Abstract
Like few other organs, the skin is continuously exposed to multiple exogenous and endogenous stressors. Superimposed on this is the impact of psychological stress on skin physiology and pathology. Here, we review the “brain–skin connection,” which may underlie inflammatory skin diseases triggered or aggravated by stress, and we summarize relevant general principles of skin neuroimmunology and neuroendocrinology. Specifically, we portray the skin and its appendages as both a prominent target of key stress mediators (such as corticotropin-releasing hormone, ACTH, cortisol, catecholamines, prolactin, substance P, and nerve growth factor) and a potent source of these prototypic, immunomodulatory mediators of the stress responses. We delineate current views on the role of mast cell-dependent neurogenic skin inflammation and discuss the available evidence that the skin has established a fully functional peripheral equivalent of the hypothalamic–pituitary–adrenal axis as an independent, local stress response system. To cope with stress-induced oxidative damage, the skin and hair follicles also express melatonin, probably the most potent neuroendocrine antioxidant. Lastly, we outline major, as-yet unmet challenges in cutaneous stress research, particularly in the study of the cross-talk between peripheral and systemic responses to psychological stress and in the identification of promising molecular targets for therapeutic stress intervention.
Psychological stress is a prevalent aspect of life, usually triggered by a stimulus (stressor), which induces a reaction in the brain (stress perception). Subsequently, additional physiological systems are activated in the body, for example, the immune, endocrine, and nervous systems (stress response) (Cacioppo et al., 1998). According to Walter Cannon's hypothesis, the stress response is an evolutionarily adaptive psychophysiological survival mechanism that allows the individual to react to an acute stress-or — for example, a predator — with either “fight or flight,” and to react to exposure to chronic stress by saving energy (Cannon, 1914). However, stressors have changed, and the present connotation of stress does not include the survival of the individual based on the putative preparedness to fight or evade predators, or to save energy. Hence, pathophysiological changes associated with the stress response are misrouted and serve as aggravating or triggering factors in the pathogenesis of many diseases, for example, inflammatory, autoimmune, and allergic diseases (Qiu et al., 1999; Chandler et al., 2002; Blois et al., 2005). In a general sense, the term “stress” is widely used, for example in the context of exposure to environmental stressors, as depicted in Figure 1. In this review, we focus predominantly on psychological stress; however, as a relationship between environmental stress and psychological stress is highly suggestive, a discussion of this form of stress has also been included (Figure 1).
Figure 1. Brain–skin cross-talk upon exposure to psychological stress or environmental stress factors.
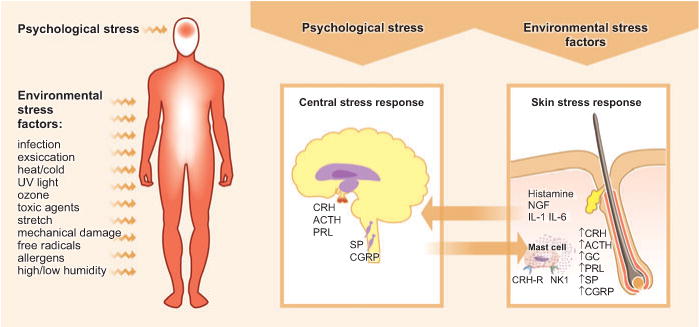
Upon perception of psychological stress, the central stress response leads to the activation of the hypothalamic–pituitary–adrenal axis, which causes the release of corticotropin-releasing hormone (CRH), ACTH, and prolactin (PRL). Further, an upregulation of substance P (SP) and calcitonin gene-related peptide (CGRP) can be observed in the dorsal root ganglia. Such stress response patterns may be translated into a skin stress response, including the local production of CRH, ACTH, and glucocorticoids (GCs), the release of inflammatory cytokines, and the sprouting of SP+ nerve fibers. In the skin response to stress, mast cells occupy a central switchboard position, as they are targets for stress-triggered factors as well as effector cells that contribute, for example, to neurogenic inflammation in the skin. Environmental factors are also capable of inducing a skin stress response, and this may be signaled to the brain, where it affects behavior and leads to an increased vulnerability to additional stress perception. NGF, nerve growth factor.
Systemic response patterns to stress: neuro-endocrine-immune circuitry
Activation of neurohormones by psychological stress occurs largely via the hypothalamic–pituitary–adrenal (HPA) axis, with subsequent upregulation of key stress hormones, such as corticotropin-releasing hormone (CRH), ACTH, and glucocorticoids (Cacioppo et al., 1998; Glaser and Kiecolt-Glaser, 2005). Via these stress-related hormones, accompanied by additional stress response mediators such as neuropeptides or neurotrophins (Webster, 2002), immune responses are profoundly altered (Glaser and Kiecolt-Glaser, 2005). For example, glucocorticoids inhibit the production of IL-12, IFN-γ, and tumor necrosis factor by antigen-presenting cells and T helper 1 (Th1) cells but upregulate the production of IL-4, IL-10, and IL-13 by Th2 cells (Wonnacott and Bonneau, 2002). This may induce the selective suppression of the Th1-mediated cellular immunity and trigger a shift toward Th2-mediated humoral immunity. It has been postulated that this Th2 shift may actually protect the organism from systemic “overshooting” with Th1/proinflammatory cytokines that have tissue-damaging potential (Elenkov et al., 1999). Besides such often-cited immunosuppressive effects of glucocorticoids, relevant examples of proinflammatory actions of CRH — which triggers the release of glucocorticoids — have recently been introduced, for example, in inflammatory arthritis, where both CRH and urocortin have been identified in the joints (McEvoy et al., 2001).
