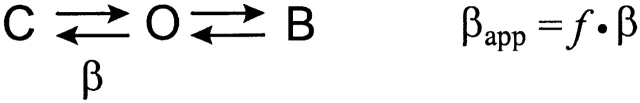Abstract
K+ channels encoded by the human ether-à-go-go-related gene (HERG) are distinguished from most other voltage-gated K+ channels by an unusually slow deactivation process that enables cardiac IKr, the corresponding current in ventricular cells, to contribute to the repolarization of the action potential. When the first 16 amino acids are deleted from the amino terminus of HERG, the deactivation rate is much faster (Wang, J., M.C. Trudeau, A.M. Zappia, and G.A. Robertson. 1998. J. Gen. Physiol. 112:637–647). In this study, we determined whether the first 16 amino acids comprise a functional domain capable of slowing deactivation. We also tested whether this “deactivation subdomain” slows deactivation directly by affecting channel open times or indirectly by a blocking mechanism. Using inside-out macropatches excised from Xenopus oocytes, we found that a peptide corresponding to the first 16 amino acids of HERG is sufficient to reconstitute slow deactivation to channels lacking the amino terminus. The peptide acts as a soluble domain in a rapid and readily reversible manner, reflecting a more dynamic regulation of deactivation than the slow modification observed in a previous study with a larger amino-terminal peptide fragment (Morais Cabral, J.H., A. Lee, S.L. Cohen, B.T. Chait, M. Li, and R. Mackinnon. 1998. Cell. 95:649–655). The slowing of deactivation by the peptide occurs in a dose-dependent manner, with a Hill coefficient that implies the cooperative action of at least three peptides per channel. Unlike internal TEA, which slows deactivation indirectly by blocking the channels, the peptide does not reduce current amplitude. Nor does the amino terminus interfere with the blocking effect of TEA, indicating that the amino terminus binding site is spatially distinct from the TEA binding site. Analysis of the single channel activity in cell-attached patches shows that the amino terminus significantly increases channel mean open time with no alteration of the mean closed time or the addition of nonconducting states expected from a pore block mechanism.We propose that the four amino-terminal deactivation subdomains of the tetrameric channel interact with binding sites uncovered by channel opening to specifically stabilize the open state and thus slow channel closing.
Keywords: excised macropatch, single channel recordings, electrophysiology, Xenopus oocyte, ion channels
INTRODUCTION
Repolarization of the ventricular cardiac action potential depends on a collection of potassium currents that control the duration of the action potential and the QT interval during each heartbeat. Temporally, IKr is the last of these currents to exert its repolarizing influence (Zeng et al. 1995). Disrupting IKr with drugs (Sanguinetti and Jurkiewicz 1990) or by mutations in the human ether-à-go-go–related gene (HERG) (Curran et al. 1995) prolongs the ventricular action potential and QT interval, leading in some cases to life-threatening cardiac arrhythmias. The polypeptide encoded by HERG forms channels in heterologous expression systems with the characteristic features of IKr (Sanguinetti et al. 1995; Trudeau et al. 1995), although the drug-binding properties of native IKr channels are more closely mimicked by hetero-oligomeric assemblies of HERG with the smaller MinK-related peptide MiRP1 (Abbott et al. 1999).
Work in several laboratories has contributed to our understanding of how the gating mechanisms of HERG channels enable IKr to fulfill its physiological role in the heart. Like other S4-containing channels, HERG channels activate and inactivate upon depolarization, and return to rest (deactivate) upon repolarization (Sanguinetti et al. 1995; Trudeau et al. 1995). But because inactivation is faster than activation, channels spend little time in the open state and outward current is effectively suppressed at the positive voltages reached during the peak of the action potential (Zhou et al. 1998). It is upon repolarization, as HERG channels recover from inactivation, revisit the open state, and slowly close, that a large outward current is evoked. This “resurgent current,” a term coined for the current through Na+ channels exhibiting an analogous gating process in cerebellar Purkinje neurons (Raman and Bean 1997), provides the terminal repolarization for the cardiac action potential. The resurgent nature of the native current that would later be identified as IKr (Sanguinetti and Jurkiewicz 1990) was originally described by Shibasaki 1987, who also accurately predicted the underlying gating mechanism.
Critical to the production of the resurgent current are a rapid, C-type inactivation mechanism (Schonherr and Heinemann 1996; Smith et al. 1996; Herzberg et al. 1998), and a slow deactivation process, about which less is known. Our previous studies suggest that a domain within the first 16 residues of the amino terminus slows deactivation by interacting with a site near the internal mouth of the pore. Removal of the first 16 amino acids dramatically increases deactivation rate (Wang et al. 1998), phenocopying a more extensive amino-terminal deletion (Δ2-354) (Schonherr and Heinemann 1996; Spector et al. 1996). The same phenotype results from the covalent modification of the S4–S5 linker by a thiol-reducing agent, suggesting that the addition of a bulky group near the internal mouth of the pore interferes with the ability of the amino terminus to slow deactivation. Conversion from the slow to the fast deactivation phenotype occurs within minutes of adding the agent, as if a dynamic process leaves the target cysteine accessible to modification when the amino-terminal domain and its receptor site are not in contact (Wang et al. 1998).
In contrast, a peptide corresponding to the first 135 amino acids of HERG, and encompassing a Per-Arnt-Sim (PAS) domain identified within its crystal structure, gradually reconstituted slow deactivation over a 24-h period when injected into oocytes expressing HERG channels lacking the amino terminus (Morais Cabral et al. 1998). Acute application of this peptide to an excised patch had no effect, and the reconstituted slow deactivation was unaffected by membrane excision, arguing against a rapid association and dissociation process and in favor of a stable, structural role for the PAS domain or other region within the peptide (Morais Cabral et al. 1998).
