Abstract
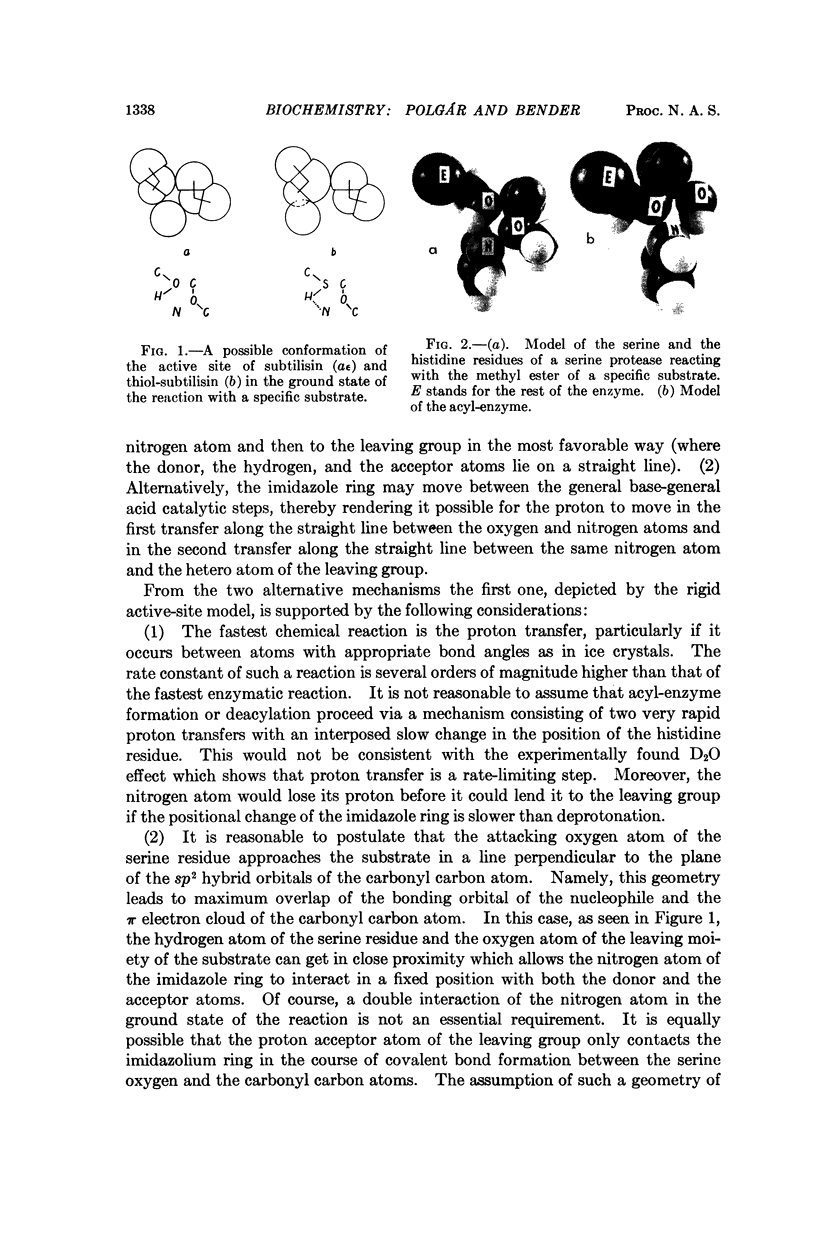
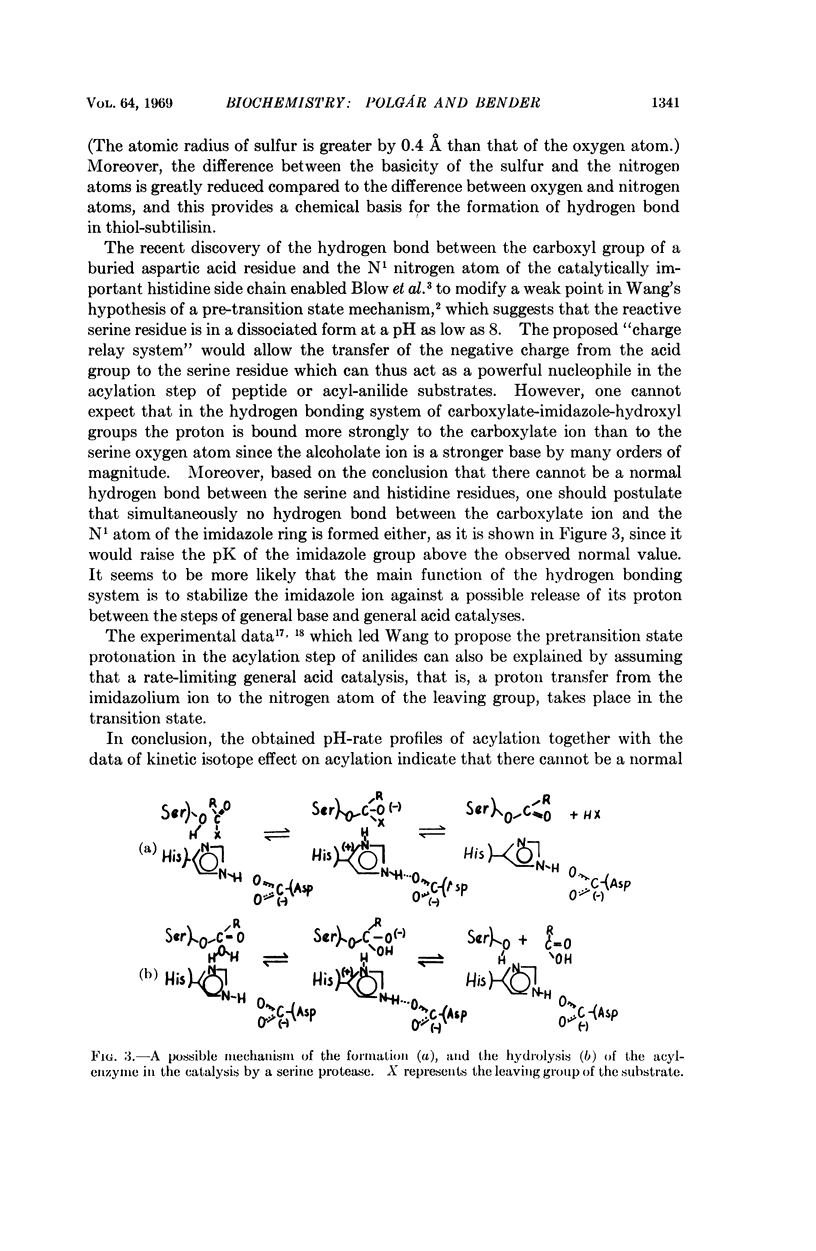
The high reactivity of the serine residue at the active site of serine proteases is often attributed to the formation of a hydrogen bond between this serine and a histidine residue. In the case of the serine protease subtilisin, the catalytic serine residue can be specifically replaced by a cysteine residue and this modified enzyme is called thiol-subtilisin. By studying the D2O effect on acyl-enzyme formation with subtilisin and thiol-subtilisin, we present evidence that thiol-subtilisin but not subtilisin may contain a hydrogen bond. Based on the comparison of the catalytic activities of subtilisin and thiol-subtilisin, a rigid active site model for the serine proteases is proposed in which the histidine residue operates in a fixed steric position both as a general base and as a general acid, and this, rather than the formation of a hydrogen bond, accounts for the high nucleophilicity of the serine residue.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDER M. L., KEZDY J. MECHANISM OF ACTION OF PROTEOLYTIC ENZYMES. Annu Rev Biochem. 1965;34:49–76. doi: 10.1146/annurev.bi.34.070165.000405. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Charney E., Bernhard S. A. Optical properties and the chemical nature of acyl-chymotrypsin linkages. J Am Chem Soc. 1967 May 24;89(11):2726–2733. doi: 10.1021/ja00987a040. [DOI] [PubMed] [Google Scholar]
- Inagami T., York S. S., Patchornik A. An electrophilic mechanism in the chymotrypsin-catalyzed hydrolysis of anilide substrates. J Am Chem Soc. 1965 Jan 5;87(1):126–127. doi: 10.1021/ja01079a027. [DOI] [PubMed] [Google Scholar]
