outer segments and olfactory receptor neurons. Continuous curves in A and B show fits of the data to the Hill equation (see materials and methods). (C) Bar graph showing K i values for genistein inhibition of closed and activated channels from rods, cones, and olfactory neurons. Data represents mean ± SEM for all of the individual experiments included in A and B.
Role of RETα and RETβ Subunits in Genistein Inhibition of Rod CNG Channels
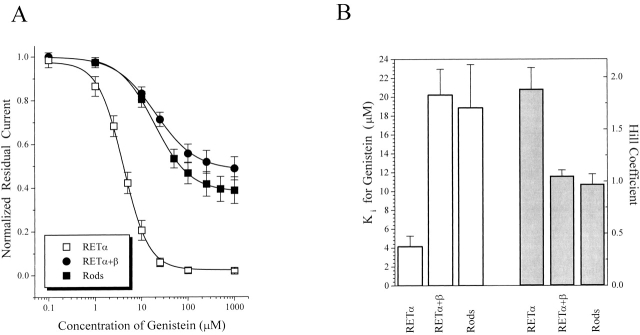
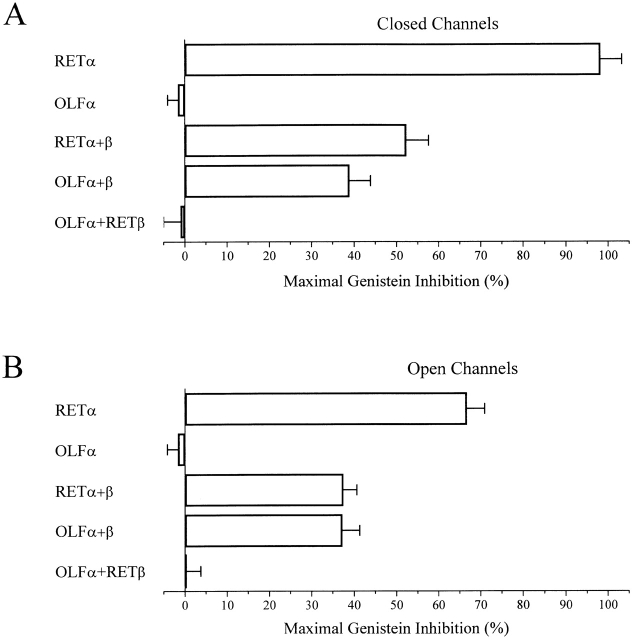
While inhibition of native rod channels is qualitatively similar to that of expressed homomeric RETα channels, there are striking quantitative differences. Thus, saturating genistein can completely suppress activation of closed RETα channels (inhibition of 98 ± 5%; n = 20), whereas activation of native channels is maximally reduced by only 61 ± 8% (n = 9). Fig. 4 shows that the RETβ subunit almost entirely accounts for this difference. Thus, heteromeric RETα+β channels behave in a manner very similar to native rod channels, with channels in their closed state exhibiting a maximal genistein inhibition of 53 ± 5% (n = 10) (Fig. 4 A). Moreover, the apparent K i of heteromeric RETα+β channels was 20 μM, a value much higher than for homomeric RETα channels (4 μM), but similar to that for native rod channels (19 μM) (Fig. 4 B). The Hill coefficient also differs for native rod CNG channels (∼1) and homomeric RETα channels (∼2), but the value for heteromeric RETα+β channels (∼2) is indistinguishable from that for native channels.
Figure 4.
Subunit dependence of genistein inhibition of closed rod CNG channels. (A) Dose-inhibition curves for genistein effect on closed native rod, homomeric RETα, and heteromeric RETα+β channels. (B) Bar graph showing K i and Hill coefficient values for genistein inhibition curves. Data represents mean ± SEM for all individual experiments included in A.