In this study, we tested the hypothesis that the small segment of 16 amino acids required for slow deactivation can act as an independent, soluble domain to dynamically restore slow deactivation to a channel lacking an amino terminus. This approach is analogous to that used to restore fast inactivation gating in Shaker channels with an amino-terminal peptide (Zagotta et al. 1990). We also asked whether the HERG amino terminus slows deactivation directly by stabilizing the open state and increasing channel open time, or indirectly by a blocking mechanism that prevents channel closure while it occupies its binding site, two possibilities that could not be distinguished in our previous studies. The latter “foot-in-the-door” hypothesis is based on the slowing of deactivation by quaternary ammonium ions in other K+ channels (Armstrong 1969, Armstrong 1971; Choi et al. 1993) and by the amino terminus peptide in Shaker channels, a secondary consequence of the inactivation mechanism (Demo and Yellen 1991). We found that a peptide corresponding to the first 16 amino acids can restore slow deactivation to truncated HERG channels, and that the amino terminus slows deactivation directly by holding the channels open longer. This gating mechanism, not previously described, may be generally important among diverse voltage-gated channels that mediate resurgent currents upon repolarization or otherwise require slow deactivation to fulfill their physiological roles.
METHODS
Expression of Channels in Xenopus Oocytes
Preparation of oocytes and RNA synthesis and injection were performed as previously described (Wang et al. 1998). The expression level of the channels was monitored daily with a two-electrode voltage clamp. The appropriate outward whole-cell current level was 50 μA for the macropatch experiments and 20 μA for the single channel recordings.
Unless otherwise noted, all experiments were carried out in an S620T background. This mutation dramatically increased expression levels. Deactivation in S620T was shown in a previous study to be regulated by the amino terminus in a manner indistinguishable from that in wild-type channels (Wang et al. 1998).
Current Recording and Data Analysis
All currents were recorded using an Axopatch 200A integrating patch clamp and pClamp 6.0.3 data acquisition software (Axon Instruments, Inc.). Recordings were digitized at 2.5 kHz unfiltered for macropatch experiment and 10 kHz filtered at 1 kHz for single channel experiments. Single channel activities were recorded in the cell-attached configuration, while the macropatch data were obtained in the inside-out patch configuration. Patch electrodes were fabricated from borosilicate glass using a Flaming/Brown micropipette puller (Sutter Instrument Co.). The tip of the electrode was heat polished with a microforge (Narishige Scientific Instruments) immediately before the recordings. For single-channel recordings, the electrode was also coated with Sylgard as close to the tip as possible to reduce capacitance. The tip opening was 3–6 μm for macropatch and 1 μm for single channel recordings. The resistance of the electrodes was 1–2 MΩ for macropatch and 5–10 MΩ for single channel recordings with the solutions indicated below. Seal resistance was at least 10 GΩ for macropatch and 50 GΩ for the single channel recordings. In macropatch experiments, once a gigaseal was formed and on-cell currents were recorded as controls, the patch was excised from the oocyte into internal bath solution.
PClamp and Origin 4.1 (Microcal Software, Inc.) were used for data analysis and generating statistic plots. Time constants (τ s) for deactivation were measured in Clampfit with a Chebyshev fit to the deactivating tail current using the equation y = A0 + A1 e − t /τ1 + A2 e − t /τ2. Deactivation rate was estimated from the time constant τ = 1/(α + β), where α is the activation rate constant and β is the deactivation rate constant for a two-state system. At −140 mV, there is little return from the closed to the open state and so deactivation rate can be reasonably inferred from τ ≅ 1/β. Time constants are represented in figures as box plots, in which the box top and bottom represent the range of the data between 1 and 99% and the middle line represents the mean value. Histograms of the open- and closed-time distribution were generated in pStat from the continuous recordings obtained with Fetchex program. Second-order exponential fitting to the histograms using the Marquardt-LSQ method generated the time constants as the mean dwell time of each state.
We observed faster deactivation kinetics in the macropatch recordings compared with those in our previous whole-cell recordings. The faster deactivation rate occurred in both on-cell and excised patch configurations, and in both S620T and S620TΔ2-354 channels. Therefore, the interpretation of our data is unaffected. Likely reasons for this change to fast kinetics include alteration of membrane mechanics and negative pressure associated with patch formation (Shcherbatko et al. 1999).
Solutions
Unless otherwise noted, the pipette and bath solution contained (mM): 100 KCl, 0.3 CaCl2, 1 MgCl2, 40 N-methyl-glucamine, and 10 HEPES, pH 7.4. The internal bath solution contained (mM): 140 KCl, 2 MgCl2, 10 EGTA, 5 HEPES, and 5 MgATP, pH 7.4. TEA-Cl was dissolved in internal bath solution, and then applied to the bath solution directly.
Peptide Synthesis and Handling
The H16 and H16s peptides were synthesized using the facilities at the University of Wisconsin Biotechnology Center. Both peptides were purified with reverse-phase HPLC. The peptide was stored as lyophilized powder at −20°C. Before each experiment, the peptide was then dissolved in internal bath solution to desired concentration, and then applied to the bath solution directly.
RESULTS
Restoration of Slow Deactivation by an Amino-Terminal Peptide
In a previous study, we found that the first 16 amino acids are necessary for slow deactivation in HERG channels (Wang et al. 1998) and that their removal fully mimics the deactivation phenotype of the more extensive amino-terminal truncation Δ2-354 (Schonherr and Heinemann 1996; Spector et al. 1996; Wang et al. 1998). To determine whether this smaller region is sufficient to restore slow deactivation to the truncated channel, we constructed a peptide corresponding to the first 16 amino acids and applied it to inside-out macropatches excised from oocytes expressing the construct Δ2-354. Fig. 1 A is a composite of three recordings of tail currents evoked at −140 mV after an activating step to 60 mV (only partially shown). The currents were sequentially recorded from the same patch, first immediately after excision, then after application of peptide (H16), and finally after wash out of the peptide. Deactivation was rapid in the on-cell recording and unchanged by excision. Application of the peptide slowed deactivation without reducing the initial tail current, an effect that was rapidly reversible upon washout (Fig. 1A). Consistent results were obtained with four patches, indicating that the first 16 amino acids comprise an independent, soluble domain that reversibly slows deactivation without pore blocking. Both exponential components characteristic of HERG deactivation were slowed by the peptide (Fig. 1 B), consistent with the effect of the native amino terminus in whole cell recordings (Wang et al. 1998). When a scrambled peptide with the same amino acid composition as the H16 peptide was applied to the excised patch, deactivation was unaffected (Fig. 1C and Fig. D), indicating that the slowing of deactivation depends on the structure dictated by the primary amino acid sequence of the peptide and not on its size or net charge.