Elegant experiments with hypophysectomized rats have further proven the effect of additional pituitary hormones — for example, prolactin (PRL) — on the immune system. In these studies, the lack of PRL resulted in abnormalities of the immune system, including increased thymic atrophy and lymphopenia, which could be reversed after grafting of syngeneic pituitary (Chikanza and Panayi, 1991). Interestingly, elevated PRL antagonizes apoptosis in thymocytes exposed to glucocorticoids in vivo, which suggests that, under conditions of increased glucocorticoids, such as during stress, elevated PRL functions physiologically to maintain survival and function of T lymphocytes (Krishnan et al., 2003).
Neurohormonal responses to stress also include an activation of the sympathetic nervous system with a subsequent increase of catecholamines, a phenomenon that has received much less attention than the stress-triggered activation of the HPA axis. For half a century it has been known that lymphoid organs are prominently innervated by noradrenergic nerve fibers (Dahlström et al., 1965), and evidence that has accumulated since then supports the idea that the immune system is regulated via the sympathetic nervous system and catecholamines at the regional, local, and systemic levels (Cacioppo et al., 1998). For example, lymphocytes express adrenergic receptors and respond to catecholamine stimulation with the development of stress-induced lymphocytosis, and distinct changes in lymphocyte trafficking, circulation, proliferation, and cytokine production (Sanders et al., 1997; Dhabhar, 2000).
Besides the classical stress-related neurohormones — such as the players of the HPA axis (including CRH, ACTH, and cortisol), PRL, and catecholamines — nerve growth factor (NGF) is now recognized as an important parameter in stress responses (Aloe et al., 2002). Besides its function as an important trophic factor for peptidergic and sympathetic neurons and their axon sprouting, NGF is increasingly recognized as a potent immunomodulator, promoting cross-talk between neuronal cells, glia, and immune cells and facilitating monocyte/macrophage migration through vascular endothelium (Levi-Montalcini et al., 1996).
Clinical relevance of stress in dermatology
The skin is exceptionally well suited to serve as a model organ for dissecting the complex neuro-endocrine-immune circuitry invoked during stress responses, for the following reasons:
Hardly any other organ is continuously exposed to such a wide range of stressors as the skin, the prototypic environmental interface organ of vertebrate life; and this exposure occurs 24 hours a day during our entire lifetime (Figure 1).
A special susceptibility of the skin to acute and/or chronic psychological stress has been described in a wide range of skin diseases, including pruritus, prurigo nodularis, atopic dermatitis, psoriasis, urticaria, acne vulgaris, lichen planus, alopecia areata, and telogen effluvium (Whitlock, 1976; Zouboulis and Böhm, 2004; Hadshiew et al., 2004; O'Leary et al., 2004).
Adaptive skin responses to acute stress, besides classical autonomic-nervous “stress indicators” of the skin such as flushing and sweating, include an enhanced skin immune function with increased intracutaneous extravasation of immunocompetent cells (Dhabhar, 2000) and increased mast-cell degranulation (Katsarou-Katsari et al., 2001).
The skin and its appendages are exquisitely well innervated, and their afferent signals to the central nervous system are relatively over-represented in the sensory cortex.
The skin and its appendages are capable of generating the same mediators that are used during systemic stress responses, and they have established a fully functional peripheral equivalent of the systemic, stress-activated HPA axis (Slominski and Wortsman, 2000; Ito et al., 2005) (Figure 2).
The course of skin disorders (listed in Figure 3) in response to stress and/or various stress-modulatory psychological or pharmacological interventions can easily be followed macroscopically, and skin biopsies for further in-depth histological, immunological, endocrinological, neurobiological, biochemical, and molecular analyses can easily be performed, thus greatly facilitating stress research in both humans and experimental animals.
Figure 2. Stress mediators and effector cells in the neuro-endocrine-immune environment of the skin.
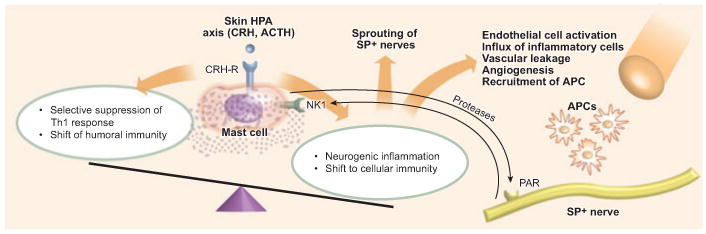
Activated mast cells may skew the immune response toward the selective suppression of an adaptive T helper 1 (Th1) response and encourage a shift to a Th2 bias, promoting humoral immunity via IL-4. On the other hand, mast-cell activation may lead to neurogenic inflammation of the skin and promote cellular immunity, accompanied by sprouting of substance P (SP)-positive nerve fibers, endothelial-cell activation, vascular leakage, and angiogenesis. Via the release of tryptase by mast cells, proteinase-activated receptors (PARs) are activated, which additionally enhance neurogenic inflammation and vascular leakage. Further, the capability of releasing corticotropin-releasing hormone (CRH) and the presence of CRH receptor (CRH-R) allow mast cells to contribute to the activation of the skin hypothalamic–pituitary–adrenal (HPA) axis. APC, antigen-presenting cell.
Figure 3. Mast cells: important regulators of neurogenic inflammation during stress responses.
