Abstract
Olfactory receptor neurons (ORNs) from the squid, Lolliguncula brevis, respond to the odors l-glutamate or dopamine with increases in internal Ca2+ concentrations ([Ca2+]i). To directly asses the effects of increasing [Ca2+]i in perforated-patched squid ORNs, we applied 10 mM caffeine to release Ca2+ from internal stores. We observed an inward current response to caffeine. Monovalent cation replacement of Na+ from the external bath solution completely and selectively inhibited the caffeine-induced response, and ruled out the possibility of a Ca2+-dependent nonselective cation current. The strict dependence on internal Ca2+ and external Na+ indicated that the inward current was due to an electrogenic Na+/Ca2+ exchanger. Block of the caffeine-induced current by an inhibitor of Na+/Ca2+ exchange (50–100 μM 2′,4′-dichlorobenzamil) and reversibility of the exchanger current, further confirmed its presence. We tested whether Na+/Ca2+ exchange contributed to odor responses by applying the aquatic odor l-glutamate in the presence and absence of 2′,4′-dichlorobenzamil. We found that electrogenic Na+/Ca2+ exchange was responsible for ∼26% of the total current associated with glutamate-induced odor responses. Although Na+/Ca2+ exchangers are known to be present in ORNs from numerous species, this is the first work to demonstrate amplifying contributions of the exchanger current to odor transduction.
Keywords: electrogenic, dichlorobenzamil, chemoreception, sodium–calcium exchange, cephalopod
INTRODUCTION
Calcium plays multiple roles in olfactory transduction. In addition to contributing to the initial transduction current (Firestein and Werblin 1987; Frings et al. 1995; Leinders-Zufall et al. 1997), increases in intracellular calcium are involved in the amplification of odor-evoked currents in vertebrate olfactory receptor neurons (ORNs) via the activation of calcium-activated chloride currents (ICl(Ca)) (Kleene and Gesteland 1991; Kurahashi and Yau 1993; Kleene 1993, Kleene 1997; Lowe and Gold 1993; Kleene et al. 1994; Kleene and Pun 1996; Nakamura et al. 1997; Hallani et al. 1998; Reuter et al. 1998). Increases in intracellular calcium have also been implicated in olfactory adaptation, either by increasing phosphodiesterase activity (Borisy et al. 1992), attenuating adenylyl cyclase activity (Wei et al. 1998; Leinders-Zufall et al. 1999), or by decreasing the sensitivity of olfactory cyclic nucleotide–gated channels for cAMP (Kurahashi and Shibuya 1990; Chen and Yau 1994; Kurahashi and Menini 1997; Leinders-Zufall et al. 1998). With so many processes controlled by intracellular calcium, regulating this second messenger is extremely important.
Both Ca2+ pumps and Ca2+ exchangers are used to regulate intracellular Ca2+. Two classes of electrogenic Ca2+ exchangers have been cloned, the Na+/Ca2+, K+ exchanger (NCKX) (Cervetto et al. 1989; Reilander et al. 1992) and the Na+/Ca2+ exchanger (NCX); for review, see Blaustein and Lederer 1999. In forward mode, these antiporters exhibit stoichiometries of either 4 Na+ in 1 Ca2+ + 1 K+ out (NCKX), or 3 Na+ in 1 Ca2+ out (NCX), each resulting in the net inward movement of one positive charge per cycle. The role of NCX has been most extensively documented in cardiac tissue, where it is the predominant method of calcium efflux following contraction, promoting relaxation of myocytes (Hryshko and Philipson 1997). In addition, NCX appears to be involved in Ca2+ entry via reverse Na–Ca exchange during the initial phases of the cardiac action potential, when internal Na+ is elevated (Hryshko and Philipson 1997). In mammalian vascular smooth muscle, neurons and astrocytes, the cardiac type NCX appears to be colocalized with underlying endoplasmic or sarcoplasmic reticulum structures, suggesting a role in tightly regulating Ca2+ released from intracellular stores (Juhaszova et al. 1996). In cultured retinal amacrine cells, NCX has been shown to be important in terminating synaptic transmission (Gleason et al. 1994). Sodium–calcium exchange has also been well studied in squid axons, where it is involved in regulating intracellular calcium concentrations (DiPolo 1973, DiPolo 1977; DiPolo and Beaugé 1993, DiPolo and Beaugé 1994a,DiPolo and Beaugé 1994b). A squid sodium–calcium exchanger, NCX-SQ1, was recently cloned from the stellate ganglia of Loligo opalescens (He et al. 1998).
In the olfactory system, sodium–calcium exchange has been localized, both by calcium imaging and immunocytochemistry, to the dendritic knob (and possibly the cilia) of rat ORNs, where it is presumed to clear calcium from the olfactory cilia and dendritic knob after odor stimulation (Noe et al. 1997). In Xenopus ORNs, Na+/Ca2+ exchange has been suggested to be present in the dendrites of ORNs (Jung et al. 1994). A more recent study shows that Na+/Ca2+ exchange is required for the termination of ICl(Ca) (Reisert and Matthews 1998), and that blocking sodium–calcium exchange prevents recovery from adaptation to odors.
Squid ORNs can be either excited or inhibited by odors (Danaceau and Lucero 1998, Danaceau and Lucero 2000; Lucero et al. 1992), and increases in intracellular calcium have been associated with both types of conductances (Piper and Lucero 1999; Danaceau and Lucero 2000). Therefore, we hypothesized that sodium–calcium exchange might be involved in calcium regulation in squid ORNs, following odor responses.
We have used nystatin-perforated patch and whole-cell voltage-clamp recordings to characterize electrogenic sodium–calcium exchange activity in squid ORNs. Forward activity of this exchanger is completely inhibited by removing external Na+, but is not affected by removal of internal or external K+. NCX currents are blocked by 2′,4′-dichlorobenzamil (DCB), an inhibitor of sodium–calcium exchange (Frelin et al. 1988). In addition, we show that the NCX current is responsible for ∼26% of the current measured after stimulation with glutamate, which activates a mixed cation conductance permeable to calcium > sodium and potassium (Danaceau and Lucero 2000). This is the first time that an electrogenic sodium–calcium exchanger has been implicated in amplification of olfactory responses in any system.
MATERIALS AND METHODS
Cell Preparation and Culture Conditions
The methods for cell dissociation were similar to those described in Danaceau and Lucero 1998. In brief, the olfactory organs of the squid, Lolliguncula brevis, were excised under a dissecting microscope and treated with nonspecific protease (10 mg ml−1, type XIV; Sigma- Aldrich) in sterile filtered artificial sea water (ASW) for 40 min. After a 3–5-min rinse in ASW, the olfactory organ was plated onto a drop of culture medium on concanavalin A (10 mg ml−1, type IV; Sigma-Aldrich)–coated glass coverslips and allowed to settle for 15 min before adding 2 ml medium and placing in the incubator at 17°C. Culture medium was changed daily and the cells were used for experiments between 1 and 3 d in culture. Only the pyriform receptor cell type (Lucero et al. 1992) was used in these experiments.
Solutions
The external bath and internal pipette solutions used in these experiments are listed in Table and Table . The external and internal solutions were set to an osmolarity of 780 mOsm and a pH of 7.4 or 7.2, respectively. The culture medium consisted of Leibovitz's L-15 (GIBCO BRL) supplemented with salts to bring the osmolarity to 780 mOsm, 2 mM HEPES, pH 7.6, 2 mM l-glutamine, 50 IU ml−1 penicillin G, and 0.5 mg ml−1 streptomycin. Nystatin stock solution contained 1 mg nystatin/20 μl DMSO; nystatin internal solution contained 3 μl nystatin stock + 2 μl 10% pluronic acid ml−1 internal solution. All internal solutions were kept on ice throughout the experiments.