Figure 1.
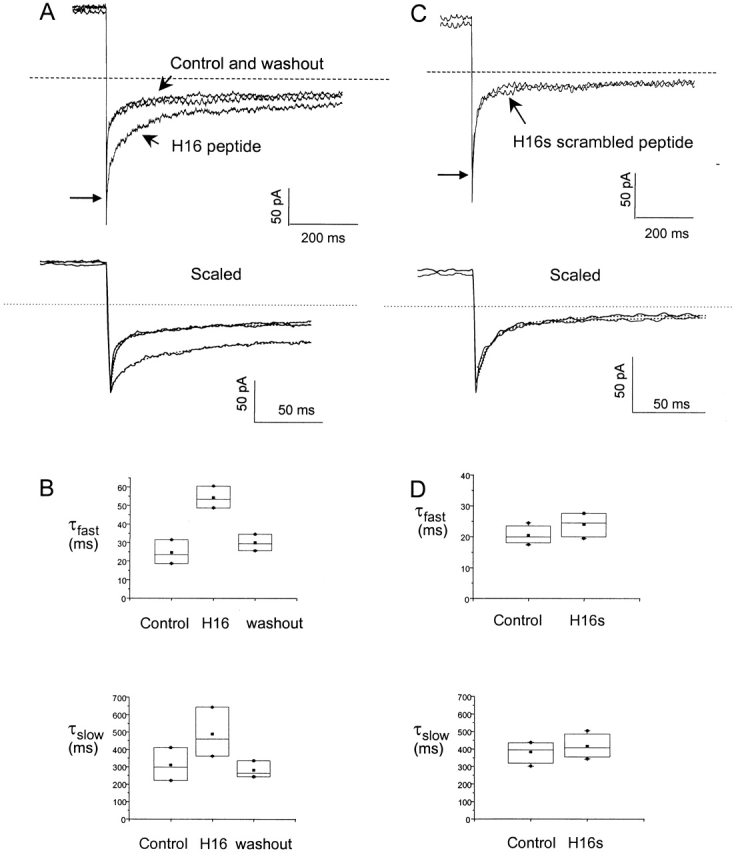
A soluble peptide corresponding to the first 16 amino acids (H16) reversibly restores slow deactivation to HERG channels lacking amino termini. (A) Tail currents from S620T Δ2-354 channels at −140 mV after a 1-s voltage step to 60 mV. (See methods regarding use of the S620T background.) Three current traces are shown, immediately after the patch was excised into the internal bath solution, after a 3-min incubation with 1 mM H16 peptide solution, and after a 5-min washout with peptide-free internal bath solution, respectively. The initial tail current amplitude, indicated by the long arrow, is unaffected by application of the peptide. The bottom panel, with expanded time scale, shows the initial tail current amplitude and includes dotted lines showing the fit with two exponential components. (B) Fast and slow mean time constants of deactivation extracted from exponential fits to the deactivating tail currents as shown in A. Values are τfast = 24.5 ± 6.5 ms, τslow = 309.8 ± 95.4 ms, A f (weight of the amplitude of the fast component represented as a percentage of total current) = 82.0 ± 8.2% for control; τfast = 54.2 ± 5.9 ms, τslow = 488.2 ± 143.5 ms A f = 73.4 ± 9.3% with the added peptide; and τfast = 29.9 ± 4.5 ms, τslow = 280.1 ± 48.3 ms, A f = 75.6 ± 6.9% upon washout of the peptide (n = 4). Using ANOVA (P < 0.01, at confidence interval = 0.05), there is a significant difference between the control and the experiment with peptide applied. (C) Tail currents before and after a 10-min incubation in the scrambled H16s peptide solution. (D) The mean time constants of both fast and slow components of the deactivation was not significantly changed by the scrambled peptide (ANOVA, P > 0.3 at confidence interval = 0.05). Values are τfast = 20.4 ± 2.9 ms, τslow = 381.9 ± 62.0 ms, A f = 89.7 ± 5.5% for control; τfast = 24.0 ± 4.2 ms, τslow = 414.7 ± 68.5 ms, A f = 81.6 ± 7.6% for the scrambled peptide (n = 3).
To determine whether the H16 peptide mimics the intact amino terminus or instead slows deactivation by an independent mechanism not intrinsic to the native channel, we tested the effect of the peptide in HERG channels with intact amino termini. In contrast to its slowing of truncated channels shown in Fig. 1 A, the peptide had no effect on the deactivation rate in channels with an intact amino terminus (Fig. 2 A), indicating that the peptide and the native amino terminus slow deactivation by the same process. The values for the mean time constants for the dominant fast component extracted from exponential fits to the deactivating tail currents from three such experiments are summarized in Fig. 2 B.
Figure 2.