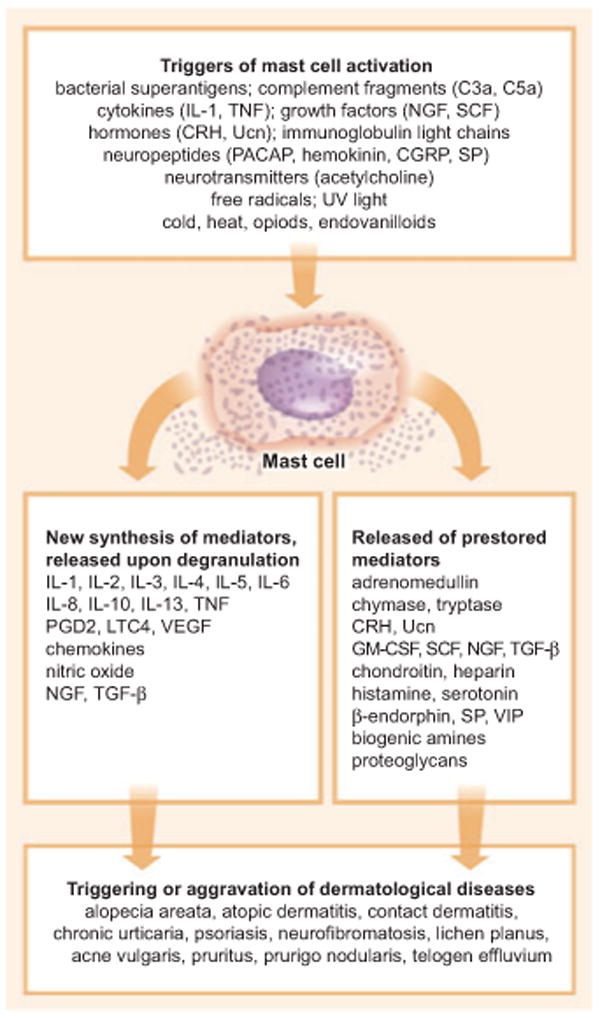
A multitude of mediators, the majority of which are released upon stress challenge, act as mast-cell activators. The activation subsequently results in the synthesis or the release of pre-stored mediators, which may then trigger a wealth of dermatological diseases. CGRP, calcitonin gene-related peptide; CRH, corticotropin-releasing hormone; cysLTs, cysteinyl leukotrienes; NGF, nerve growth factor; PACAP, pituitary adenylate cyclase-activating polypeptide; PGD2, prostaglandin D2; SCF, stem cell factor; SP, substance P; TGF-β, transforming growth factor-β; TNF, tumor necrosis factor; Ucn, urocortin; VEGF, vascular endothelial growth factor; VIP, vasoactive intestinal peptide.
The skin has established a fully functional peripheral equivalent of the hypothalamic–pituitary–adrenal axis
The skin has its own neuroendocrine system, which is tightly linked into systemic neuroendocrine axes, probably in order to coordinate peripheral responses to stress and to maintain cutaneous and global homeostasis (Slominski and Wortsman, 2000). These activities are organized by the intracutaneous neuroendocrine axes, composed chiefly of a cutaneous equivalent of the HPA axis (Slominski et al., 2002; Slominski et al., 2005b) (Figures 1 and 2). Recently, a fully functional peripheral equivalent of the HPA axis could be demonstrated in microdissected, organ-cultured human scalp hair follicles, which were capable of synthesizing cortisol and showing fully functional feedback controls (Ito et al., 2005).
Strikingly, human skin also expresses CRH and, additionally, urocortin mRNA and protein (Slominski et al., 2002). Murine skin contains CRH protein but has not been shown to contain CRH mRNA (though it does express both transcripts and protein for the CRH receptor ligand urocortin). Hence, the neural delivery of CRH into the skin must also be considered as a possible means by which CRH protein can be transported into the skin in a highly localized fashion (Roloff et al., 1998). Most recently, the expression of urocortin II (stresscopin-related peptide) mRNA in both human and mouse skin could be detected, and corresponding receptors for these ligands were also identified in skin cells (Pisarchik and Slominski, 2001). CRH-like immunoreactivity is further present in the dorsal horn of the spinal cord and dorsal root ganglia (Skofitsch et al., 1985), as well as in sympathetic ganglia (Merchenthaler et al., 1983). Strikingly, acute stress increases the skin content of CRH, which may derive from dorsal root ganglia (Theoharides et al., 2004).
Mapping of the cutaneous CRH signaling system in humans revealed that the CRH receptor type 1 (CRH-R1) is expressed in all major cellular populations of epidermis, dermis, and subcutis. CRH-R1 appears to be the most prevalent isoform of CRH-R, and the CRH-R2 gene is expressed solely in the dermis and adnexal structures. In mouse skin, the CRH-R2 gene and protein are widely expressed in all cutaneous compartments.
The pathophysiological relevance of CRH-R1 may be reflected by the observation that CRH-R1 is involved in stress-induced exacerbation of chronic contact dermatitis in rats (Kaneko et al., 2003). Interestingly, affected scalp skin areas from patients with alopecia areata, which may be precipitated by psychological stress, show increased expression of CRH-R2 around hair follicles (Katsarou-Katsari et al., 2001). Furthermore, affected skin areas from patients with contact dermatitis and chronic urticaria have increased expression of CRH-R1, as compared with normal control skin (TC Theoharides, unpublished data). Although the importance of CRH-R in the skin stress response is highly probable, the functional role of CRH-R expression in pathological human skin conditions remains to be identified.
Hypothalamic CRH production and secretion represent the most proximal end of the systemic HPA axis. CRH activates CRH-R1 and induces production and release of the proopiomelanocortin (POMC)-derived peptides ACTH, α-melanocyte-stimulating hormone, and β-endorphin in the pituitary gland (Slominski and Wortsman, 2000). ACTH, in turn, stimulates the production and secretion of cortisol or corticosterone by the adrenal cortex, which counteract the effect of the stressors by suppression of the HPA axis through a negative feedback mechanism (Slominski and Wortsman, 2000). Mammalian skin expresses POMC and produces the POMC-derived peptides β-endorphin, ACTH, α-melanocyte-stimulating hormone, and β-lipocortin. The first full demonstration of the cutaneous expression of POMC was accomplished in mouse skin (Slominski et al., 1991) and this was followed by insights from human skin (Schauer et al., 1994). The presence of β-endorphin, ACTH, α-melanocyte-stimulating hormone, and their modified forms in the skin was confirmed both by reverse-phase (RP)-HPLC separation and by liquid-chromatography mass spectrometry.