Table 1.
Composition of External Bath Solutions
| Bath solution | Na+ | K+ | Ca2+ | Mg2+ | Li+ | Cl− | Tris+ | Glucose |
|---|---|---|---|---|---|---|---|---|
| ASW | 344 | 10 | 10 | 35 | 0 | 440 | 0 | 17.5 |
| Tris ASW | 0 | 0 | 10 | 35 | 0 | 410 | 376 | 10 |
| Li ASW | 0 | 10 | 10 | 35 | 345 | 445 | 0 | 17.5 |
All solutions contained 10 mM HEPES, were set to pH 7.4 with NaOH or TMAOH and set to an osmolality of 780 mOsm kg−1 using d-glucose.
Table 2.
Composition of Internal Pipette Solutions
| Internal solution | Na+ | K+ | Cl− | F− | Gluc− | TMA+ | TEA+ |
|---|---|---|---|---|---|---|---|
| Na Gluc with MgATP | 328 | 25 | 61 | 25 | 326 | 0 | 52 |
| 60 TEA Na Gluc | 355 | 0 | 61 | 25 | 330 | 0 | 60 |
| Li Gluc | 0 | 25 | 61 | 25 | 330 | 0 | 60 |
| TMA Gluc | 25 | 0 | 61 | 25 | 270 | 270 | 60 |
| TMA Cl | 25 | 0 | 365 | 25 | 0 | 365 | 0 |
All solutions were set to pH 7.2 with NaOH or TMAOH and were set to an osmolality of 780 mOsm kg−1 with d-glucose. Pipette solutions also contained 0.5 mM Ca2+, 10 mM HEPES, and 1.5 mM EGTA. Nystatin was added to various internal solutions, as described in materials and methods. Gluc, gluconate.
Chemicals
All chemicals were obtained from Sigma-Aldrich except for DCB, which was obtained from Molecular Probes. DCB was dissolved in DMSO as a 50-mM stock solution before dilution to 50 or 100 μM in ASW.
Nystatin Perforated-Patch Voltage-Clamp Recordings
Nystatin voltage- and current-clamp experiments were essentially similar to those described in Danaceau and Lucero 1998. In brief, 2–5 MΩ resistance electrodes pulled from thick-walled borosilicate filament glass (Sutter Instrument Co.) were filled with nystatin internal solution. Fresh nystatin internal solution was made every 2 h and nystatin stock was remade every 5 h. Gentle suction was applied to form a gigaohm seal. Recordings were made ∼5–20 min after seal formation. Bath solutions were perfused through the recording chamber at a rate of 1–2 ml min−1. Test solutions were delivered with an SF-77 rapid solution changer (Warner Instrument Corp.). The application of test solutions was calibrated with an open pipette. There was a delay of ∼90 ms between the beginning of the electronic stimulus and the current recorded by the open pipette. We did not correct the data for this delay. A 3-M KCl agar bridge was used to ground the recording chamber.
Whole-Cell Voltage-Clamp Recordings
Whole-cell voltage-clamp recordings were similar to the nystatin patch recordings. The same 2–5-MΩ resistance electrodes were filled with either Na+-gluconate internal containing Mg2+-ATP or with Li+-internal solution. The electrode tip was lowered onto the soma of the ORN and gentle suction was applied to form a gigaohm seal. Once the seal was formed, more suction was applied to rupture the membrane patch and achieve whole-cell access. Bath and test solutions were delivered as described for nystatin-perforated patch recordings.
Data Acquisition
Voltage-clamp and current-clamp data were acquired with an Axon Instruments 200A patch clamp amplifier (TL-1-125 Interface; Axon Instruments, Inc.) and a 486, 33 MHz computer using either PClamp 5.6 or Axotape 2.0.2. The data in Fig. 1, Fig. 2, and Fig. 3 B were sampled at 250 Hz and filtered off line with a digital eight-pole lowpass Bessel filter (Clampfit 8; Axon Instruments, Inc.) using a −3 dB filter cutoff frequency of 35 Hz. The data in Fig. 3 A, 5, and 6 were sampled at 500 Hz and digitally filtered off line with a −3 dB filter cutoff frequency of 50 Hz. The data in Fig. 4 were acquired at 10 kHz and filtered on line with a low pass four-pole Bessel filter cutoff at 5 kHz. Data for capacitance and series resistance (R s) measurements were sampled at 100 kHz and also filtered on line at 10 kHz (data not shown). The R s of nystatin-perforated patched cells averaged 39 ± 13 M (SD; n = 18). Approximately 60–75% of R s was electrically compensated. The voltage errors associated with the remaining R s as well as liquid junction potentials were corrected for off line as described in Danaceau and Lucero 1998. All averages are presented as the mean ± SD (n = number of cells).
Figure 1.
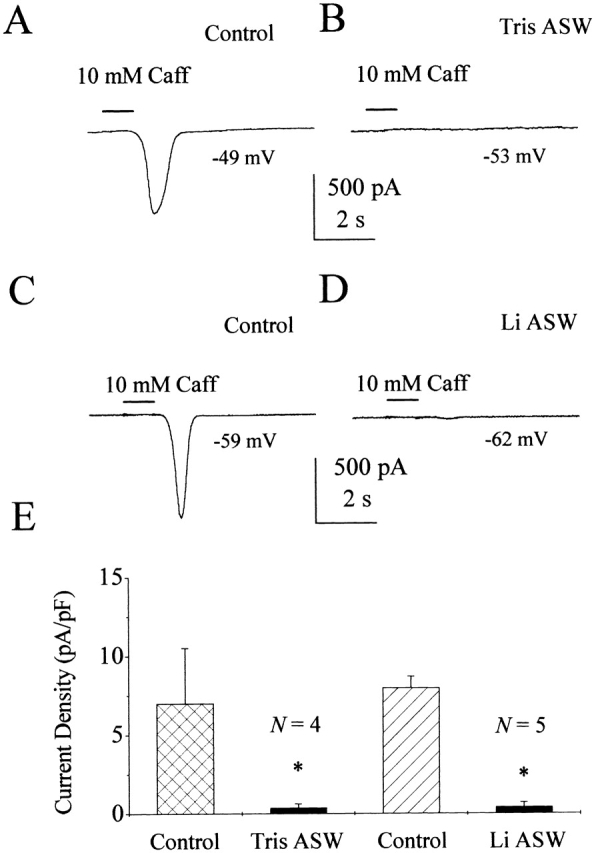
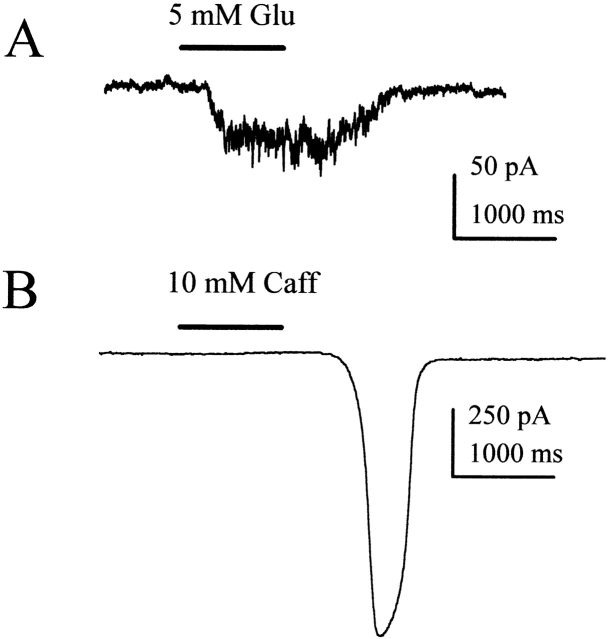
10 mM caffeine activates a sodium-dependent current in nystatin-perforated patched squid ORNs. (A) Current trace from a nystatin-perforated patched ORN during a 1-s application of 10 mM caffeine. Solutions ASW external (ext)/TMA Cl internal (int). (B) In the same cell, replacing external Na+ with Tris+ completely eliminated the caffeine-induced current. Solutions: Tris ASW ext/TMA Cl int. (C) A caffeine-activated current response in ASW from a different cell. Same solutions as in A. (D) Current trace from the cell in C in Li+ ASW. (E) Average current density of caffeine-induced current responses from squid ORNs in ASW, Tris+ ASW, ASW, and Li+ ASW, respectively. The holding potential has been corrected for liquid junction potential and is indicated in each panel. Error bars indicate SD. *P < 0.05.