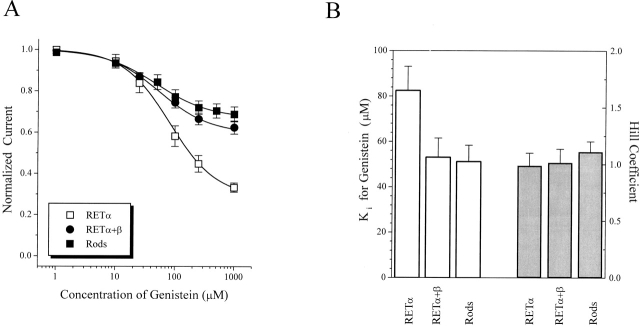
The same is true of rod channels in their open state. Genistein inhibition of native rod channels more closely resembles inhibition of heteromeric RETα+β than of homomeric RETα channels (Fig. 5 A). In the presence of saturating cGMP (2 mM), genistein inhibits native, homomeric RETα, and heteromeric RETα+β channels by 31 ± 4% (n = 5), 67 ± 4% (n = 18), and 37 ± 3% (n = 9), respectively. Fig. 5 B shows that K i values for genistein on fully activated channels was ∼51 μM for native, 82 μM for RETα, and 53 μM for RETα+β. The Hill coefficient was similar for inhibition of each of the open channels, with values near 1 for each. However, while the Hill coefficients for inhibition of native and heteromeric RETα+β are the same for open and closed channels (∼1 for each), for homomeric RETα channels, the Hill coefficient changes from 2 when the channels are closed to 1 when the channels are fully activated.
Figure 5.
Subunit dependence of genistein inhibition of fully activated rod CNG channels. (A) Dose-inhibition curves for genistein effect on native and homomeric RETα, and heteromeric RETα+β channels. All channels were fully activated with saturating (2 mM) cGMP before genistein application. (B) Bar graph showing K i and Hill coefficient values for genistein inhibition curves. Data represents mean ± SEM for all individual experiments included in A.
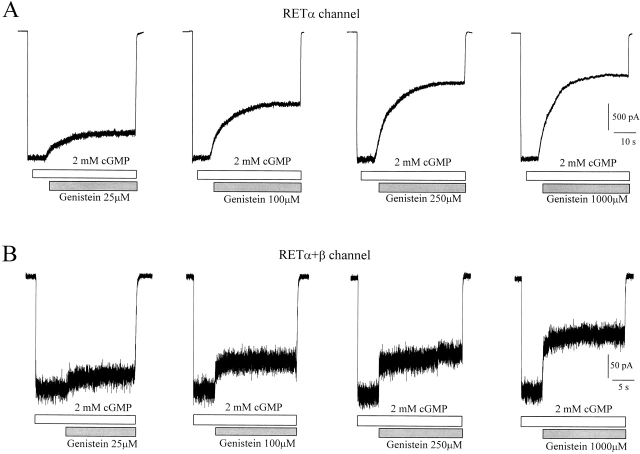
The β subunit also accounts for differences in the kinetics of genistein inhibition. Genistein inhibition of open native rod channels is relatively fast, with a time constant of 1.2 s for fully activated channels (see Fig. 1 C). In contrast, the time course of genistein inhibition of open homomeric RETα channels is much slower (Fig. 6 A), with a time constant of 9 s. The kinetics of inhibition of heteromeric RETα+β channels (Fig. 6 B) are similar to that of native channels with a time constant of ∼1 s. The kinetics of genistein inhibition are similar at a range of genistein concentrations from 25 to 1,000 μM. This suggests that the rate-limiting step in inhibition is not genistein binding, but rather the interaction between the PTK and channel subunits.
Figure 6.
Kinetics of inhibition of fully activated homomeric RETα (A) and heteromeric RETα+β (B) channels by various concentrations of genistein.
Role of OLFα and OLFβ Subunits in Genistein Inhibition of Olfactory CNG Channels
Like rod CNG channels, native olfactory CNG channels are heteromers containing OLFα and OLFβ subunits, in addition to a splice variant of the RETβ subunit (Sautter et al. 1998; Bonigk et al. 1999). To determine whether OLFα, like RETα, is the primary target for genistein inhibition, we examined the effect of genistein on homomeric OLFα channels. Fig. 7 shows that genistein fails to inhibit both closed (A) and fully activated (B) OLFα channels, at concentrations up to 500 μM (n = 4). In contrast, heteromeric channels, formed by coexpressing OLFα and OLFβ subunits were inhibited to an even greater extent than were native olfactory channels.