In light of the existence of a local, neuroendocrine skin axis, it has been proposed that the cutaneous defense against stressors is organized similarly to the classical HPA axis. Effectors of this axis (CRH, urocortin, and POMC peptides) are capable of regulating skin pigmentary, immune, epidermal, dermal, and adnexal systems (see Böhm et al., 2006, this issue). In this context, environmental challenges such as UV light and biological or chemical stress (Figure 1) trigger multiple pathways involving structuralized or simultaneous local production of CRH-related and POMC-derived messages (Slominski et al., 2005a), possibly to counteract the local effects of the environmental stress. This complex response would be susceptible to feedback inhibition by cortisol and/or corticosterone, which are produced locally in the skin (Slominski et al., 2005a; Ito et al., 2005).
The skin expresses the molecular apparatus necessary for the synthesis of adrenal and adrenal-like glucocorticosteroids. Specifically, production of deoxycorticosterone, corticosterone, and cortisol was documented in vitro (Slominski et al., 2005a). Hence there is now experimental evidence that CRH triggers a functional cascade structured hierarchically along the same algorithm as in the classical HPA axis: CRH activates CRH-R1, stimulates cAMP accumulation, and increases production of ACTH with enhanced production of cortisol and corticosterone in melanocytes, of corticosterone but not cortisol in fibroblasts (Slominski et al., 2005b), and of ACTH and cortisol in human scalp hair follicle epithelium in situ (Ito et al., 2005).
The skin expresses additional neuroendocrine stress-related signals: prolactin, melatonin, catecholamines
This intracutaneous equivalent of the systemic HPA axis is complemented by additional neuroendocrine activities of the skin, whose role in cutaneous stress responses remains to be determined. For example, murine skin and hair follicles as well as human scalp hair follicles also express PRL, another key neuroendocrine signal whose level rises sharply during psychological stress responses (Sonino et al., 2004).
In addition, the skin and its pilosebaceous units display a complete local serotoninergic/melatoninergic system (Slominski et al., 2005b), which includes the capacity to synthesize melatonin (Kobayashi et al., 2005), the most effective neurohormonal scavenger of reactive oxygen species (Fischer and Elsner, 2001). Whether or not recently discovered elements of a pituitary–thyroid axis, which are also expressed in the skin (Slominski et al., 2002), and the intracutaneous synthesis of catecholamines also feed into skin responses to psychological stress can currently only be speculated on and constitutes an important open question for future neuroendocrine and neuroimmunological research into cutaneous stress responses.
Skin mast cells and their functional role as “switchboards” of neurogenic inflammation during stress responses
Human mast cells are activated by a plethora of mast-cell secretagogues and other mediators (Figure 3) (Theoharides and Conti, 2004). This includes the stress hormones ACTH and CRH, as skin mast cells express five CRH-R1 isoforms as well as CRH-R2α (Pisarchik and Slominski, 2001). Strikingly, besides hair follicles, skin mast cells may be among the richest sources of CRH outside the brain (Kempuraj et al., 2001). Thus, skin mast cells not only are highly sensitive to modulation of their activities by classical stress hormones but also generate key stress hormones (at least CRH) themselves.
Conversely, once mast-cell degranulation has been induced by stress, the itch induced by one prototypic mast-cell product, histamine, elicits tonic sensory stimuli that differ from the central nervous system sensations triggered by pain and stimulates distinct cortical regions and brain processes (Walter et al., 2005). Thus, stress-induced mast-cell degranulation in the skin probably has immediate and prominent central nervous system effects, which may sustain a vicious, stress-induced cycle of proinflammatory events (Figures 2 and 3).
Mast-cell activation is well known for its key role in allergic and anaphylactic reactions; furthermore, mast cells are capable of synthesizing and secreting over 50 biologically potent molecules, including most cytokines (Figure 3). Hence, mast cells may easily induce a multitude of tissue effects necessary for the initiation of inflammation (Figures 2 and 3), the most prominent being vasodilation, vascular endothelial molecule expression, and chemoattraction. In fact, mast cells have now been implicated in innate immunity (Galli et al., 2005). The vasodilatory, angiogenic, proinflammatory, and neurosensitizing mediators of mast cells include histamine, heparin, kinins, neuropeptides such as vasoactive intestinal peptide, and proteases such as chymase and tryptase (preformed), as well as chemoattractants, cytokines, growth factors, leukotrienes, prostaglandins, nitric oxide, stem cell factor, and vascular endothelial growth factor (Theoharides and Conti, 2004).
The most abundant mast-cell mediator is tryptase, which causes microvascular leakage, activation of proteinase-activated receptors, and inflammation, as well as activation of neuropeptide precursors and direct proteolytic damage. Mast cells are also particularly rich in tumor necrosis factor and IL-6, both of which are implicated in inflammation and could perpetuate local inflammatory processes in response to psychological, chemical, mechanical, or oxidative stress.
Mast cells are located perivascularly in close proximity to neurons, and a functional association between mast cells and neurons has been reported (Arck et al., 2003a). Mast cells could also be activated by antidromic nerve stimulation, as shown for trigeminal or cervical ganglion stimulation that may result in neuropeptide secretion (Dimitriadou et al., 1991). Substance P (SP)-reactive fibers were localized close to mast cells (Arck et al., 2003b), and release of SP from sensory afferents could stimulate mast-cell secretion in vivo.