Figure 2.
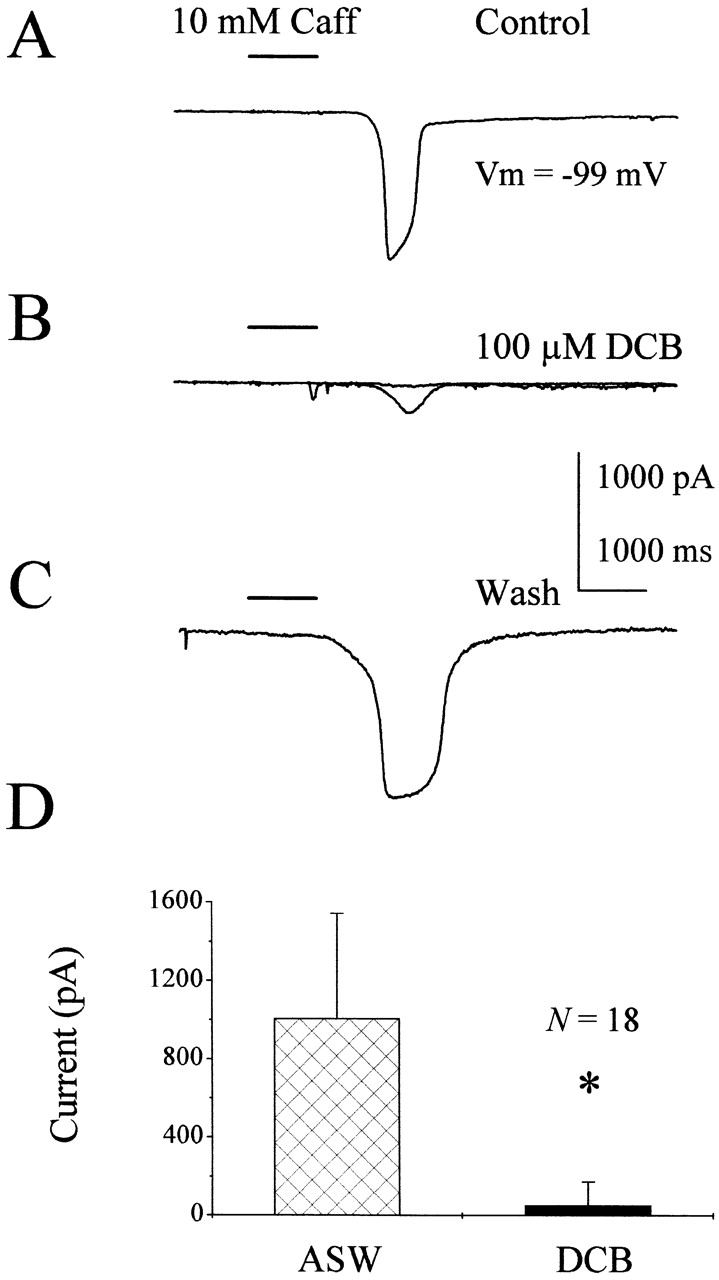
DCB reversibly eliminates Na+/Ca2+ exchange in squid ORNs. (A) 10 mM caffeine elicits a large Na+/Ca2+ exchange current. (B) After 12 s in the presence of 100 μM DCB, INCX is partially blocked and, after 42 s, INCX is completely eliminated. (C) After removing DCB and allowing the cell to recover for 2 min, INCX is restored. (D) In all 18 cells tested, DCB eliminated INCX. Solutions: ASW ext./Na gluconate int. *P < 0.001.
Figure 3.
Caffeine-induced currents are delayed relative to odor-induced currents. (A) This current trace was obtained from a nystatin-perforated patched squid ORN during a 1-s application of 5 mM glutamate. (B) In the same cell, the current response to a 1-s application of 10 mM caffeine is markedly delayed. Solutions: ASW ext/TMA gluconate int.
Figure 4.
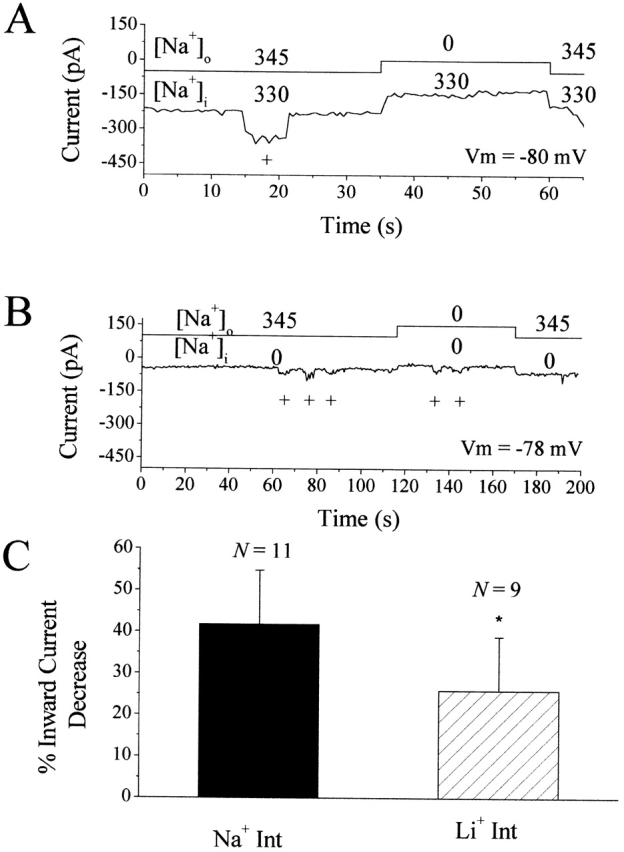
Na+/Ca2+ exchange currents are reversible in squid ORNs. (A) Current traces from a squid ORN under whole-cell voltage-clamp conditions. The solid line above the current trace indicates the switch from ASW to Tris+ ASW. +, 20-mV hyperpolarization. External and internal sodium concentrations are noted above and below the solid line in millimoles per liter−1. Solutions: ASW ext (Tris+ ASW)/Na+ gluconate int with 4 mM Mg2+ and 2 mM ATP. (B) A different ORN exposed to the same protocol as in A, but with Li+ gluconate internal solution to eliminate reverse Na+/Ca2+ exchange. The solid line indicates the switch from ASW to Tris+ ASW and back again. Numbers indicate external and internal sodium concentrations in millimoles per liter−1. +, 20-mV hyperpolarizations. Solutions: ASW ext (Tris+ ASW)/Li+ gluconate int; Rs = 19 MΩ, equilibration time = 5 min). (C) Mean percent decrease in the steady state inward current measured during the switch to Tris ASW under the conditions in A (Na+ int) and B (Li+ int). *The mean current reduction in Li+ gluconate is significantly smaller than Na+ gluconate int P < 0.05. Error bars indicate SD; n = numbers of cells.