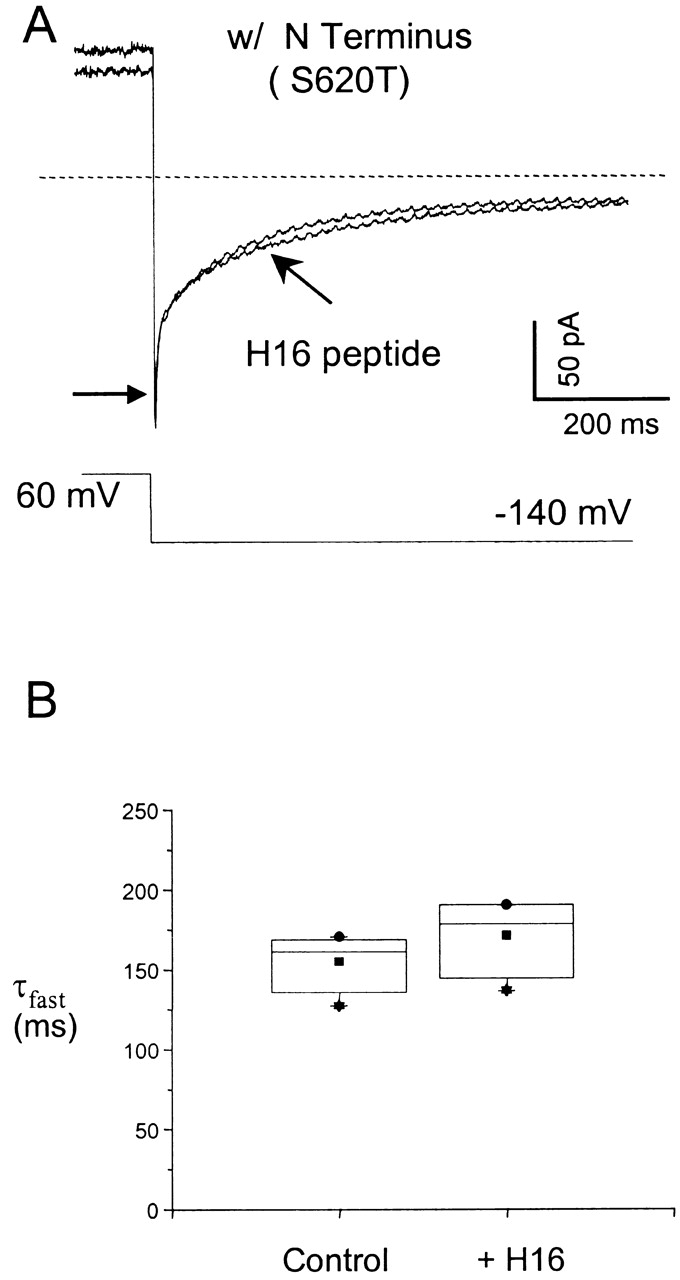
The H16 peptide has no effect on deactivation rate in channels with intact amino termini, indicating that the peptide acts by the same mechanism as the native amino terminus. (A) Tail currents from S620T channels evoked at −140 mV after a voltage step to 60 mV, before and after a 5-min incubation in 1 mM H16 peptide. (Arrow) Initial tail current amplitude. (B) Time constants of dominant fast components of the deactivation extracted from exponential fits to the tail currents were 137.6 ± 16.5 ms, A f = 90.0 ± 2.3% for the controls, and 167.7 ± 21.5 ms, A f = 87.6 ± 4.7% after application of the peptide (n = 3). There is no significant difference when tested with ANOVA. (P > 0.3 at confidence level of 0.05.)
Multiple Amino Terminal Peptides Cause Slow Deactivation
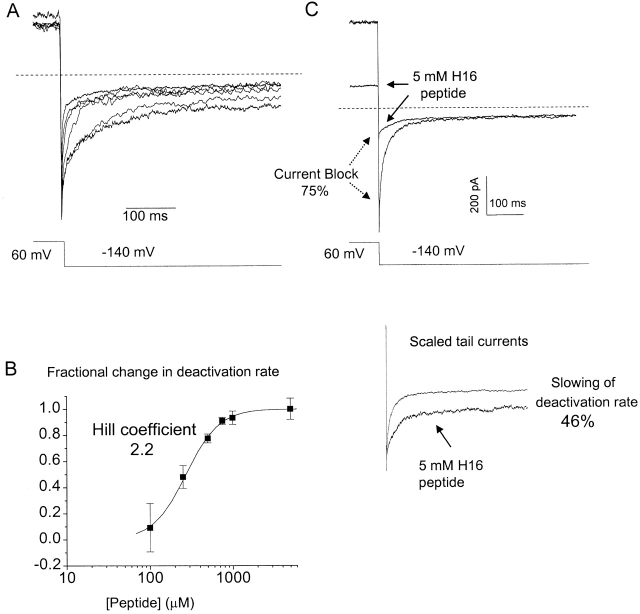
How many amino termini are required to slow the deactivation rate of a single channel? The peptide slowed deactivation in a dose-dependent manner (Fig. 3A and Fig. B), giving a Hill coefficient of 2.2 ± 0.1. Thus, three or more amino termini probably mediate the slowing effect. Restoration of slow deactivation was not complete, with the time constant saturating at a value approximately halfway between the corresponding values for the wild-type and truncated channels (see discussion).
Figure 3.
Multiple amino termini are required to slow deactivation in the HERG channel. (A) Tail currents from S620T Δ2-354 channels show progressive slowing in solutions of increasing H16 peptide concentration. From top to bottom, the concentrations are 0, 100, 250, 500, 750, and 1,000 μM. (B) Dose–response curve plotted as a fractional slowing of deactivation rate [Δ(1/τ)/Δ(1/τ)max] against peptide concentration (n = 3 for each peptide concentration up to 1 mM, n = 2 for 5 mM). τ is the time constant of the dominant fast component, which contributes >90% of the deactivating current. Fitting the data to the Hill equation [ln(1 − y)/y] = −N(lnK − ln[peptide]) yielded a Hill coefficient of 2.2 ± 0.1, indicating possible cooperative interactions among at least three amino termini. (C) High concentrations (5 mM) of peptide blocked the current (top), but caused no further slowing of deactivation (scaled currents, bottom).
At significantly higher concentrations (5 mM), the peptide has an inhibitory effect (Fig. 3 C). We wondered whether channel block by the peptide might reflect a physiological process that could account for the fractional slowing of deactivation not restored by the peptide. Based on a model of open-channel block, in which the degree of slowing of deactivation is directly proportional to the degree of current block (see detailed description in Fig. 4, legend), the resulting inhibition of current predicts that the deactivation rate will be slowed by 82.3 ± 6.8%. We found that deactivation is slowed only by 47.6 ± 9.3%, similar to that observed with the lower, saturating concentration of peptide. The inhibition is therefore an artifact not unexpected from such high concentrations of synthetic peptide and cannot account for the additional component of slowing.
Figure 4.