In contrast to the degranulation typically observed in anaphylactic reactions, in which most of the 500 secretory granules (1,000 nm each in diameter) release their contents in an explosive manner, mast cells can undergo secretion of the content of individual granules. Mast cells also have the ability to release some mediators selectively without degranulation. This unique phenomenon was described first for biogenic amines and recently for IL-6 in response to the prototypic inflammatory cytokine IL-1; this process involves the selective release of IL-6, without histamine or tryptase, through small vesicles (50–70 nm each in diameter) (Kandere-Grzybowska et al., 2003). Such selective release of mediators could be involved in a variety of processes, including stress response, angiogenesis, and cancer growth (Theoharides and Conti, 2004). In fact, elevation of serum IL-6 in response to acute stress is entirely mast cell-dependent (Huang et al., 2003). Such selective release of mast-cell mediators is often associated with ultrastructural alterations of their electron dense granular core — which are indicative of secretion — but without degranulation; this process is termed “activation” (Kandere-Grzybowska et al., 2003).
Skin innervation and neurogenic inflammation in stress response
The skin is highly innervated by a plenitude of nerve fiber subpopulations, each carrying one or more neuronal mediators into the skin, which may be released upon central or peripheral stimulation (Peters et al., 2005). Skin nerve fibers also carry neuronal mediators, and the involvement of such neuronal mediators — that is, the sensory neuropeptide SP (Paus et al., 1994) or the neurotrophin NGF — in the stress response has recently been acknowledged (Peters et al., 2004).
Stress and the skin: working concepts
Established mouse models for investigation of the impact of psychological stress on skin homeostasis are now available (Arck et al., 2001; Arck et al., 2003b). In one model, exposure to sound stress severely impairs mechanisms of immune tolerance in various organs — for example, the Th1/Th2 cytokine ratio, the expression of indoleamine 2,3-dioxygenase, and the presence of CD4+CD25bright regulatory T cells (Blois et al., 2005). Further, leukocyte function-associated antigen-1 acts as a key peripheral blood marker for stress perception (Blois et al., 2005). This stress model is now widely used in neuroimmunological stress research, for example, to evaluate the effects of stress on bronchial hyperreactivity (Joachim et al., 2004) or on experimental colitis (Qiu et al., 1999). Using this model, it has been shown that stress exerts severe skin inflammation, affecting, for instance, hair growth or the aggravation of experimental atopic dermatitis (Arck et al., 2003b; Peters et al., 2005). In a distinct, complementary rodent stress model, mice exposed to foot shock showed similar inhibition of hair growth in a similar way (Aoki et al., 2003).
In this accepted murine model of chronic “psychological” stress, exposure to sound stress induces neurogenic skin inflammation characterized by increased NGF expression, upregulated perifollicular mast-cell degranulation, and perifollicular accumulation of antigen-presenting cells, thus inhibiting hair growth by downregulating proliferation and upregulating apoptosis of hair follicle keratinocytes and by prematurely triggering hair follicle regression (catagen) (Arck et al., 2001; Peters et al., 2004). In the absence of functional mast cells or the neurokinin-1 receptor (Arck et al., 2005), and in the presence of neurokinin-1 receptor blockers (Arck et al., 2003b) or NGF-neutralizing antibodies (Peters et al., 2004), exposure to sound stress fails to induce premature catagen entry, neurogenic skin inflammation, and keratinocyte apoptosis. Intriguingly, stress exposure is associated with an upregulation not only in the number of intracutaneous SP+ nerve fibers, but also in SP immunoreactivity in dorsal root ganglia, as measured by retrograde tracing (Peters et al., 2004). Therefore, in this model, psychological stress activates a defined, hierarchically organized cascade of events in which NGF, SP, and mast cells play key roles. It remains to be determined whether CRH and/or ACTH reside upstream of NGF and/or SP.
The obvious question raised by these findings is how NGF and/or SP trigger or exacerbate deleterious inflammatory events such as mast-cell activation or migration of antigen-presenting cells. Indeed, NGF directly stimulates activation of mast cells via functional neurotrophin receptors (Marshall et al., 1999), thus aggravating cutaneous neurogenic inflammation (Figure 2). Furthermore, in response to stress, mast cells may secrete proteinases such as tryptase and trypsin, which may subsequently trigger additional cytokine release, cell migration, recruitment of leukocytes, and endothelial-cell activation (Steinhoff et al., 2000). Tryptase-releasing mast cells can be found in close proximity to proteinase-activated receptor-2-expressing cells such as keratinocytes, dermal endothelial cells, and C fibers during inflammation.
These mechanisms probably act synergistically with NGF and SP to upregulate the neurogenic inflammation cascade. Moreover, the release of other neuropeptides — for example, calcitonin gene-related peptide — may also be upregulated upon stress exposure (A Kuhlmei and P C Arck, unpublished observations), and this may result in subsequent proteinase-activated receptor activation. Further, NGF is also implicated in the HPA axis (Scaccianoce et al., 1993). Hence, stress-induced neurogenic skin inflammation may affect the HPA axis, thus affecting behavior or leading to even higher stress perception (Figure 1).
Perspectives: long-term damage of skin stem cells induced by stress?
Future research in the analysis of cutaneous stress response patterns should focus on crucial skin-cell populations that may be especially vulnerable to stress and that are critical to skin homeostasis and regeneration. One such cell population could be hair follicle epithelial stem cells, which are vital for maintenance and cyclic renewal of the pilosebaceous apparatus (Panteleyev et al., 2001). Sound stress in mice upregulates the number of apoptotic cells in the bulge region of the hair follicle and attracts dense, potentially autodestructive perifollicular cell infiltrates consisting mainly of activated macrophages to this crucial epithelial stem-cell region of the hair follicle (Arck et al., 2001; Arck et al., 2003). This places the hair follicle at risk of irreversible damage by induction of programmed hair follicle organ deletion.