These experiments comply with NIH publication 86-23, “Principles of Animal Care,” revised 1985, and with the current laws of the Unites Stated regarding animal research.
RESULTS
Caffeine Activates a Sodium-dependent Inward Current
Ca2+-imaging experiments have shown that caffeine releases calcium from internal stores in squid ORNs (Piper and Lucero 1999). When we applied 10 mM caffeine to nystatin-perforated patched squid ORNs, we observed a large inward current (Fig. 1 A). To test whether the caffeine-induced inward current was Na+ dependent or selective, we substituted either Tris+ or Li+ for external Na+. Tris+ is a relatively large organic cation that does not permeate Na+ channels and should not support either Na+-selective or -dependent currents. Li+, on the other hand, is a small, inorganic cation that permeates most Na+ channels better than Na+, but cannot replace Na+ at Na+-dependent transporters. Li+ should augment Na+-selective responses and fail to support Na+-dependent responses. Replacing external Na+ with Tris+ completely eliminated the caffeine-induced current (Fig. 1 B). The average magnitude of the caffeine-induced current was −694 ± 349 pA in ASW and −38 ± 23 pA in Tris ASW, a significant 95% reduction (SD; n = 4; paired t test, P < 0.05). By dividing the current by the cell capacitance, we obtained the current density under each condition. The current density was 7 ± 4 pA/pF in ASW and 0.4 ± 0.2 pA/pF in Tris+ ASW (Fig. 1 E). In some cells, a small (0.7 ± 0.6 pA/pF; SD; n = 16) chloride-selective component that activated earlier than the large inward current was observed. The early Cl− current was blocked by 100 μM niflumic acid, reversed at ECl during ion substitution experiments, and was unaffected by Tris+ substitution (data not shown).
Replacing external Na+ with Li+ also eliminated the caffeine-induced inward current (Fig. 1 D). Fig. 1C and Fig. D, shows traces from a single squid ORN treated with 10 mM caffeine in normal ASW and Li+ ASW, respectively. In this cell, the maximum amplitude of the current was reduced from −807 to −22 pA. In normal ASW, these caffeine-induced currents averaged −788 ± 316 pA (SD; n = 5) and had an average current density of 8 ± 1 pA/pF at −50 mV. However, in Li+ ASW, the size of the current was significantly reduced by 94% to −44 ± 50 pA and a density of 0.4 ± 0.3 pA/pF (SD; n = 5; paired t test, P < 0.01; Fig. 1 E). Inhibition by both Tris+ and Li+ indicates that the caffeine-induced inward current is Na+ dependent, as would be expected for forward Na+/Ca2+ exchange rather than a Na+-selective conductance such as a Ca2+-activated cation channel or a cyclic nucleotide–gated channel. The presence or absence of K+ in the internal solution did not affect the caffeine-induced current (Fig. 1 A), suggesting that the NCKX is not contributing to the caffeine-induced responses.
DCB Inhibits the Caffeine-activated Current
The dependence of caffeine-activated currents on external Na+, along with caffeine's ability to liberate internal calcium stores in squid ORNs (Piper and Lucero 1999), suggested to us that the depolarizing current could be due to an electrogenic sodium–calcium exchanger. To further confirm this hypothesis, we tested the effects of DCB, a blocker of sodium–calcium exchange (Frelin et al. 1988; Kleyman and Cragoe 1988). Fig. 2 A shows a control response to a 1-s application of 10 mM caffeine in ASW. The two current traces obtained in the presence of DCB are shown in Fig. 2 B. The bath was switched to ASW + 100 μM DCB for 12 s before the first caffeine application in the presence of DCB (Fig. 2 B). The first caffeine + DCB application elicited a reduced current response while the second, which was obtained 30-s later, showed no response. The effect of DCB on this cell was reversible as shown in Fig. 2 C. The caffeine-induced current after DCB wash was 181% of the control; however, the other cells tested either died during DCB washout or did not recover. For the 18 cells treated with caffeine and DCB, the sodium–calcium exchange current was significantly reduced by 95% from an average control current density of 11 ± 7 to 1 ± 1 pA/pF (SD; n = 18; paired t test; P < 0.001) (Fig. 2 D). This reduction was statistically significant. The block of the caffeine-induced responses by DCB, along with their elimination in the absence of external Na+, lead us to conclude that caffeine, by liberating internal calcium stores, activates an electrogenic Na+/Ca2+ exchanger, which generates net inward currents. This caffeine-induced Na+/Ca2+ exchange current will be referred to as INCX.
Time Course of INCX
In squid ORNs, odor responses peak within 300–400 ms (Danaceau and Lucero 2000). The onset of INCX, however, was delayed relative to odor-induced currents (Fig. 3A and Fig. B). The cell in Fig. 3 illustrates a typical example from a single cell of the delayed onset of the caffeine-induced currents relative to glutamate-induced currents. Note that the caffeine application is over before any NCX current is observed. The time to peak and time to half peak of glutamate-induced responses averaged 382 ± 106 ms (SD; n = 12) and 228 ± 64 ms, respectively, at −99 mV. In ASW, the times to peak and half peak of caffeine-induced INCX averaged 2.0 ± 0.4 and 1.6 ± 0.4 s, respectively, at −88 mV (SD; n = 9). We did not correct any data for the ∼90-ms delay between the beginning of the trigger of the solution changer and the arrival of test solutions at the cell (see materials and methods). Unlike voltage-gated ion channels, the time to peak for caffeine responses did not change significantly across the voltage range tested (−88 to +52 mV). The marked delay in the onset and time to peak of caffeine responses is presumably due to the time it takes caffeine to diffuse to calcium stores, and for store-released Ca2+ to reach high enough concentrations at the cell membrane to activate the exchanger.
INCX Is Reversible
Some Na+/Ca2+ exchangers are capable of acting in either forward or reverse mode (DiPolo 1989). In forward mode, three Na+ ions are exchanged for one Ca2+ ion, resulting in a net inward flow of current. In reverse mode, the stoichiometry remains the same, but the directions of transport and current flow are reversed. To characterize the reverse mode of Na+/Ca2+ exchange in squid ORNs, we combined whole-cell recording techniques with a high sodium internal solution that also included 4 mM Mg2+ and 2 mM ATP to counteract Na+-dependent inactivation of the exchanger (Collins et al. 1992). After achieving whole-cell access (R s = 42 ± 12 MΩ, SD; n = 11), the intracellular and pipette solutions were allowed to equilibrate for 13.5 ± 7.6 min before the external bath solution was switched from ASW to Tris+ ASW. The combination of high internal sodium, low internal calcium, zero external sodium, and 10 mM external calcium was expected to stimulate reverse Na+/Ca2+ exchange, resulting in extrusion of Na+, uptake of Ca2+, and an outward exchanger current. Under these conditions, the removal of external sodium stimulated outward currents (seen as a reduction in the steady state inward leak current) that we attribute to reverse Na+/Ca2+ exchange (Fig. 4 A). As expected, the inward current reduction observed upon removal of external Na+ was smaller when a Li+ internal solution was used (Fig. 4 B; R s = 34 ± 12 MΩ; equilibration time = 3.9 ± 1.9 min; n = 8). To quantify these results, we calculated the percent decrease in the magnitude of the resting inward current obtained when the external bath solution was switched from ASW to Tris+ ASW. With the Li+ internal solution, the steady state inward current was reduced by 26 ± 13% (SD; n = 9), a significantly smaller decrease than the 42 ± 13% (SD; n = 11) reduction measured with high Na+ internal solution (Independent Student's t test; P < 0.05; Fig. 4 C). Presumably, a longer more complete equilibration of the Li+ internal would have further reduced the effect on the inward current. Indeed, the cell shown in Fig. 4 B had equilibrated and the switch to Tris+ ASW had very little effect. These results indicate that reverse Na+/Ca2+ exchange can be stimulated by high internal Na+ concentrations combined with Na+ free external solution, and that reverse INCX can be reduced by replacing internal Na+ with Li+.