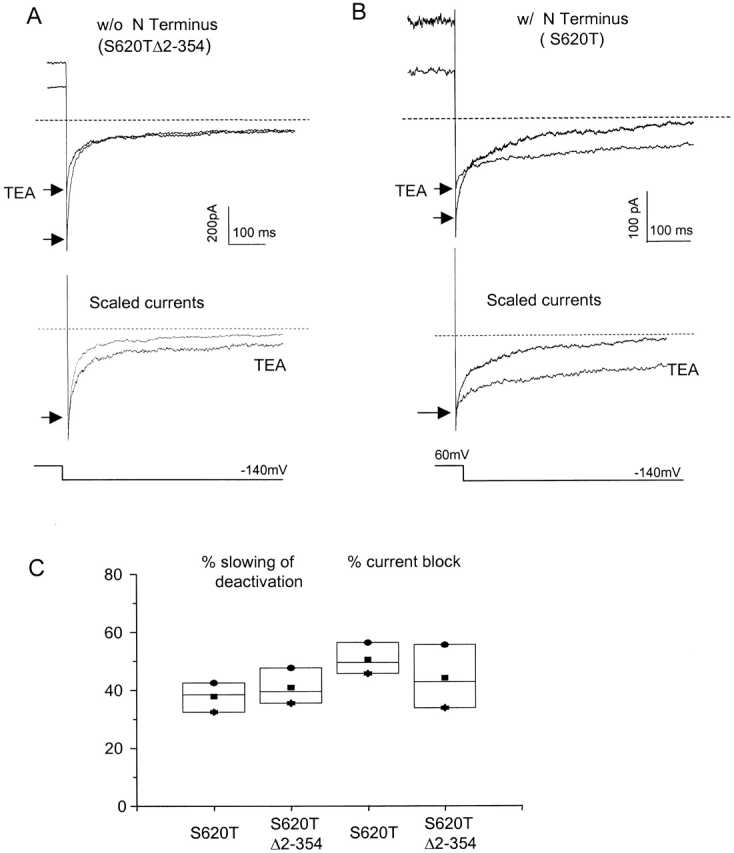
The amino terminus does not alter the ability of TEA to block current or to slow deactivation. (A, top) Tail currents from S620TΔ2-354 truncated channels evoked at −140 mV after depolarization to 60 mV, before and after application of 1 mM TEA to the internal bath solution. Arrows point to the instantaneous tail current levels. (Bottom) The same currents scaled to their peak amplitudes to illustrate the slowing of apparent deactivation rate by TEA. (B, top) Tail currents from channels with amino termini intact under same conditions as in A. (Bottom) The same currents scaled. (C) Box plot shows that mean values for percent slowing of deactivation (left) are predicted by corresponding percent current block (right) for both S620TΔ2-354 truncated channels and S620T channels with intact amino termini, indicating a lack of competition between the amino terminus and TEA in open-channel block (n = 3 for each construct, P > 0.2). The competition models describe a channel that cannot close until the blocking particle vacates its binding site (Scheme I), where C, O, and B are the closed, open, and blocked states, respectively, and β is the rate constant of the open-to-closed transition. SCHEME I Channels in the blocked state are unavailable for closing until they return to the open, unblocked state, and so the apparent deactivation rate βapp is reduced according to the expression βapp = f · β, where f is the fraction of channels in the open state. At the very negative voltages studied, we assume little return from the closed to the open state and βapp ≅ 1/τfast (see methods). The forward and reverse rates of block are assumed to be fast relative to β.
The Amino Terminus Does Not Slow Deactivation by a Pore-blocking Mechanism
Note that the slowing of deactivation by the peptide shown in Fig. 1 and Fig. 3 is accompanied by an increase in the current integral, or total charge moving through the channels, implying that the peptide increases the channel mean open time. If, in contrast, the amino terminus had slowed the apparent deactivation rate by occluding the pore, as proposed for Shaker channels (Demo and Yellen 1991), we would have seen an initial reduction in current amplitude and a proportional decrease in apparent deactivation rate as predicted by competition models (Beam 1976; Yeh and Narahashi 1977; Neher and Steinbach 1978; Grissmer and Cahalan 1989; Choi et al. 1991; see Fig. 4 legend).
Like other voltage-gated K+ channels, HERG channels are blocked by internal TEA (Smith et al. 1996). Consistent with this block, the deactivation time course was slowed when TEA was applied to the inner aspect of Δ2-354 truncated channels in excised macropatches. TEA slowed the apparent deactivation rate by 44.1 ± 10.9%, close to the 50.5 ± 5.4% predicted by the fractional current block obtained in this experiment (Fig. 4A and Fig. C). The tail currents have been scaled to their peaks in Fig. 4A, bottom, for comparison of deactivation rates.
To determine whether the amino terminus competes with TEA to slow deactivation, reflecting overlapping binding sites in the permeation pathway, we applied TEA to the channels with intact amino termini (Fig. 4 B). TEA slowed the apparent deactivation rate by 39.6 ± 4.0%, similar to the 42.4 ± 5.5% predicted by current block. Quantitatively similar effects were observed in the truncated channel (summarized in Fig. 4 C). The additive and noncompetitive effects of TEA and the amino terminus indicate that they slow deactivation using different mechanisms and different sites.
The Amino Terminus Slows Deactivation by Stabilizing the Open State
The slowing of macroscopic currents by peptide in the excised macropatch, with no evidence of channel block, suggests that a preferential stabilization of the open state underlies slow deactivation. To test this hypothesis directly, we determined the life time of each conformational state inferred from recordings of single channels with intact (S620T) or truncated (S620TΔ2-354) amino termini. Under steady state conditions at −80 mV, bursting behavior is prominent in both channels, but the truncated channels exhibit shorter openings (Fig. 5A and Fig. B). Ensemble tail currents constructed from multiple trials of single channel recordings elicited by a repolarizing step are consistent with the corresponding whole-cell currents for each construct, confirming the identity of the single channels (Fig. 5C and Fig. D).
Figure 5.