Theoretically, stress may also affect melanocyte stem cells — for example, in the hair follicle during premature, “stress-induced” graying or alopecia areata, or in the epidermis during vitiligo, a depigmentation disorder that, in a minority of patients, may be triggered or aggravated by acute psychoemotional stress (Nishimura et al., 2005).
Perspectives: from bench to bedside
No specific pharmacological interventions are as yet available to prevent or treat stress-triggered skin disorders in humans. However, based on the wealth of data that has recently accumulated in this field, reasonable pharmacological treatment options are slowly coming into sight.
Abrogation of mast-cell activation seems to be a promising approach in this endeavor, but to date, few if any clinically available molecules can effectively inhibit mast-cell activation. Disodium cromoglycate was shown to inhibit rodent mast cells but was a very weak inhibitor of mast-cell cytokine release (Kempuraj et al., 2002). Increasing recent evidence indicates that certain flavonols, such as quercetin, are powerful inhibitors of both prestored and newly synthesized mediators from human mast cells. The combination of such flavonoids with proteoglycans, such as chondroitin sulphate, appears to provide synergistic efficacy by inhibiting both activation and secretion of mast cells (Theoharides and Bielory, 2004). Appropriate CRH-R antagonists, when available, might also provide a unique therapeutic approach in skin conditions precipitated or worsened by stress.
An effective therapeutic intervention — for example, to abrogate stress-triggered telogen effluvium — would have to prolong the anagen phase of the hair cycle, thus preventing the premature onset of catagen, which forms the basis of stress-induced telogen effluvium. As external application of the potassium channel opener minoxidil to human scalp skin over many months can prolong anagen, it is interesting to note that topical minoxidil also very effectively counteracts the stress-induced, hair growth-inhibitory changes in murine skin in vivo, including the inhibition of mast-cell activation (Arck et al., 2003a).
Further, the prototypic stress-associated neuropeptide SP may be blocked by the application of a high-affinity neurokinin-1 receptor antagonist (Arck et al., 2003b). Thus, neurokinin-1 receptor antagonists might be useful in alleviating stress-induced hair loss and skin inflammation. NGF receptor p75 antagonists also deserve systematic exploration as candidate “anti-stress” drugs in the treatment of stress-triggered or stress-aggravated skin disorders, such as psoriasis and stress-induced telogen effluvium. However, one evidently must be very cautious in translating results from murine models to humans.
In conclusion, there is now solid evidence for the existence of both an intrafollicular equivalent of the HPA axis and a defined brain–skin axis, whose modulation by chronic psychological stress is of profound dermatological interest. A new era in neuroimmunology must begin, and the primary goal is clearly to dissect proximal from distal mediators within the hierarchy of factors released in the stress response. Only integrative approaches and interdisciplinary research will make this possible.
Lastly, dermatologists should become far more attentive to the effect of psychological stress on skin disorders, not only for the benefit of their patients, but also because the skin serves as a very clinically relevant model system for exploring the neuroimmunology of peripheral and central stress responses.
Editor's Note.
Several laboratories worldwide have independently revealed that our skin is an unexpectedly prominent target organ for numerous neuroendocrine, neurotrophic, neurotransmitter, and neuropeptide signals, which have a profound impact on skin biology in health and disease. Even more importantly, they have identified the skin as a potent factory for the same signals. This, and the recognition that skin and nervous system share a common molecular syntax and jointly exploit the immune system to provide additional signaling and regulatory input, are beginning to revolutionize how we think about skin. As a consequence, a new specialty — cutaneous neuroendocrinology — has emerged that explores the “brain–skin connection,” overlapping richly with the inseparable fields of skin neurobiology, neuroimmunology, and neuropharmacology.
The editors expect cutaneous neuroendocrinology to develop into one of the fastest-moving, most exciting branches of skin research, impacting the course of classical neuro- and psychodermatology, and our understanding of pruritus and the role of psychoemotional stress in many inflammatory skin diseases. It will also influence the development of novel (adjuvant?) strategies for the management of skin diseases long recognized for their neuronal influences (for example, psoriasis, atopic dermatitis, lichen planus, and prurigo nodularis). Therefore, we have organized two groups of Perspectives articles. The first installment sketches the broad outlines of the field by focusing on the impact of psychoemotional stress on the skin (Arck et al.), on the neurophysiology, neuroimmunology, and neuroendocrinology of itch (Steinhoff et al.), and on the central role of neurotrophins in skin biology (Botchkarev et al.).
Next month's issue will complement this with in-depth coverage of cholinergic, adrenergic, and peptidergic signaling, and the role of melanocortin receptor ligands.
Ralf Paus
Acknowledgments
The writing of this review and the authors' own experimental work cited herein were made possible by grants provided by the German Research Foundation to P.C.A., E.M.J.P., and R.P., as well as grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (National Institutes of Health, USA) to T.C.T. and from the National Institutes of Health (USA) to A.S.
Abbreviations
- CRH
corticotropin-releasing hormone
- CRH-R
CRH receptor
- HPA
hypothalamic–pituitary–adrenal
- NGF
nerve growth factor
- POMC
proopiomelanocortin
- PRL
prolactin
- SP
substance P
- Th
T helper
Footnotes
CONFLICT OF INTEREST: The authors state no conflict of interest.