INCX Amplifies Odor-induced Currents
To determine if INCX plays a role in olfactory transduction, we tested its effect on glutamate-activated currents. Glutamate activates a nonselective, calcium-permeable conductance in squid ORNs (Danaceau and Lucero 2000), and increases in [Ca2+]i during glutamate responses might be sufficient to activate forward exchange. Fig. 5 A shows current responses of a single ORN treated with glutamate in the presence and absence of 50 μM DCB. In the presence of DCB, the size of the glutamate response in this cell was reduced by 44% from −149 to −83 pA. In the 18 cells tested, the size of the glutamate-induced current was reduced by 26 ± 10% (n = 18), a statistically significant decrease from control conditions (paired t test; P < 0.05; Fig. 5 B). We were able to obtain recovery (93 ± 10%) of the original glutamate-induced current in all nine cells that survived long enough for a second control application of glutamate. There was no significant difference between the first and second control glutamate-induced currents (paired t test; P > 0.05) confirming that the current reduction seen in the presence of DCB was not due to rundown. The averaged data in Fig. 5 B includes experiments where DCB was preapplied for 1 min (n = 10) and experiments where DCB was coapplied with glutamate (n = 8). There was no difference between data obtained under the two conditions, confirming that the brief preapplication of DCB was not elevating intracellular Ca2+, which could mediate the subsequent reduction in the glutamate response.
Figure 5.
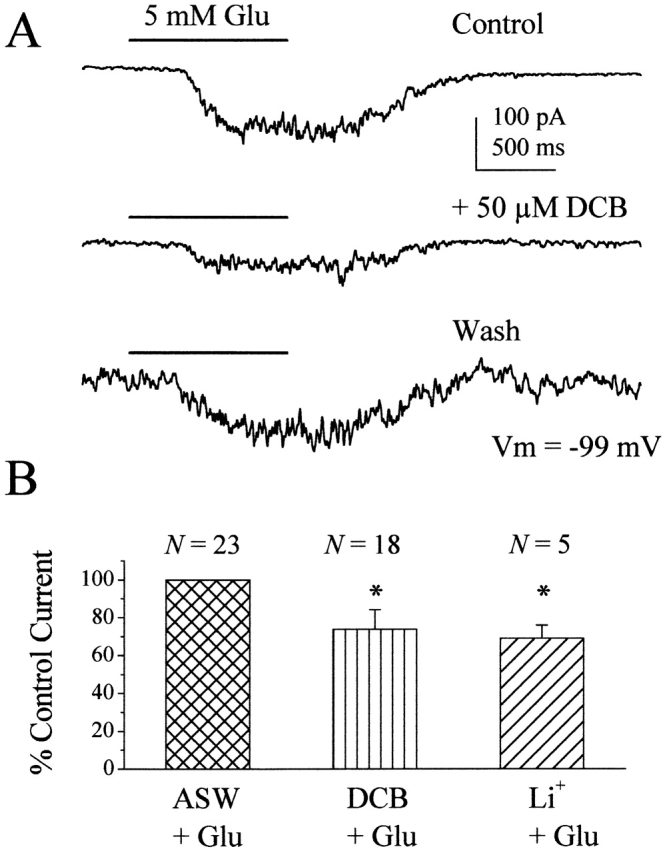
INCX amplifies odorant responses in squid ORNs. (A) Three successive responses to 5 mM glutamate, 5 mM glutamate in 50 μM DCB, and 5 mM glutamate, respectively, are shown for a nystatin-perforated patched squid ORN. This cell was exposed to DCB for 36 s before application of glutamate + DCB. When DCB is removed, the size of the glutamate-induced current returns to control levels. (B) The magnitude of the peak current was normalized to the peak of the control responses and plotted as a bar graph. DCB was either coapplied with glutamate (n = 8) or pre-incubated for 58 ± 21 s (n = 10). The combined DCB data show that the response to 5 mM glutamate is reduced by 26 ± 10% (n = 18; vertical stripes). In the absence of external Na+ (Li+ + Glu, diagonal stripes), 5 mM glutamate responses were reduced by 31 ± 7%. Error bars indicate SD. *P < 0.05. Solutions: ASW ext/60 TEA Na gluconate int.
Although 2′4′DCB has been established as an inhibitor of Na+/Ca2+ exchange, a closely related compound, 3′4′DCB can inhibit cyclic nucleotide–gated channels (Gomez and Nasi 1997). We were concerned that the current reductions shown in Fig. 5 could be resulting from a simple block of the glutamate-activated currents by 2′4′DCB. To eliminate this possibility, we compared glutamate responses in ASW and DCB to those in Li+ ASW. Fig. 5 B shows the average relative glutamate-induced currents in the presence of 50 μM DCB (DCB + Glu) and in the absence of external Na+ (Li++ Glu). In both conditions, we eliminated NCX and reduced the total glutamate-induced current by similar amounts. In Li+ ASW, the glutamate-induced current was reduced by 31 ± 7% (n = 5), a statistically significant decrease from control conditions (paired t test; P < 0.05), but not different from the 26 ± 10% reduction observed with DCB (independent t test, P > 0.05). The similarity in current reduction when using either Na-free ASW or DCB suggests that DCB is inhibiting INCX rather than blocking a channel.
To further confirm the specificity of DCB, we applied it during glutamate responses in Li+ ASW. Under these conditions, there should be no Na+/Ca2+ exchange because of the lack of external sodium. Thus, any reduction of the glutamate-activated currents would be due solely to block by DCB. Fig. 6A and Fig. B, shows glutamate-induced current traces from a single squid ORN in Li+ ASW in the absence and presence of DCB, respectively, and show that DCB has no effect on the size of glutamate-activated currents. The current responses from six cells were normalized to the response to glutamate alone and plotted in Fig. 6 C. In Li+ ASW, the magnitude of the responses to glutamate in the presence of DCB was 98 ± 14% of the responses to glutamate, a difference that was not significant at the P > 0.05 level. These results confirm that Na+/Ca2+ exchange acts to augment or amplify odor responses to glutamate in squid ORNs.
Figure 6.