- Neet K. E., Koshland D. E., Jr The conversion of serine at the active site of subtilisin to cysteine: a "chemical mutation". Proc Natl Acad Sci U S A. 1966 Nov;56(5):1606–1611. doi: 10.1073/pnas.56.5.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neet K. E., Nanci A., Koshland D. E., Jr Properties of thiol-subtilisin. The consequences of converting the active serine residue to cysteine in a serine protease. J Biol Chem. 1968 Dec 25;243(24):6392–6401. [PubMed] [Google Scholar]
- Parker L., Wang J. H. On the mechanism of action at the acylation step of the alpha-chymotrypsin-catalyzed hydrolysis of anilides. J Biol Chem. 1968 Jul 10;243(13):3729–3734. [PubMed] [Google Scholar]
- Polgar L., Bender M. L. Chromatography and activity of thiol-subtilisin. Biochemistry. 1969 Jan;8(1):136–141. doi: 10.1021/bi00829a019. [DOI] [PubMed] [Google Scholar]
- Polgar L., Bender M. L. The reactivity of thiol-subtilisin, an enzyme containing a synthetic functional group. Biochemistry. 1967 Feb;6(2):610–620. doi: 10.1021/bi00854a032. [DOI] [PubMed] [Google Scholar]
- Sigler P. B., Blow D. M., Matthews B. W., Henderson R. Structure of crystalline -chymotrypsin. II. A preliminary report including a hypothesis for the activation mechanism. J Mol Biol. 1968 Jul 14;35(1):143–164. doi: 10.1016/s0022-2836(68)80043-9. [DOI] [PubMed] [Google Scholar]
- Wang J. H. Facilitated proton transfer in enzyme catalysis. It may have a crucial role in determining the efficiency and specificity of enzymes. Science. 1968 Jul 26;161(3839):328–334. doi: 10.1126/science.161.3839.328. [DOI] [PubMed] [Google Scholar]
- Wright C. S., Alden R. A., Kraut J. Structure of subtilisin BPN' at 2.5 angström resolution. Nature. 1969 Jan 18;221(5177):235–242. doi: 10.1038/221235a0. [DOI] [PubMed] [Google Scholar]