Figure 7.
Subunit dependence of genistein inhibition of olfactory CNG channels. Dose-inhibition curves for genistein effect on closed (A) and fully activated (B) native (n = 3–5), homomeric OLFα (n = 4), and heteromeric OLFα+β (n = 6) channels.
Studies on hybrid RET+OLF channels
The observation that OLFβ is necessary for genistein inhibition suggests that the PTK responsible for indirectly conferring genistein sensitivity can selectively associate with OLFβ, rather than with OLFα subunits. Do the two RET subunits, RETα and RETβ, also differ in their ability to interact with the PTK and confer genistein inhibition? To address this question, we took advantage of the observation that OLFα is apparently unable to interact with the PTK. If RETβ can interact with the PTK, one might expect that hybrid heteromeric channels (OLFα+RETβ) generated by coexpressing OLFα with RETβ would be subject to genistein inhibition. Expression of such hybrid channels was confirmed by examining their sensitivity to l-cis diltiazem, which has a heightened affinity for blocking channels that contain the RETβ subunit. Fig. 8 shows that genistein has no effect on these hybrid channels, strongly suggesting that the RETβ subunit does not bind to the PTK responsible for genistein inhibition. Hence we propose that genistein inhibition of rod CNG channels is mediated selectively by the interaction between the PTK and the RETα subunit.
Figure 8.
Summary data for genistein inhibition of various expressed homomeric and heteromeric channels in closed (A) and fully activated (B) states. n = 5–8 for each channel type.
DISCUSSION
Multimolecular Complexes between CNG Channels and PTKs
There is a growing realization that ion channels do not exist in isolation, but rather coexist with other proteins in macromolecular complexes. Protein partners of ion channels include cytoskeletal components, anchored via PDZ and other domains (Sheng and Wyszynski 1997) and regulatory enzymes, such as protein kinases and phosphatases (Levitan 1999). Biochemical evidence for such regulatory complexes includes copurification or coimmunoprecipitation of various kinases and phosphatases, including PTKs and PTPs, with ion channels (Rehm et al. 1989; Holmes et al. 1996; Yu et al. 1997; Tsai et al. 1999). Electrophysiological evidence for regulatory complexes comes from the ability of ATP to modulate channel gating after isolation from the cytoplasmic environment, either by excising membrane patches or by reconstituting the channels in artificial lipid bilayers. Moreover, modulation can be prevented by application of specific inhibitors of kinases and/or phosphatases (Biefeld and Jackson 1994; Reinhart and Levitan 1995; Molokanova et al. 1997; Wilson et al. 1998). These studies suggest that channels, kinases, and phosphatases are often involved in an intimate, rather than just a casual relationship.
Our studies strongly suggest that CNG channels are part of a regulatory complex that contains PTKs and PTPs. The cGMP sensitivity of homomeric RETα channels expressed in Xenopus oocytes increases after patch excision, owing to tyrosine dephosphorylation by PTPs (Molokanova et al. 1997). Conversely, application of ATP decreases cGMP sensitivity, owing to tyrosine phosphorylation by PTKs. The observation that modulation can be elicited repeatedly without decrement, even many minutes after patch excision and superfusion, suggests that the PTKs and PTPs responsible for modulation are tightly associated with RETα channels.
Application of PTP and PTK inhibitors to intact rods shows that the sensitivity of native rod CNG channels is also modulated by changes in tyrosine phosphorylation (Molokanova et al. 1997). However, we have found that in the absence of Ca2+, CNG channels in excised patches from rod outer segments do not exhibit spontaneous changes in cGMP sensitivity and are unaffected by ATP application. In recent studies, we find that extrinsic growth factors, such as IGF-1, can trigger changes in cGMP sensitivity by initiating a cascade that ultimately activates PTPs in rods (Savchenko, Kraft, Molokanova, Kramer, manuscript in preparation). Hence it has been unclear whether the native rod CNG channels are tightly, or only loosely, associated with PTKs and PTPs.