References
- Aloe L, Alleva E, Fiore M. Stress and nerve growth factor: findings in animal models and humans. Pharmacol Biochem Behav. 2002;73:159–66. doi: 10.1016/s0091-3057(02)00757-8. [DOI] [PubMed] [Google Scholar]
- Aoki E, Shibasaki T, Kawana S. Intermittent foot shock stress prolongs the telogen stage in the hair cycle of mice. Exp Dermatol. 2003;12:371–7. doi: 10.1034/j.1600-0625.2002.120403.x. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a ‘brain-hair follicle axis (BHA)’: inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J. 2001;15:2536–8. doi: 10.1096/fj.00-0699fje. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Kuhlmei A, Peters EM, Knackstedt M, Peter A, et al. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med. 2005;83:386–96. doi: 10.1007/s00109-004-0627-z. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Peters EM, Hagen E, Klapp BF, Paus R. Topical minoxidil counteracts stress-induced hair growth inhibition in mice. Exp Dermatol. 2003a;12:580–90. doi: 10.1034/j.1600-0625.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Arck PC, Handjiski B, Peters EM, Peter AS, Hagen E, Fischer A, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003b;162:803–14. doi: 10.1016/S0002-9440(10)63877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blois SM, Tometten M, Kandil J, Hagen E, Klapp BF, Margni RA, et al. ICAM-1/LFA-1 cross talk is a proximate mediator capable of disrupting immune integration and tolerance mechanism at the feto-maternal interface in murine pregnancies. J Immunol. 2005;174:1820–9. doi: 10.4049/jimmunol.174.4.1820. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, et al. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Ann NY Acad Sci. 1998;840:664–73. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The emergency function of the adrenal medulla in pain and the major emotions. Am J Physiol. 1914;33:356–72. [Google Scholar]
- Chandler N, Jacobson S, Esposito P, Connolly R, Theoharides TC. Acute stress shortens the time of onset of experimental allergic encephalomyelitis (EAE) in SJL/J mice. Brain Behav Immun. 2002;16:757–63. doi: 10.1016/s0889-1591(02)00028-4. [DOI] [PubMed] [Google Scholar]
- Chikanza IC, Panayi GS. Hypothalamic-pituitary mediated modulation of immune function: prolactin as a neuroimmune peptide. Br J Rheumatol. 1991;30:203–7. doi: 10.1093/rheumatology/30.3.203. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Mya-Tu M, Fuxe K, Zetterström BE. Observations on adrenergic innervation of dog heart. Am J Physiol. 1965;209:689–92. doi: 10.1152/ajplegacy.1965.209.4.689. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann NY Acad Sci. 2000;917:876–93. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC. Trigeminal sensory fiber stimulation induces morphologic changes reflecting secretion in rat dura mast cells. Neuroscience. 1991;44:97–112. doi: 10.1016/0306-4522(91)90253-k. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Webster EL, Torpy DJ, Chrousos GP. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann NY Acad Sci. 1999;876:1–11. doi: 10.1111/j.1749-6632.1999.tb07618.x. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Elsner P. The antioxidative potential of melatonin in the skin. Curr Probl Dermatol. 2001;29:165–74. doi: 10.1159/000060665. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–42. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–51. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Hadshiew IM, Foitzik K, Arck PC, Paus R. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol. 2004;123:455–7. doi: 10.1111/j.0022-202X.2004.23237.x. [DOI] [PubMed] [Google Scholar]
- Huang M, Pang X, Karalis K, Theoharides TC. Stress-induced interleukin-6 release in mice is mast cell-dependent and more pronounced in Apolipoprotein E knockout mice. Cardiovasc Res. 2003;59:241–9. doi: 10.1016/s0008-6363(03)00340-7. [DOI] [PubMed] [Google Scholar]
- Ito N, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–4. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- Joachim RA, Sagach V, Quarcoo D, Dinh QT, Arck PC, Klapp BF. Neurokinin-1 receptor mediates stress-exacerbated allergic airway inflammation and airway hyperresponsiveness in mice. Psychosom Med. 2004;66:564–71. doi: 10.1097/01.psy.0000132878.08780.93. [DOI] [PubMed] [Google Scholar]
- Kandere-Grzybowska K, Letourneau R, Boucher W, Bery J, Kempuraj D, Poplawski S, et al. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol. 2003;171:4830–6. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Kawana S, Arai K, Shibasaki T. Corticotropin-releasing factor receptor type 1 is involved in the stress-induced exacerbation of chronic contact dermatitis in rats. Exp Dermatol. 2003;12:47–52. doi: 10.1034/j.1600-0625.2003.120106.x. [DOI] [PubMed] [Google Scholar]
- Katsarou-Katsari A, Singh LK, Theoharides TC. Alopecia areata and affected skin CRH receptor upregulation induced by acute emotional stress. Dermatology. 2001;203:157–61. doi: 10.1159/000051732. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Huang M, Kandere K, Boucher W, Letourneau R, Jeudy S, et al. Azelastine is more potent than olopatadine in inhibiting interleukin-6 and tryptase release from human umbilical cord blood derived cultured mast cells. Ann Allergy Asthma Immunol. 2002;88:501–6. doi: 10.1016/s1081-1206(10)62389-7. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Papadopoulou NG, Lytinas M, Huang M, Kandere-Grzybowska K, Madhappan B, et al. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–8. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kromminga A, Dunlop TW, Tychsen B, Conrad F, Suzuki N, et al. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005;19:1710–2. doi: 10.1096/fj.04-2293fje. [DOI] [PubMed] [Google Scholar]