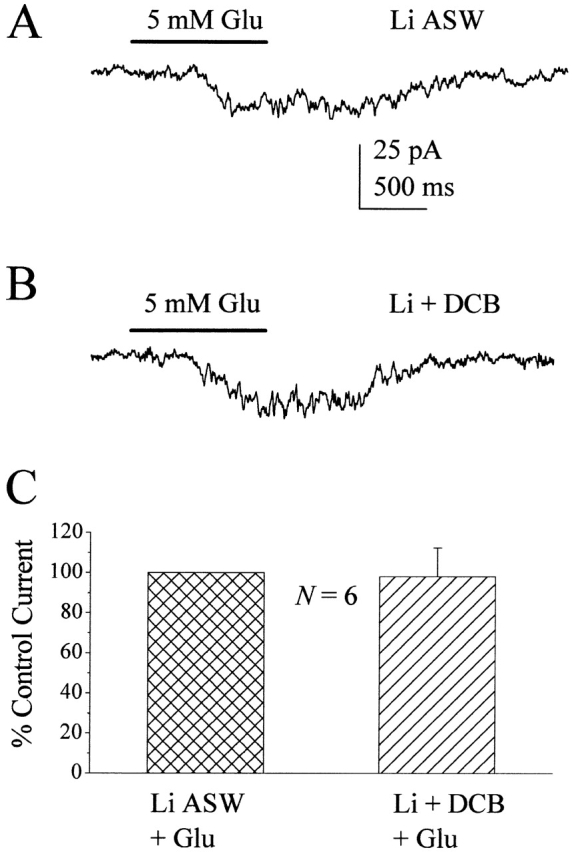
DCB has no effect on glutamate-induced currents in Li+ ASW. (A) Current trace from a nystatin-perforated patched squid ORN treated with 5 mmol liter−1 glutamate in Li+ ASW. (B) Current trace from the same cell in the presence of 50 μM DCB. Note that the magnitude of the current is unchanged. (C) Bar graph comparing the average, normalized current responses to glutamate from squid ORNs in Li+ ASW (crosshatch) and in Li+ ASW + 50 μM DCB (diagonal stripes). In the presence of DCB, responses to glutamate were 98 ± 14% of the control glutamate responses in Li+ ASW, and were not significantly different from each other (P > 0.05). Error bar indicates SD. Solutions: Li+ ASW ext/60 TEA Na+ gluconate int.
DISCUSSION
INCX Is Present in Squid ORNs
We have identified a sodium–calcium exchanger in squid ORNs that is activated by increases in intracellular calcium, dependent upon external sodium, inhibited by DCB, a blocker of sodium–calcium exchange, and which amplifies glutamate-induced inward currents. There was a significant delay in the onset of INCX activated by caffeine compared with odor responses. The sequential steps required for caffeine-induced INCX responses could cause this delay. First, caffeine must reach the cell and diffuse through the plasma membrane and the cytosol to reach intracellular Ca2+ stores. Then caffeine binds and activates ryanodine receptors that are presumably present and responsible for liberating calcium stores. Finally, the released calcium must diffuse through the cytosol to activate the sodium–calcium exchanger proteins. These steps are contrasted with odor transduction, where, presumably, all of the transduction machinery is located in very close proximity, minimizing diffusion times and resulting in faster kinetics. With regard to the amplification of glutamate responses by INCX, there was no delay seen for the portion of the current attributed to NCX. The glutamate-induced current and INCX were concurrent (see Fig. 5). These results suggest that some NCX proteins are located in very close proximity to the transduction channels for glutamate-induced odor responses, and that the delay between caffeine application and INCX is due to the several diffusion and binding steps mentioned above and not to any property of the sodium–calcium exchanger itself. In support of this hypothesis, our recent immunological study using a polyclonal antibody against the cloned squid NCX (NCX-SQ1) showed specific staining localized to the cilia of the pyriform cell type used in the present studies (Lucero et al. 2000).
Ionic Dependence of INCX
We have shown that forward Na+/Ca2+ exchange in squid ORNs is blocked by the replacement of external Na+ with either Tris+ or Li+. In addition, there does not appear to be any dependence upon internal K+. In all of the experiments except for those presented in Fig. 4, there was no K+ present in the internal pipette solutions. These ionic requirements are consistent with the NCX type Na+/Ca2+ exchangers, including the mammalian cardiac exchanger, NCX1, and the recently cloned NCX-SQ1 from squid (He et al. 1998; Hryshko and Philipson 1997). Furthermore, the lack of a requirement for internal K+ argues that this exchanger is not related to the Na+/Ca2+, K+ exchanger from rod photoreceptors (Cervetto et al. 1989; Reilander et al. 1992). Thus, squid ORNs possess a classical NCX-type Na+/Ca2+ exchanger.
Reversal of INCX
In addition to forward Na+/Ca2+ exchange stimulated by caffeine application, we have also demonstrated reverse Na+/Ca2+ exchange in squid ORNs. This, again, is consistent with results seen for other NCX-type exchangers and with the squid Na+/Ca2+ exchanger, NCX-SQ1 (He et al. 1998). Upon removal of external sodium, we observed a decrease in the inward holding current of squid ORNs in the presence of a high Na+ internal solution containing Mg2+ and ATP. We attribute the reduction in recorded inward current to the generation of a net outward current during reverse NCX. Under conditions that should have eliminated reverse NCX (Li+ internal solutions), the removal of external Na+ resulted in a significantly smaller reduction in the holding current compared with the Na+ internal solution. Incomplete dialysis of the Li+ internal solution may account for the residual reverse NCX observed. The difference in current reduction between the two internal conditions reflects the relative amount of reverse Na+/Ca2+ exchange generated by increasing internal Na+.
Amplification of Odor Responses
Olfactory receptor potentials in several species have been shown to consist of at least two current responses, an initial transduction current, followed by (or coupled with) an amplification current. In vertebrate ORNs, calcium-activated chloride currents amplify the initial transduction current (Kleene and Gesteland 1991; Kleene 1993; Kurahashi and Yau 1993; Lowe and Gold 1993; Kleene and Pun 1996; Kleene 1997; Nakamura et al. 1997; Reuter et al. 1998). Amplification of olfactory signals has been reported in invertebrates as well. In lobster, initial odor-activated conductances that are permeable to sodium and calcium (Fadool and Ache 1992) are amplified by sodium-activated cation channels (Zhainazarov and Ache 1997; Zhainazarov et al. 1998). In addition, a calcium-activated cation channel has been described in ORNs from the moth Manduca sexta that may be involved in augmenting the initial depolarizing odor response (Zufall et al. 1991). To our knowledge, this is the first report of direct amplification of an odor response by an electrogenic ion exchanger.
The reduction in the glutamate-induced current in the presence of Li+ or DCB was not due to a buildup of intracellular Ca2+ acting directly on the transduction machinery because coapplication of glutamate and DCB produced the same inhibition as a 1 min preincubation in DCB. In addition, we showed that in the absence of NCX, DCB had no effect on the glutamate-induced current (Fig. 6). Thus, the component of glutamate-induced current inhibited by DCB or Li+ application appears to be INCX and not an indirect effect of the experimental conditions.
In frog, sodium–calcium exchange appears to be involved in terminating olfactory responses. By removing [Ca]i, the size and duration of ICl(Ca) is reduced. Conversely, the duration of ICl(Ca) is prolonged when sodium–calcium exchange is blocked by eliminating external Na+ (Reisert and Matthews 1998). In the present study, we see an opposite role for sodium–calcium exchange. Instead of contributing towards adaptation, the squid sodium–calcium exchanger is involved in amplifying odor responses. Furthermore, we show that this amplification is due to the electrogenic properties of the exchanger itself, as opposed to Rana, where only the effects of removing [Ca2+]i were reported (Reisert and Matthews 1998).