We have used the PTK inhibitor genistein to probe the interaction between PTKs and CNG channels. In previous studies (Molokanova et al. 1999a), we showed that genistein inhibits gating of expressed RETα channels even in the absence of ATP, indicating that the inhibition does not require catalytic activity of PTKs. However, the genistein effect was suppressed by PTK inhibitors that do not directly interact with CNG channels (AMP-PNP and erbstatin), strongly suggesting that genistein does not directly bind to the channel, but rather affects channel gating indirectly by binding to a PTK. From these studies, we concluded that the PTK can allosterically influence channel gating even without catalyzing phosphorylation. In this paper, we find characteristics of genistein inhibition of native rod CNG channels that are entirely consistent with our previous results on expressed channels, supporting the notion that a similar macromolecular complex, including at least CNG channels and a PTK, must exist in rod outer segments. Since we observe at least some degree of genistein inhibition not only in rods, but also in cones and olfactory neurons, CNG channels in all of these sensory cells may occur in macromolecular complexes with PTKs. The placement of regulatory enzymes, such as PTKs and PTPs, in close apposition with substrate proteins, such as CNG channels, may be important for efficient and rapid signaling. We suggest that, in rods, a complex of CNG channels, PTKs, and perhaps PTPs may be part of a larger “transducisome,” also containing other proteins involved in phototransduction (Koerschen et al. 1999).
Role of α and β Subunits in Genistein Inhibition
Since homomeric RETα channels are strongly inhibited by genistein, it appears that the RETα subunit alone is sufficient for binding the PTK. For heteromeric RETα+β channels, genistein has a lower apparent affinity, smaller maximal effect, and a smaller Hill coefficient for inhibition of closed channels. These observations are consistent with the possibility that genistein inhibition of RETα+β channels is solely mediated by interaction between the PTK and the RETα subunit, with the RETβ subunit unable to bind. However, it also possible that the PTK binds to both subunits, but in the context of the heteromeric RETα+β channel, binding of the PTK to each subunit is less favorable. The observation that genistein has no effect on hybrid OLFα+RETβ channels helps distinguish between these possibilities. Thus, the RETβ subunit is unable to confer genistein sensitivity when coexpressed with OLFα subunits, which by itself is insensitive. The OLFβ subunit does confer genistein sensitivity when coexpressed with OLFα, indicating that OLFα is not simply “dominant negative” for genistein inhibition. Hence, we conclude that genistein inhibition of rod channels is exclusively mediated by an interaction between the PTK and the RETα subunit. The RETβ subunit does appear to alter the kinetics of this interaction (Fig. 6), perhaps through an allosteric influence on RETα subunits.
The situation is different for olfactory CNG channels. Homomeric OLFα channels are unaffected by genistein, whereas heteromeric OLFα+β channels are inhibited, suggesting that the OLFβ subunit, rather than the OLFα, interacts with the PTK. Hence, whereas the α subunit interacts with the PTK in rod CNG channels, the β subunit interacts with the PTK in olfactory CNG channels. It should be noted that the nomenclature of CNG channel subunits (α vs. β) is not based on sequence homology, but rather is based on the chronology of subunit cloning, such that the first cloned subunits were named “α” and the second named “β.” In fact, the two subunits that are unable to interact with the PTK (OLFα and RETβ) share a homologous domain in the NH2 terminus that enables these subunits to interact with Ca2+/calmodulin (Chen et al. 1994; Liu et al. 1994). It remains to be determined whether this region somehow inhibits PTK binding to these subunits, rendering them genistein insensitive. It also remains to be determined whether the RETα and OLFβ subunits share regions that allow interaction with the PTK, enabling genistein inhibition. In addition, it should be noted that native olfactory CNG channels appear to contain not only OLFα and OLFβ subunits, but also a splice variant of the RETβ subunit (Sautter et al. 1998; Bonigk et al. 1999). In our expressed heteromeric OLFα+β channels (Fig. 8), the lack of RETβ subunit, which apparently does not bind PTK, may account for the greater degree of genistein inhibition as compared with native olfactory channels.