- Krishnan N, Thellin O, Buckley DJ, Horseman ND, Buckley AR. Prolactin suppresses glucocorticoid-induced thymocyte apoptosis in vivo. Endocrinology. 2003;144:2102–10. doi: 10.1210/en.2003-0053. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Skaper SD, Dal Toso R, Petrelli L, Leon A. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514–20. doi: 10.1016/S0166-2236(96)10058-8. [DOI] [PubMed] [Google Scholar]
- Marshall JS, Gomi K, Blennerhassett MG, Bienenstock J. Nerve growth factor modifies the expression of inflammatory cytokines by mast cells via a prostanoid-dependent mechanism. J Immunol. 1999;162:4271–6. [PubMed] [Google Scholar]
- McEvoy AN, Bresnihan B, FitzGerald O, Murphy EP. Corticotropin-releasing hormone signaling in synovial tissue from patients with early inflammatory arthritis is mediated by the type 1a corticotropin-releasing hormone receptor. Arthritis Rheum. 2001;44:1761–7. doi: 10.1002/1529-0131(200108)44:8<1761::AID-ART311>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Hynes MA, Vingh S, Schally AV, Petrusz P. Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat spinal cord. Brain Res. 1983;275:373–7. doi: 10.1016/0006-8993(83)91001-6. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–4. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- O'Leary CJ, Creamer D, Higgins E, Weinman J. Perceived stress, stress attributions and psychological distress in psoriasis. J Psychosom Res. 2004;57:465–71. doi: 10.1016/j.jpsychores.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Panteleyev AA, Jahoda CA, Christiano CA. Hair follicle predetermination. J Cell Sci. 2001;114:3419–31. doi: 10.1242/jcs.114.19.3419. [DOI] [PubMed] [Google Scholar]
- Paus R, Heinzelmann T, Schultz KD, Furkert J, Fechner K, Czarnetzki BM. Hair growth induction by substance P. Lab Invest. 1994;71:134–40. [PubMed] [Google Scholar]
- Peters EM, Handjiski B, Kuhlmei A, Hagen E, Bielas H, Braun A, et al. Neurogenic inflammation in stress-induced termination of murine hair growth is promoted by nerve growth factor. Am J Pathol. 2004;165:259–71. doi: 10.1016/S0002-9440(10)63294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EM, Kuhlmei A, Tobin DJ, Muller-Rover S, Klapp BF, Arck PC. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav Immun. 2005;19:252–62. doi: 10.1016/j.bbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Pisarchik A, Slominski A. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–6. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- Qiu BS, Vallance BA, Blennerhassett PA, Collins SM. The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med. 1999;5:1178–82. doi: 10.1038/13503. [DOI] [PubMed] [Google Scholar]
- Roloff B, Fechner K, Slominski A. Hair cycle-dependent expression of corticotropin-releasing factor (crf) and crf receptors in murine skin. FASEB J. 1998;12:287–97. doi: 10.1096/fasebj.12.3.287. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol. 1997;158:4200–10. [PubMed] [Google Scholar]
- Scaccianoce S, Cigliana G, Nicolai R, Muscolo LA, Porcu A, Navarra D, et al. Hypothalamic involvement in the activation of the pituitary–adrenocortical axis by nerve growth factor. Neuroendocrinology. 1993;58:202–9. doi: 10.1159/000126534. [DOI] [PubMed] [Google Scholar]
- Schauer E, Trautinger F, Kock A, Schwarz A, Bhardwaj R, Simon M, et al. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93:2258–62. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skofitsch G, Zamir N, Helke CJ, Savitt JM, Jacobowitz DM. Corticotropin-releasing factor-like immunoreactivity in sensory ganglia and capsaicin sensitive neurons of the rat central nervous system: colocalization with other neuropeptides. Peptides. 1985;6:307–18. doi: 10.1016/0196-9781(85)90057-9. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Mazurkiewicz J. Pro-opiomelanocortin expression and potential function of pro-opiomelanocortin products during induced hair growth in mice. Ann NY Acad Sci. 1991;642:459–61. doi: 10.1111/j.1749-6632.1991.tb24417.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–87. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Kohn L, Ain KB, Venkataraman GM, Pisarchik A, et al. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol. 2002;119:1449–55. doi: 10.1046/j.1523-1747.2002.19617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005a;19:176–94. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005b;288:E701–6. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- Sonino N, Navarrini C, Ruini C, Fallo F, Boscaro M, Fava GA. Life events in the pathogenesis of hyperprolactinemia. Eur J Endocrinol. 2004;151:61–5. doi: 10.1530/eje.0.1510061. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–8. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Bielory L. Mast cells and mast cell mediators as targets of dietary supplements. Ann Allergy Asthma Immunol. 2004;93(Suppl):S24–34. doi: 10.1016/s1081-1206(10)61484-6. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol. 2004;25:235–41. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–8. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Walter B, Sadlo MN, Kupfer J, Niemeier V, Brosig B, Stark R, et al. Brain activation by histamine prick test-induced itch. J Invest Dermatol. 2005;125:380–2. doi: 10.1111/j.0022-202X.2005.23817.x. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–63. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Whitlock FA. Psychophysiological aspects of skin disease. In: Rook A, editor. Major Problems in Dermatology. Vol. 8. Saunders; London: 1976. pp. 1–36. [Google Scholar]
- Wonnacott KM, Bonneau RH. The effects of stress on memory cytotoxic T lymphocyte-mediated protection against herpes simplex virus infection at mucosal sites. Brain Behav Immun. 2002;16:104–17. doi: 10.1006/brbi.2001.0624. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Böhm M. Neuroendocrine regulation of sebocytes: a pathogenetic link between stress and acne. Exp Dermatol. 2004;13 4:31–5. doi: 10.1111/j.1600-0625.2004.00254.x. [DOI] [PubMed] [Google Scholar]