Use of NCX during glutamate responses in squid ORNS results in a 1.5× amplification of the original Ca2+ signal. This observation can be verified algebraically. We start with the simple equation:
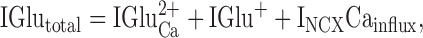 |
1 |
where IGlutotal = the total current recorded during glutamate application, IGluCa 2+ is the glutamate-induced current carried by Ca2+ influx through the transduction channel, IGlu+ is the glutamate-induced current carried by monovalent cations through the transduction channel, and INCXCa influx is the net inward current of the exchanger activated by Ca2+ influx. If we take the extreme case where Ca2+ carries all of the current that enters the transduction channel during a glutamate response, then IGlu+ reduces to zero and becomes:
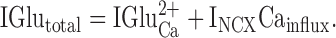 |
2 |
For every 2+ charges that enter the cell as Ca2+, 3+ charges enter as Na+ on NCX, resulting in a 1.5× amplification of the Ca2+ signal. Assuming that the only Ca2+ available to the exchanger is via influx, then
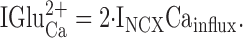 |
3 |
Since under the hypothetical conditions of a Ca2+-selective transduction channel, two thirds of IGlutotal is carried by Ca2+, we can substitute % values into , where ():
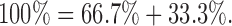 |
4 |
This means that if all of the current through the transduction channel were carried by Ca2+, then total block of INCX would reduce IGlutotal by 33.3%. If reduction of IGlutotal was <33.3% then, by substituting into 1 and rearranging we see that the contribution of monovalent cations (IGlu+) to the total glutamate-induced current is ():
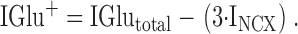 |
5 |
If block of INCX reduces IGlutotal by >33.3%, then, in addition to Ca2+ influx, Ca2+ released from intracellular stores must contribute to INCX (INCXCastores) and the total INCX (INCXtotal) becomes ():
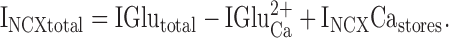 |
6 |
Rearranging and including contributions from monovalent cations gives the final form of the equation describing the glutamate-induced current ():
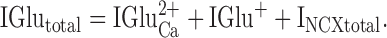 |
7 |
We showed that regardless of whether INCX was blocked by DCB or not supported by Li+ replacement of external Na+, IGlutotal was reduced on average by 26–31%. These findings indicate that the majority (70% or more) of the current through the glutamate-induced transduction channel is carried by Ca2+, and that there is a small contribution (22% or less) by monovalent cations. If we look at individual cells, we see that in some cells, INCX total contributes >40% of the current. In these cases, we suggest that Ca2+ released from intracellular stores is contributing to the total Ca2+ available to the exchanger and further amplifying the current. In general, the contribution of Ca2+ by store release was modest and only observed in a subset of cells (4/18 cells in DCB, 2/5 cells in Li+). Thus, activation of NCX during glutamate responses appears to be mainly dependent on Ca2+ influx through a glutamate-activated transduction channel (IGluCa 2+). These observations correlate well with our earlier studies showing that the glutamate-induced current in squid ORNs has a much higher selectivity for Ca2+ over Na+ or K+ (Danaceau and Lucero 2000). We do not know yet whether the glutamate-induced transduction channel is an integral part of the glutamate receptor (ionotropic receptor) or a cyclic nucleotide–gated channel; however, we do see similar currents with internal perfusion of cAMP (Lucero and Piper 1994).
In addition to depolarizing odor responses, squid ORNs respond to some odors (all aversive so far) with hyperpolarizing responses (Lucero et al. 1992; Danaceau and Lucero 1998). One hyperpolarizing odor, betaine, activates a chloride conductance that is calcium independent (Danaceau and Lucero 1998; Piper and Lucero 1999), while a different odor, dopamine, induced hyperpolarizing responses that are associated with increases in intracellular calcium (Piper and Lucero 1999). The sodium–calcium exchanger described in this paper could contribute to olfactory adaptation of a hyperpolarizing, calcium-dependent response in two ways. First, olfactory responses that are dependent upon increases in intracellular calcium would be attenuated as the exchanger cleared intracellular calcium from the cytosol. Second, the depolarizing electrogenic properties of the exchanger would oppose the hyperpolarizing responses elicited by dopamine, counteracting membrane hyperpolarization. Future experiments will be needed to investigate these hypotheses.
Acknowledgments
We thank J.D. Lucero, T. Dang, and Dr. C. Hegg for technical assistance and the National Resource Center for Cephalopods (Galveston, TX) for providing squids. We also thank Drs. W.C. Michel, H.M. Brown, D. Piper, and K. Spitzer for helpful comments on the manuscript.
This work was supported by National Institutes of Health (NIH) National Institute on Deafness and Other Communication Disorders grant DC02587 to M.T. Lucero.
Footnotes
Abbreviations used in this paper: ASW, artificial sea water; DCB - 2′,4′ dichlorobenzamil; ext, external; int, internal; NCKX, Na+/Ca2+, K+ exchanger; NCX, sodium calcium exchange; ORN, olfactory receptor neuron.
References
- Blaustein M.P., Lederer W.J. Sodium/calcium exchangeits physiological implications. Physiol. Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Borisy F.F., Ronnett G.V., Cunningham A.M., Juilfs D., Beavo J., Snyder S.H. Calcium/calmodulin-activated phosphodiesterase expressed in olfactory receptor neurons. J. Neurosci. 1992;12:915–923. doi: 10.1523/JNEUROSCI.12-03-00915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Lagnado L., Perry R.J., Robinson D.W., McNaughton P.A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989;337:740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Chen T.-Y., Yau K.-W. Direct modulation by Ca2+-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Collins A., Somlyo A.V., Hilgemann D.W. The giant cardiac membrane patch methodstimulation of outward Na–Ca exchange current by MgATP. J. Physiol. 1992;454:27–57. doi: 10.1113/jphysiol.1992.sp019253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaceau J.P., Lucero M.T. Betaine activates a hyperpolarizing chloride conductance in squid olfactory receptor neurons. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1998;183:225–235. doi: 10.1007/s003590050250. [DOI] [PubMed] [Google Scholar]
- Danaceau J.P., Lucero M.T. Mixture interactions of glutamate and betaine in single squid olfactory neurons. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 2000;186:57–67. doi: 10.1007/s003590050007. [DOI] [PubMed] [Google Scholar]
- DiPolo R. Calcium efflux from internally dialyzed squid giant axons. J. Gen. Physiol. 1973;62:575–589. doi: 10.1085/jgp.62.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R. Characterization of the ATP-dependent calcium efflux in dialyzed squid giant axons. J. Gen. Physiol. 1977;69:795–813. doi: 10.1085/jgp.69.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R. The sodium–calcium exchange in intact cells. In: Allen T.J.A., Noble D., Reuter H., editors. Sodium–Calcium Exchange. Oxford University Press; New York, NY: 1989. pp. 5–26. [Google Scholar]
- DiPolo R., Beaugé L. Effects of some metal-ATP complexes on Na+–Ca2+ exchange in internally dialyzed squid axons. J. Physiol. 1993;462:71–86. doi: 10.1113/jphysiol.1993.sp019544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Cardiac sarcolemmal Na/Ca-inhibiting peptides XIP and FMRF-amide also inhibit Na/Ca exchange in squid axons Am. J. Physiol. Cell Physiol. 363 1994. C307 C311a [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Effects of vanadate on MgATP stimulation of Na/Ca exchange support kinase-phosphatase modulation in squid axons Am. J. Physiol. Cell Physiol. 363 1994. C1382 C1391b [DOI] [PubMed] [Google Scholar]