Stoichiometry of Interactions between PTKs and CNG Channel Subunits
Fig. 9 A presents one possible mechanism for how PTKs might interact with the subunits of CNG channels. The Hill coefficient for genistein inhibition of closed homomeric RETα channels is 2. If this accurately reflects the number of genistein binding sites, it would suggest that two PTKs are involved in channel inhibition. Since the homomeric channel contains four identical subunits, it is possible that each PTK simultaneously interacts with two neighboring subunits (Fig. 9 A, left). Many receptor-type PTKs, such as growth factor receptors, either contain repeating domains or are dimeric proteins (Fantl et al. 1993), providing a possible structural basis for PTK binding to identical sites on adjacent channel subunits. However, it is possible that the Hill coefficient underestimates the number of genistein binding sites. Thus, more than two PTKs (e.g., four PTKs) may underlie genistein inhibition, with each subunit individually inhibited by its own enzyme. To illustrate the uncertainty in the number of PTKs involved (two or four), the illustrated PTKs are divided in half with a dotted line.
Figure 9.
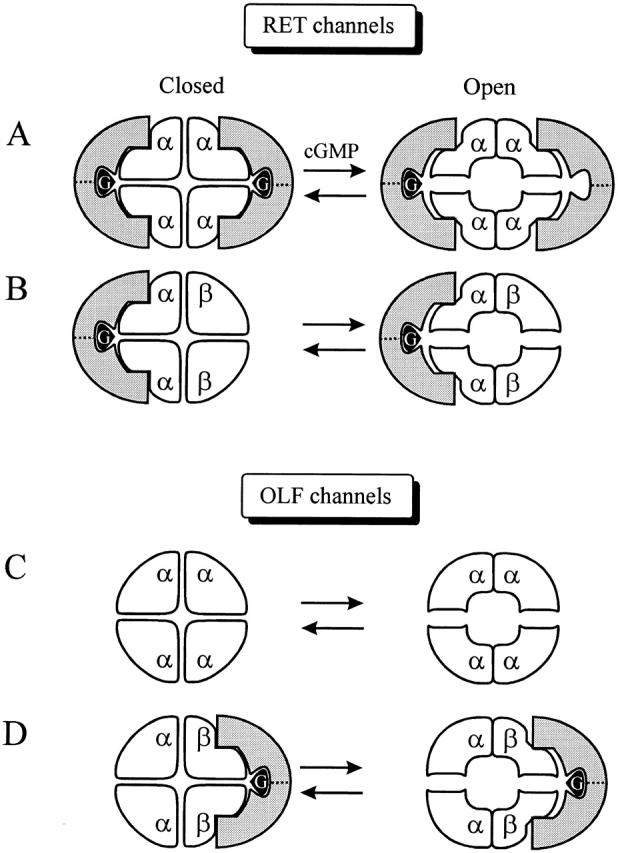
Schematic diagram of genistein inhibition. Homomeric and heteromeric RET and OLF channels and their interactions with PTKs, symbolized with the shaded crescent symbol. Genistein (G) is depicted bound to the PTK. In the closed state of the homomeric RETα channel (A), two PTKs, and thus two genistein molecules, are required to completely inhibit the channel. cGMP triggers channel opening, which functionally dimerizes RETα subunits. With the PTK spanning two functional dimers, only one PTK, and therefore one genistein, is sufficient to inhibit the channel, although two are still able to bind. In addition, the affinity of the closed channel for the PTK is higher than for the open channel, hence a tighter fit between the channel and the PTK symbol is depicted for the closed channel. For the RETα+β channel (B), only the RETα binds the PTK. Hence there is no change in the number of PTK required to blocked the closed and open channels. The homomeric OLFα channel (C) is unaffected by genistein because OLFα subunits do not contain PTK binding sites. The heteromeric OLFα+OLFβ (D) channel binds one PTK, via the binding sites on the OLFβ subunits.