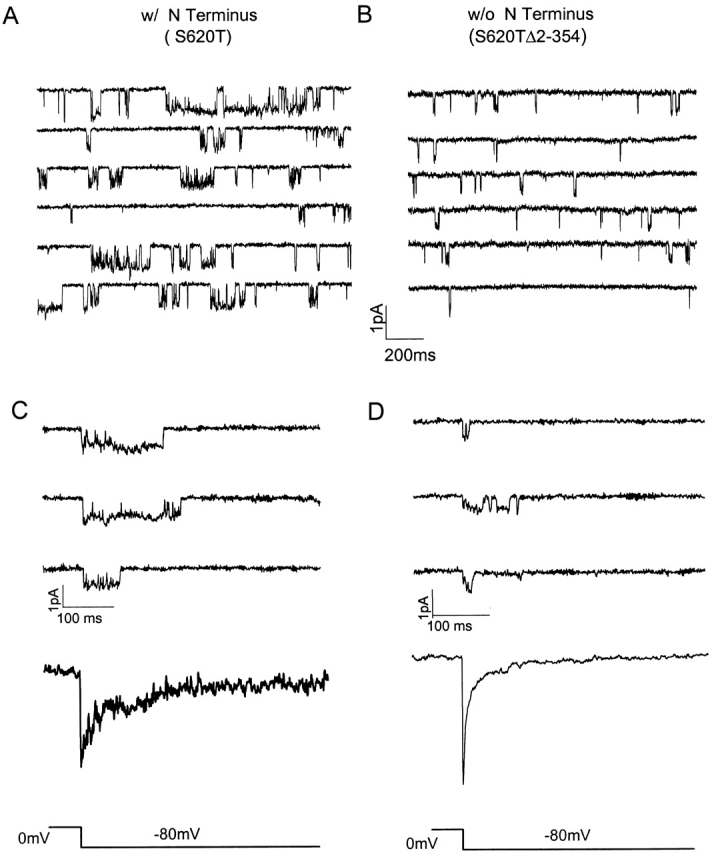
Single-channel activities at negative (repolarizing) potentials display longer open times when the amino terminus is present. During a continuous voltage command of −80 mV, lasting for 3−5 min, single-channel activities were recorded in cell-attached patch with 100 mM potassium in the pipette. Representative segments of the records from S620T and S620TΔ2-354 channels are shown in A and B, with longer openings apparent in S620T channels (n = 3 for each construct). (C and D) Ensemble tail currents and representative single trials of activities from which the ensemble averages were constructed for S620T and S620TΔ2-354 channels, respectively, during a 2-s repolarizing command to −80 mV after depolarization to 60 mV. Deactivation of the ensemble currents displays typical slow phenotype for S620T channel and fast phenotype for S620TΔ2-354 channels (n = 3 for each construct).
Fits to the histograms of the open- and closed-time distributions (Fig. 6A and Fig. B) yielded two exponential components for open and closed states for both channels, indicating two open and two closed states. Both mean open times, as represented by the time constants of the open time histogram, are significantly prolonged in the presence of the amino terminus, whereas neither mean closed time is affected. This selective increase of the mean open time is consistent with a mechanism by which the amino terminus stabilizes the open state to hold the channel open longer. The lack of additional nonconducting states attributable to the presence of the amino terminus also further confirms the absence of pore block.
Figure 6.
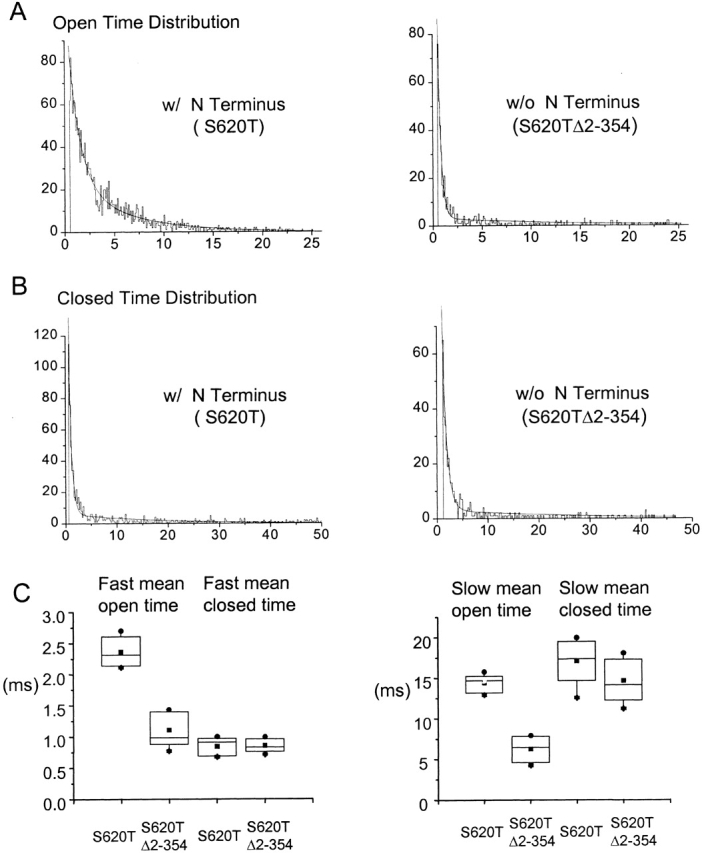
The amino terminus specifically increases the mean open time of the channel. Representative histograms of open- (A) and closed-time (B) distribution for both S620T and S620TΔ2-354 channels are shown with the fitting of the second-order exponential superimposed onto the plot (solid smooth lines). Time constants extracted from the fittings were taken as the mean open and closed dwell time and plotted in C. Both fast and slow mean open times are reduced when the amino terminus are deleted (values are τfast = 2.4 ± 0.2 ms, τslow = 14.4 ± 1.1 ms for S620T channels; τfast = 1.1 ± 0.3 ms, τslow = 6.3 ± 1.7 ms for S620TΔ2-354 channels; n = 3 for each construct, P < 0.01), while the two mean closed times remain unaffected (values are τfast = 0.8 ± 0.1 ms, τslow = 17.1 ± 2.9 ms for S620T channels; τfast = 0.9 ± 0.1 ms, τslow = 14.6 ± 2.7 ms for S620TΔ2-354 channels; n = 3 for each construct, P > 0.3).
DISCUSSION
In this study, we found that a small amino terminal domain of HERG can reversibly reconstitute slow deactivation as a soluble peptide. The amino terminal domain slows deactivation directly by increasing channel open time, rather than indirectly slowing apparent deactivation rate by a pore blocking mechanism. We envision the amino terminus interacting with the open “teepee” conformation suggested by recent structural and functional studies of the KcsA bacterial K+ channel (Doyle et al. 1998; Perozo et al. 1999). For example, the peptide might intercalate between the staves of the teepee formed by the S6 transmembrane domain like the cadmium ion that links a substituted cysteine with a nearby histidine residue to stabilize the open state in Shaker channels (Holmgren et al. 1998). Alternatively, the peptide domain could stabilize the open teepee conformation by inserting between residues in S5 and S6 uncovered by channel opening.