- Fadool D.A., Ache B.W. Plasma membrane inositol 1,4,5-trisphosphate-activated channels mediate signal transduction in lobster olfactory receptor neurons. Neuron. 1992;9:907–918. doi: 10.1016/0896-6273(92)90243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S., Werblin F.S. Gated currents in isolated olfactory receptor neurons of the larval tiger salamander. Proc. Natl. Acad. Sci. USA. 1987;84:6292–6296. doi: 10.1073/pnas.84.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C., Barbry P., Vigne P., Chassande O., Cragoe E.J., Jr., Lazdunski M. Amiloride and its analogs as tools to inhibit Na+ transport via the Na+ channel, the Na+/H+ antiport and the Na+/Ca2+ exchanger. Biochimie. 1988;70:1285–1290. doi: 10.1016/0300-9084(88)90196-4. [DOI] [PubMed] [Google Scholar]
- Frings S., Seifert R., Godde M., Kaupp U.B. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide–gated channels. Neuron. 1995;15:169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Gleason E., Borges S., Wilson M. Control of transmitter release from retinal amacrine cells by Ca2+ influx and efflux. Neuron. 1994;13:1109–1117. doi: 10.1016/0896-6273(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Gomez M.P., Nasi E. Antagonists of the cGMP-gated conductance of vertebrate rods block the photocurrent in scallop ciliary photoreceptors. J. Physiol. 1997;500:367–378. doi: 10.1113/jphysiol.1997.sp022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallani M., Lynch J.W., Barry P.H. Characterization of calcium-activated chloride channels in patches excised from the dendritic knob of mammalian olfactory receptor neurons. J. Membr. Biol. 1998;161:163–171. doi: 10.1007/s002329900323. [DOI] [PubMed] [Google Scholar]
- He Z., Tong Q., Quednau B.D., Philipson K.D., Hilgemann D.W. Cloning, expression, and characterization of the squid Na+–Ca2+ exchanger (NCX-SQ1) J. Gen. Physiol. 1998;111:857–873. doi: 10.1085/jgp.111.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryshko L.V., Philipson K.D. Sodium-calcium exchangerecent advances. Basic Res. Cardiol. 1997;92:45–51. doi: 10.1007/BF00794067. [DOI] [PubMed] [Google Scholar]
- Juhaszova M., Shimizu H., Borin M., Yip R.K., Santiago E.M., Lindenmayer G.E., Blaustein M.P. Localization of the Na+–Ca2+ exchanger in vascular smooth muscle, and in neurons and astrocytes. Ann. NY Acad. Sci. 1996;779:318–335. doi: 10.1111/j.1749-6632.1996.tb44804.x. [DOI] [PubMed] [Google Scholar]
- Jung A., Lischka F.W., Engel J., Schild D. Sodium/calcium exchanger in olfactory receptor neurones of Xenopus laevis . Neuroreport. 1994;5:1741–1744. doi: 10.1097/00001756-199409080-00013. [DOI] [PubMed] [Google Scholar]
- Kleene S.J. Origin of the chloride current in olfactory transduction. Neuron. 1993;11:123–132. doi: 10.1016/0896-6273(93)90276-w. [DOI] [PubMed] [Google Scholar]
- Kleene S.J. High-gain, low-noise amplification in olfactory transduction. Biophys. J. 1997;73:1110–1117. doi: 10.1016/S0006-3495(97)78143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene S.J., Gesteland R.C. Calcium-activated chloride conductance in frog olfactory cilia. J. Neurosci. 1991;11:3624–3629. doi: 10.1523/JNEUROSCI.11-11-03624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene S.J., Gesteland R.C., Bryant S.H. An electrophysiological survey of frog olfactory cilia. J. Exp. Biol. 1994;195:307–328. doi: 10.1242/jeb.195.1.307. [DOI] [PubMed] [Google Scholar]
- Kleene S.J., Pun R.Y.K. Persistence of the olfactory receptor current in a wide variety of extracellular environments. J. Neurophysiol. 1996;75:1386–1391. doi: 10.1152/jn.1996.75.4.1386. [DOI] [PubMed] [Google Scholar]
- Kleyman T.R., Cragoe E.J. Amiloride and its analogs as tools in the study of ion transport. J. Membr. Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Kurahashi T., Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- Kurahashi T., Shibuya T. Ca2+-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Res. 1990;515:261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- Kurahashi T., Yau K.W. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993;363:71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T., Greer C.A., Shepherd G.M., Zufall F. Visualizing odor detection in olfactory cilia by calcium imaging. Ann. NY Acad. Sci. 1998;855:205–207. doi: 10.1111/j.1749-6632.1998.tb10567.x. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T., Ma M., Zufall F. Impaired odor adaptation in olfactory receptor neurons after inhibition of Ca2+/calmodulin kinase II. J. Neurosci. 1999;19:1–6. doi: 10.1523/JNEUROSCI.19-14-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T., Rand M.N., Shepherd G.M., Greer C.A., Zufall F. Calcium entry through cyclic nucleotide–gated channels in individual cilia of olfactory receptor cellsspatiotemporal dynamics. J. Neurosci. 1997;17:4136–4148. doi: 10.1523/JNEUROSCI.17-11-04136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G., Gold G.H. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366:283–286. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- Lucero M.T., Horrigan F.T., Gilly W.F. Electrical responses to chemical stimulation of squid olfactory receptor cells. J. Exp. Biol. 1992;162:231–249. [Google Scholar]
- Lucero M.T., Piper D.R. IP3 and cyclic nucleotides elicit opposite membrane potential changes in squid olfactory receptor neurons Chem. Senses. 19 1994. 509(Abstr.) [Google Scholar]
- Lucero M.T., Huang W., Dang T. Immunohistochemical evidence for the Na+/Ca2+ exchanger in squid olfactory neurons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000;In press doi: 10.1098/rstb.2000.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Nishida N., Kaneko H. Direct measurement of concentration of chloride in the newt olfactory cell Chem. Senses. 22 1997. 757 758(Abstr.) [Google Scholar]
- Noe J., Tareilus E., Boekhoff I., Breer H. Sodium/calcium exchanger in rat olfactory neurons. Neurochem. Int. 1997;30:523–531. doi: 10.1016/s0197-0186(96)00090-3. [DOI] [PubMed] [Google Scholar]
- Piper D.R., Lucero M.T. Calcium signalling in squid olfactory receptor neurons. Biol. Signals Recept. 1999;8:329–337. doi: 10.1159/000014606. [DOI] [PubMed] [Google Scholar]
- Reilander H., Achilles A., Friedel U., Maul G., Lottspeich F., Cook N.J. Primary structure and functional expression of the Na/Ca,K exchanger from bovine rod photoreceptors. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:1689–1695. doi: 10.1002/j.1460-2075.1992.tb05219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J., Matthews H.R. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor neurons. J. Gen. Physiol. 1998;112:529–535. doi: 10.1085/jgp.112.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter D., Zierold K., Schroder W.H., Frings S. A depolarizing chloride current contributes to chemoelectrical transduction in olfactory sensory neurons in situ. J. Neurosci. 1998;18:6623–6630. doi: 10.1523/JNEUROSCI.18-17-06623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Zhao A.Z., Chan G.C.K., Baker L.P., Impey S., Beavo J.A., Storm D.R. Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in neuronsa mechanism for attenuation of olfactory signals. Neuron. 1998;21:495–504. doi: 10.1016/s0896-6273(00)80561-9. [DOI] [PubMed] [Google Scholar]
- Zhainazarov A.B., Ache B.W. Gating and conduction properties of a sodium-activated cation channel from lobster olfactory receptor neurons. J. Membr. Biol. 1997;156:173–190. doi: 10.1007/s002329900199. [DOI] [PubMed] [Google Scholar]
- Zhainazarov A.B., Doolin R.E., Ache B.W. Sodium-gated cation channel implicated in the activation of lobster olfactory receptor neurons. J. Neurophysiol. 1998;79:1349–1359. doi: 10.1152/jn.1998.79.3.1349. [DOI] [PubMed] [Google Scholar]
- Zufall F., Hatt H., Keil T.A. A calcium-activated nonspecific cation channel from olfactory receptor neurones of the silkmoth Antheraea polyphemus . J. Exp. Biol. 1991;161:455–468. doi: 10.1242/jeb.161.1.455. [DOI] [PubMed] [Google Scholar]