What accounts for the curious decrease in the value of the Hill coefficient from two to one as RETα channels go from closed to open during activation? Recent studies suggest that rather than acting independently or in a completely concerted manner, the four subunits of some CNG channels may function as a pair of independent dimers. Thus, for each functional dimer, the two participating channel subunits cooperatively bind cyclic nucleotide and activate in a concerted manner (Liu et al. 1998; Shammat and Gordon 1999). We propose that functional dimerization of RETα subunits is dependent on binding of cyclic nucleotide. Hence, whereas all the RETα subunits are equivalent when the channel is closed, cGMP binding and/or channel opening triggers association of adjacent channel subunits, allowing them to function as a dimer. The minimal number of genistein molecules required to inhibit a channel containing such a pair of functional dimers is 1. This assumes that the PTK preferentially interacts “perpendicularly” with respect to the functional dimers; that is, by binding to RETα subunits on two distinct functional dimers (Fig. 9 A, right). Since the PTK can bind to the channels in their closed state, binding of the PTK to two adjacent RETα subunits may actually determine which RETα subunits functionally dimerize, namely the RETα subunits that are not already joined by a PTK. In this scenario, PTK-dependent subunit linkage between subunits and cGMP-dependent functional dimerization are mutually exclusive.
In the heteromeric RETα+β channel (Fig. 9 B), only the RETα subunits are capable of binding the PTK. Hence, if the channel stoichiometry is indeed RETα2, RETβ2, a single PTK dimer could interact with adjacent RETα subunits, fully accounting for genistein inhibition because the RETβ subunits appear to be immune to genistein inhibition. This scenario is consistent with our observed Hill coefficient for genistein inhibition of 1, and also accounts for the reduced maximal effect of genistein. Moreover, the proposed subunit arrangement is supported by recent studies by Shammat and Gordon 1999, who established that α subunits in heteromeric RET channels are adjacent rather than diagonally opposed.
The role of α and β subunits with respect to genistein inhibition is reversed in the OLF channel. Homomeric OLFα channels are unaffected by genistein, suggesting that the OLFα subunit cannot interact with the PTK (Fig. 9 C). However, the OLFβ subunit confers genistein sensitivity, suggesting that OLFβ subunits contain a PTK binding site. The Hill coefficient of heteromeric OLFα+β channels is one for both closed and fully activated channels, consistent with PTK spanning two functional dimers via interaction with two adjacent β subunits (Fig. 9 D).
These diagrams are speculative and are only a starting point for considering the stoichiometric relationships between PTKs and subunits of CNG channels. Estimates of stoichiometries based on Hill coefficients need to be confirmed with more direct biochemical experiments. Various factors, including heterogeneous populations of channels (Ruiz et al. 1999) and positive or negative cooperativity between channel subunits could alter Hill coefficients, leading to an over or under estimate of the number of receptor sites.
Differences in the ability of α and β subunits of rod and olfactory CNG channels to interact with PTKs has implications beyond genistein inhibition. It is likely that susceptibility to genistein inhibition is related to a channel's ability to be modulated by tyrosine phosphorylation. Thus, channels containing only subunits that are insensitive to genistein (e.g., OLFα homomers) might be insensitive to neuromodulators that signal through PTPs or PTKs, whereas channels that contain genistein-sensitive subunits would be susceptible to such neuromodulators. Biochemical and electrophysiological studies show that expression of olfactory CNG channel subunits is especially widespread, in the nervous system and elsewhere, but channels in different locations may differ in their subunit compositions (Berghard et al. 1996; Wiesner et al. 1998), potentially providing for differential modulation by PTKs.
Footnotes
Dr. Savchenko's present address is Affymax Research Institute, Palo Alto, CA 94304.
Abbreviations used in this paper: AMP-PNP, adenylylimidodiphosphate; CNG, cyclic nucleotide-gated; PTK, protein tyrosine kinase; PTP, protein tyrosine phosphatase.