It remains to be determined why we were able to rapidly restore slow deactivation using the 16-mer peptide in the excised macropatch when a longer, 135-amino acid polypeptide, which included the initial 16 amino acids, had no effect under the same conditions (Morais Cabral et al. 1998). Perhaps in the latter study, the small deactivation subdomain was buried in the larger structure, the proper conformation of which was only slowly achieved after interaction of the polypeptide with the rest of the channel, thus accounting for the extended period of time (∼24 h) required to reconstitute slow deactivation. It therefore seems unlikely that the PAS domain identified in the crystal structure of the HERG amino terminus is involved on a rapid time scale in the regulation of deactivation rate. On the other hand, alteration of its structure can disrupt slow deactivation (Morais Cabral et al. 1998; Chen et al. 1999), perhaps by preventing the deactivation subdomain from reaching its own receptor site.
Another puzzle is why the 16-mer peptide only partially restores slow deactivation to the truncated channel. Although deletion of this region is sufficient to fully disable the amino-terminal slowing of deactivation (Wang et al. 1998), it may be just one component of a more complex structure that is needed for full restoration of function. In this case, we might expect the peptide to fully restore slow deactivation in the smaller truncation mutant Δ2-16, but unfortunately we were unable to express this mutant at sufficiently high levels for excised macropatch recordings. The larger, 135 amino acid peptide containing the PAS domain gave quantitatively similar results, slowing deactivation to a rate about halfway between that of wild-type and truncated channels (Morais Cabral et al. 1998). Both peptides may lack the structural attributes required to fully restore slow deactivation, due either to misfolding or the absence of sequences downstream in the greater part of the amino terminus not examined in either study.
Analysis of the dose dependence of the peptide effect indicates that three or more amino termini bind to hold each channel open. This finding is consistent with our previous study of heteromeric mouse ether-à-go-go–related gene 1 (Merg1) channels composed of two types of subunits arising from alternative splicing, one lacking what we now recognize as the deactivation subdomain (London et al. 1997). The intermediate deactivation rates observed were inconsistent with a single ball-and-chain mechanism expected to give rise to a preponderance of slowly closing channels. Based on the stoichiometric predictions of the current study, and the presumed tetrameric structure of the channel, it is reasonable to suggest that four amino termini interact with the open channel to slow deactivation. The physiological role of alternative splicing of the Merg or HERG orthologs is unknown, but the faster deactivation obtained in heteromultimers is expected to reduce the magnitude of the resurgent current. Perhaps selective expression or coassembly of these naturally occurring splice variants lacking the deactivation subdomain helps control IKr magnitude developmentally or in a tissue-specific manner. When slow deactivation is called for, four of the longer isoforms may assemble, each contributing a deactivation subdomain to stabilize the open state and ensure that IKr is of sufficient magnitude to fulfill its physiological role.
Previous studies have shown that deactivation of voltage-dependent K+ currents can be slowed by an amino-terminal blocking mechanism, as in Shaker (Demo and Yellen 1991) or by internal blocking ions that prevent channel closure (Armstrong 1969, Armstrong 1971; Choi et al. 1993). The blocker effectively competes for the open state, reducing the number of channels available to close at any given time, thus slowing the apparent deactivation rate by mass action. Although there is no apparent inactivating phenotype characteristic of channel block by the amino terminus in HERG channels (Schonherr and Heinemann 1996; Spector et al. 1996; Wang et al. 1998), we were unable in previous studies to rule out a fast amino-terminal blocking effect with slow recovery by comparing macroscopic current from truncated and wild-type channels. In this study, we show that slow deactivation is not associated with block by comparing initial tail currents from the same channel before and after application of peptide. Instead, the integral of the deactivating current is increased, indicating that the amino terminus stabilizes the channel in its open state during repolarization. Furthermore, the additive effects of TEA and the deactivation subdomain indicate that spatially discrete receptor sites mediate their respective modulatory effects on the time course of deactivation.
The slow deactivation process in HERG channels has provided an opportunity to investigate an unusual gating mechanism that was not previously apparent in studies of rapidly deactivating channels more commonly studied. We do not yet know exactly how the first 16 amino acids stabilize the open state in HERG, or whether the action of the deactivation subdomain will emerge as a common gating mechanism used by members of other channel families. However, it is clear that for many channels, including HERG, the amino terminus is a hub of activity for a diverse array of mechanisms modulating gating such as phosphorylation (e.g., Covarrubias et al. 1994), interactions with beta subunits (Sewing et al. 1996), alternative splicing (Timpe et al. 1988; Lees-Miller et al. 1997; London et al. 1997), and carboxy-terminal interactions (Jerng and Covarrubias 1997; Varnum and Zagotta 1997; Kupershmidt et al. 1998). Our study of the regulation of deactivation in HERG brings one more element to the functional diversity of the amino terminus, and underscores the importance of more detailed structural and functional analyses of its interactions with the gating machinery.
Acknowledgments
We thank Erin McCarthy for preparation of oocytes, Dr. Jeff Walker for advice on peptide synthesis, and Drs. Cynthia Czajkowski, Anne Lynn Gillian, and Robert Pearce for critically reading a previous version of this manuscript.
This work was supported by National Institutes of Health grant HL-55973, a National Science Foundation Career award, and an American Heart Association (AHA) Established Investigator Award (G.A. Robertson), as well as by a predoctoral fellowship from AHA-Wisconsin (J. Wang).
Footnotes
Abbreviations used in this paper: HERG, human ether-à-go-go-related gene; PAS, Per-Arnt-Sim.