References
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Berghard A., Buck L.B., Liman E.R. Evidence for distinct signaling mechanisms in two mammalian olfactory sense organs. Proc. Natl. Acad. Sci. USA. 1996;93:2365–2369. doi: 10.1073/pnas.93.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biefeld K., Jackson M.B. Phosphorylation and dephosphorylation modulate a Ca2+-activated K+ channel in rat peptidergic nerve terminals. J. Physiol. 1994;475:241–254. doi: 10.1113/jphysiol.1994.sp020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonigk W., Bradley J., Muller F., Sesti F., Boekhoff I., Ronnett G.V., Kaupp U.B., Frings S. The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. J. Neurosci. 1999;19:5332–5347. doi: 10.1523/JNEUROSCI.19-13-05332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J., Li J., Davidson N., Lester H.A., Zinn K. Heteromeric olfactory cyclic nucleotide-gated channelsa subunit that confers increased sensitivity to cAMP. Proc. Natl. Acad. Sci. USA. 1994;91:8890–8894. doi: 10.1073/pnas.91.19.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.Y., Peng Y.W., Dhallan R.S., Ahamed B., Reed R.R., Yau K.W. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 1993;362:764–767. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- Chen T.Y., Illing M., Molday L.L., Hsu Y.T., Yau K.-W., Molday R.S. Subunit 2 (or beta) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca2+-calmodulin modulation. Proc. Natl. Acad. Sci. USA. 1994;91:11757–11761. doi: 10.1073/pnas.91.24.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhallan R.S., Yau K.-W., Schrader K.A., Reed R.R. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1992;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Fantl W.F., Johnson D.E., Williams L.T. Signalling by receptor tyrosine kinases. Annu. Rev. Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- Finn J.T., Kraytwurst D., Schroeder J.E., Chen T.-Y., Reed R.R., Yau K.-W. Functional co-assembly among subunits of cyclic nucleotide-activated nonselective cation channels, and across species from nematode to human. Biophys. J. 1998;74:1333–1345. doi: 10.1016/S0006-3495(98)77846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S.E., Brautigan D.L., Zimmerman A.L. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 1992;9:739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- Grunwald M.E., Yu W.P., Yu H.H., Yau K.-W. Identification of a domain on the beta-subunit of the rod cGMP-gated cation channel that mediates inhibition by calcium-calmodulin. J. Biol. Chem. 1998;273:9148–9157. doi: 10.1074/jbc.273.15.9148. [DOI] [PubMed] [Google Scholar]
- Holmes T.C., Fadool D.A., Ren R., Levitan I.B. Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science. 1996;274:2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- Imoto M., Umezawa K., Isshiki K., Kunimoto S., Sawa T., Takeuchi T., Umezawa H. Kinetic studies of tyrosine kinase inhibition by erbstatin. J. Antibiot. (Tokyo) 1987;40:1471–1473. doi: 10.7164/antibiotics.40.1471. [DOI] [PubMed] [Google Scholar]
- Kaupp U.B., Niidome T., Tanabe T., Terada S., Bonigk W., Stühmer W., Cook N.J., Kangawa K., Matsuo H., Hirode T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cGMP-gated channel. Nature. 1989;342:762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Koerschen H.G., Illing M., Seifert R., Sesti F., Williams A., Gotzes S., Colville C., Muller F., Dose A., Godde M. A 240 kDa protein represents the complete beta subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron. 1995;15:627–636. doi: 10.1016/0896-6273(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Koerschen H.G., Beyermann M., Muller F., Heck M., Vantler M., Koch K.W., Kellner R., Wolfrum U., Bode C., Hofmann K.P., Kaupp U.B. Interaction of glutamic-acid-rich proteins with the cGMP signalling pathways in rod photoreceptors. Nature. 1999;400:761–766. doi: 10.1038/23468. [DOI] [PubMed] [Google Scholar]
- Levitan I.B. Modulation of ion channels by protein phosphorylation. Adv. Second Messenger Phosphoprotein Res. 1999;33:3–22. doi: 10.1016/s1040-7952(99)80003-2. [DOI] [PubMed] [Google Scholar]
- Liman E.R., Buck L.B. A second subunit of the olfactory cyclic nucleotide-gated channel confers high sensitivity to cAMP. Neuron. 1994;13:611–621. doi: 10.1016/0896-6273(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Liu D.T., Tibbs G.R., Paoletti P., Siegelbaum S.A. Constraining ligand-binding site stoichiometry suggests that a cyclic nucleotide-gated channel is composed of two functional dimers. Neuron. 1998;21:235–248. doi: 10.1016/s0896-6273(00)80530-9. [DOI] [PubMed] [Google Scholar]
- Liu M., Chen T.-Y., Ahamed B., Li J., Yau K.-W. Calcium-calmodulin modulation of the olfactory cyclic nucleotide-gated cation channel. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- Molokanova E., Trivedi B., Savchenko A., Kramer R.H. Modulation of rod photoreceptor cyclic nucleotide-gated channels by tyrosine phosphorylation. J. Neurosci. 1997;17:9068–9076. doi: 10.1523/JNEUROSCI.17-23-09068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E., Savchenko A., Kramer R.H. Noncatalytic inhibition of cyclic-nucleotide–gated channels by tyrosine kinase induced by genistein J. Gen. Physiol 113 1999. 45 56a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E., Maddox F., Luetje C.W., Kramer R.H. Activity-dependent modulation of rod photoreceptor cyclic nucleotide-gated channels mediated by phosphorylation of a specific tyrosine residue J. Neurosci. 19 1999. 4786 4795b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F., Bonigk W., Sesti F., Frings S. Phosphorylation of mammalian olfactory cyclic nucleotide-gated channels increases ligand sensitivity. J. Neurosci. 1998;18:164–173. doi: 10.1523/JNEUROSCI.18-01-00164.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm H., Pelzer S., Cochet C., Chambaz E., Tempel B.L., Trautwein W., Pelzer D., Lazdunski M. Dendrotoxin-binding brain membrane protein displays a K+ channel activity that is stimulated by both cAMP-dependent and endogenous phosphorylations. Biochemistry. 1989;28:6455–6460. doi: 10.1021/bi00441a044. [DOI] [PubMed] [Google Scholar]
- Reinhart P.H., Levitan I.B. Kinase and phosphatase activities intimately associated with a reconstituted calcium-dependent potassium channel. J. Neurosci. 1995;15:4572–4579. doi: 10.1523/JNEUROSCI.15-06-04572.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M., Brown R.L., He Y., Haley T.L., Karpen J.W. The single-channel dose–response relation is consistently steep for rod cyclic nucleotide-gated channelsimplications for the interpretation of macroscopic dose–response relations. Biochemistry. 1999;38:10642–10648. doi: 10.1021/bi990532w. [DOI] [PubMed] [Google Scholar]
- Sautter A., Zong X., Hofmann F., Biel M. An isoform of the rod photoreceptor cyclic nucleotide-gated channel beta subunit expressed in olfactory neurons. Proc. Natl. Acad. Sci. USA. 1998;95:4696–4701. doi: 10.1073/pnas.95.8.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammat I.M., Gordon S.E. Stoichiometry and arrangement of subunits in rod cyclic nucleotide-gated channels. Neuron. 1999;23:809–819. doi: 10.1016/s0896-6273(01)80038-6. [DOI] [PubMed] [Google Scholar]
- Shapiro M.S., Zagotta W.N. Stoichiometry and arrangement of heteromeric olfactory cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. USA. 1998;95:14546–14551. doi: 10.1073/pnas.95.24.14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Wyszynski M. Ion channel targeting in neurons. Bioessays. 1997;19:847–853. doi: 10.1002/bies.950191004. [DOI] [PubMed] [Google Scholar]
- Tsai W., Morielli A.D., Cachero T.G., Peralta E.G. Receptor protein tyrosine phosphatase alpha participates in the M1 muscarinic acetylcholine receptor-dependent regulation of Kv1.2 channel activity. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:109–118. doi: 10.1093/emboj/18.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner B., Weiner J., Middendorff R., Hagen V., Kaupp U.B., Weyand I. Cyclic nucleotide–gated channels on the flagellum control Ca2+ entry into sperm. J. Cell. Biol. 1998;142:473–484. doi: 10.1083/jcb.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G.F., Magoski N.S., Kaczmarek L.K. Modulation of a calcium-sensitive nonspecific cation channel by closely associated protein kinase and phosphatase activities. Proc. Natl. Acad. Sci. USA. 1998;95:10938–10943. doi: 10.1073/pnas.95.18.10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.M., Askalan R., Keil G.J., Salter M.W. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]