References
- Abbott G.W., Sesti F., Splawski I., Buck M.E., Lehmann M.H., Timothy K.W., Keating M.T., Goldstein S.A. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- Armstrong C.M. Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J. Gen. Physiol. 1969;54:553–575. doi: 10.1085/jgp.54.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C.M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 1971;58:413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K.G. A quantitative description of end-plate currents in the presence of two lidocaine derivatives. J. Physiol. 1976;258:301–322. doi: 10.1113/jphysiol.1976.sp011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Zou A., Splawski I., Keating M.T., Sanguinetti M.C. Long QT syndrome-associated mutations in the Per-Arnt-Sim (PAS) domain of HERG potassium channels accelerate channel deactivation. J. Biol. Chem. 1999;274:10113–10118. doi: 10.1074/jbc.274.15.10113. [DOI] [PubMed] [Google Scholar]
- Choi K.L., Aldrich R.W., Yellen G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc. Natl. Acad. Sci. USA. 1991;88:5092–5095. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.L., Mossman C., Aube J., Yellen G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 1993;10:533–541. doi: 10.1016/0896-6273(93)90340-w. [DOI] [PubMed] [Google Scholar]
- Covarrubias M., Wei A., Salkoff L., Vyas T.B. Elimination of rapid potassium channel inactivation by phosphorylation of the inactivation gate. Neuron. 1994;13:1403–1412. doi: 10.1016/0896-6273(94)90425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran M.E., Splawski I., Timothy K.W., Vincent G.M., Green E.D., Keating M.T. A molecular basis for cardiac arrhythmiaHERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- Demo S.D., Yellen G. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron. 1991;7:743–753. doi: 10.1016/0896-6273(91)90277-7. [DOI] [PubMed] [Google Scholar]
- Doyle D.A., Morais Cabral J., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. The structure of the potassium channelmolecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Grissmer S., Cahalan M. TEA prevents inactivation while blocking open K+ channels in human T lymphocytes. Biophys. J. 1989;55:203–206. doi: 10.1016/S0006-3495(89)82793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg I.M., Trudeau M.C., Robertson G.A. Transfer of rapid inactivation and E-4031 sensitivity from HERG to M-EAG Channels. J. Physiol. 1998;511:3–14. doi: 10.1111/j.1469-7793.1998.003bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren M., Shin K.S., Yellen G. The activation gate of a voltage-gated K+ channel can be trapped in the open state by an intersubunit metal bridge. Neuron. 1998;21:617–621. doi: 10.1016/s0896-6273(00)80571-1. [DOI] [PubMed] [Google Scholar]
- Jerng H.H., Covarrubias M. K+ channel inactivation mediated by the concerted action of the cytoplasmic N- and C-terminal domains. Biophys J. 1997;72:163–174. doi: 10.1016/S0006-3495(97)78655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupershmidt S., Snyders D.J., Raes A., Roden D.M. A K+ channel splice variant common in human heart lacks a C-terminal domain required for expression of rapidly activating delayed rectifier current. J. Biol. Chem. 1998;273:27231–27235. doi: 10.1074/jbc.273.42.27231. [DOI] [PubMed] [Google Scholar]
- Lees-Miller J.P., Kondo C., Wang L., Duff H.J. Electrophysiological characterization of an alternatively processed ERG K+ channel in mouse and human hearts. Circ. Res. 1997;81:719–726. doi: 10.1161/01.res.81.5.719. [DOI] [PubMed] [Google Scholar]
- London B., Trudeau M.C., Newton K.P., Beyer A.K., Copeland N.G., Gilbert D.J., N.A.Jenkins C.A., Satler, Robertson G.A. Two isoforms of the mouse ether-à-go-go-related gene coassemble to form channels with properties similar to the rapidly activating component of the cardiac delayed rectifier K+ current. Circ. Res. 1997;81:870–878. doi: 10.1161/01.res.81.5.870. [DOI] [PubMed] [Google Scholar]
- Morais Cabral J.H., Lee A., Cohen S.L., Chait B.T., Li M., Mackinnon R. Crystal structure and functional analysis of the HERG potassium channel N terminusa eukaryotic PAS domain. Cell. 1998;95:649–655. doi: 10.1016/s0092-8674(00)81635-9. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J.H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J. Physiol. 1978;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E., Cortes D.M., Cuello L.G. Structural rearrangements underlying K+-channel activation gating. Science. 1999;285:73–78. doi: 10.1126/science.285.5424.73. [DOI] [PubMed] [Google Scholar]
- Raman I.M., Bean B.P. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J. Neurosci. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M.C., Jiang C., Curran M.E., Keating M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmiaHERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M.C., Jurkiewicz N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonherr R., Heinemann S.H. Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J. Physiol. 1996;493:635–642. doi: 10.1113/jphysiol.1996.sp021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewing S., Roeper J., Pongs O. Kv beta 1 subunit binding specific for Shaker-related potassium channel alpha subunits. Neuron. 1996;16:455–463. doi: 10.1016/s0896-6273(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Shcherbatko A., Ono F., Mandel G., Brehm P. Voltage-dependent sodium channel function is regulated through membrane mechanics. Biophys. J. 1999;77:1945–1959. doi: 10.1016/S0006-3495(99)77036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J. Physiol. 1987;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.L., Baukrowitz T., Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- Spector P.S., Curran M.E., Zou A., Keating M.T., Sanguinetti M.C. Fast inactivation causes rectification of the IKr channel. J. Gen. Physiol. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpe L.C., Jan Y.N., Jan L.Y. Four cDNA clones from the Shaker locus of Drosophila induce kinetically distinct A-type potassium currents in Xenopus oocytes. Neuron. 1988;1:659–667. doi: 10.1016/0896-6273(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Trudeau M.C., Warmke J.W., Ganetzky B., Robertson G.A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- Varnum M.D., Zagotta W.N. Interdomain interactions underlying activation of cyclic nucleotide-gated channels. Science. 1997;278:110–113. doi: 10.1126/science.278.5335.110. [DOI] [PubMed] [Google Scholar]
- Wang J., Trudeau M.C., Zappia A.M., Robertson G.A. Regulation of deactivation by an amino terminal domain in HERG potassium channels. J. Gen. Physiol. 1998;112:637–647. doi: 10.1085/jgp.112.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J.Z., Narahashi T. Kinetic analysis of pancuronium interaction with sodium channels in squid axon membranes. J. Gen. Physiol. 1977;69:293–323. doi: 10.1085/jgp.69.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W.N., Hoshi T., Aldrich R.W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990;250:568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]
- Zeng J., Laurita K.R., Rosenbaum D.S., Rudy Y. Two components of the delayed rectifier K+ current in ventricular myocytes of the guinea pig type. Theoretical formulation and their role in repolarization. Circ. Res. 1995;77:140–152. doi: 10.1161/01.res.77.1.140. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Gong Q., Ye B., Fan Z., Makielski J.C., Robertson G.A., January C.T. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys. J. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]