Abstract
The role played by ribosomal conformation in codon-anticodon recognition has been studied using streptomycin as a probe, inasmuch as streptomycin is known to cause misreading of the genetic code. Changes in ribosomal structure have been followed by the method of hydrogen-tritium exchange. The results show that streptomycin induces two types of change in the hydrogen exchange pattern. At low molar ratios of streptomycin to ribosomes, a stimulation of the hydrogen exchange rate (“loosening” of ribosomal structure) is observed, with a small inhibition of polypeptide synthesis. As the streptomycin: ribosome ratio is increased, a maximum exchange rate is reached, after which the rate decreases (“tightening” of structure); in this region, inhibition of peptide synthesis increases sharply, and misreading of the code begins. None of these effects is observed with streptomycin-resistant ribosomes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIES J., GILBERT W., GORINI L. STREPTOMYCIN, SUPPRESSION, AND THE CODE. Proc Natl Acad Sci U S A. 1964 May;51:883–890. doi: 10.1073/pnas.51.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Davis B. D. Misreading of ribonucleic acid code words induced by aminoglycoside antibiotics. The effect of drug concentration. J Biol Chem. 1968 Jun 25;243(12):3312–3316. [PubMed] [Google Scholar]
- Davies J., Gorini L., Davis B. D. Misreading of RNA codewords induced by aminoglycoside antibiotics. Mol Pharmacol. 1965 Jul;1(1):93–106. [PubMed] [Google Scholar]
- Davies J., Jones D. S., Khorana H. G. A further study of misreading of codons induced by streptomycin and neomycin using ribopolynucleotides containing two nucleotides in alternating sequence as templates. J Mol Biol. 1966 Jun;18(1):48–57. doi: 10.1016/s0022-2836(66)80075-x. [DOI] [PubMed] [Google Scholar]
- ENGLANDER S. W. A HYDROGEN EXCHANGE METHOD USING TRITIUM AND SEPHADEX: ITS APPLICATION TO RIBONUCLEASE. Biochemistry. 1963 Jul-Aug;2:798–807. doi: 10.1021/bi00904a030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander S. W., Crowe D. Rapid microdialysis and hydrogen exchange. Anal Biochem. 1965 Sep;12(3):579–584. doi: 10.1016/0003-2697(65)90225-3. [DOI] [PubMed] [Google Scholar]
- FLAKS J. G., COX E. C., WHITE J. R. Inhibition of polypeptide synthesis by streptomycin. Biochem Biophys Res Commun. 1962 May 11;7:385–389. doi: 10.1016/0006-291x(62)90320-0. [DOI] [PubMed] [Google Scholar]
- FLAKS J. G., COX E. C., WITTING M. L., WHITE J. R. Polypeptide synthesis with ribosomes from streptomycin-resistant and dependent E. coli. Biochem Biophys Res Commun. 1962 May 11;7:390–393. doi: 10.1016/0006-291x(62)90321-2. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Nomura M. Initiation of protein synthesis: a critical test of the 30S subunit model. Nature. 1968 Jul 20;219(5151):232–235. doi: 10.1038/219232a0. [DOI] [PubMed] [Google Scholar]
- Heintz R., McAllister H., Arlinghaus R., Schweet R. Formation and function of the active ribosome complex. Cold Spring Harb Symp Quant Biol. 1966;31:633–639. doi: 10.1101/sqb.1966.031.01.082. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Meselson M., Raskas H. J. Cyclic dissociation into stable subunits and re-formation of ribosomes during bacterial growth. J Mol Biol. 1968 Jan 28;31(2):277–289. doi: 10.1016/0022-2836(68)90444-0. [DOI] [PubMed] [Google Scholar]
- Kaji H., Tanaka Y. Binding of dihydrostreptomycin to ribosomal subunits. J Mol Biol. 1968 Mar 14;32(2):221–230. doi: 10.1016/0022-2836(68)90006-5. [DOI] [PubMed] [Google Scholar]
- Kohler R. E., Ron E. Z., Davis B. D. Significance of the free 70 s ribosomes in Escherichia coli extracts. J Mol Biol. 1968 Aug 28;36(1):71–82. doi: 10.1016/0022-2836(68)90220-9. [DOI] [PubMed] [Google Scholar]
- Kurland C. G. The requirements for specific sRNA binding by ribosomes. J Mol Biol. 1966 Jun;18(1):90–108. doi: 10.1016/s0022-2836(66)80079-7. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Schlessinger D. Polyribosome metabolism in Escherichia coli. I. Extraction of polyribosomes and ribosomal subunits from fragile, growing Escherichia coli. J Mol Biol. 1966 Sep;20(1):123–143. doi: 10.1016/0022-2836(66)90122-7. [DOI] [PubMed] [Google Scholar]
- Modolell J., Davis B. D. Rapid inhibition of polypeptide chain extension by streptomycin. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1279–1286. doi: 10.1073/pnas.61.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
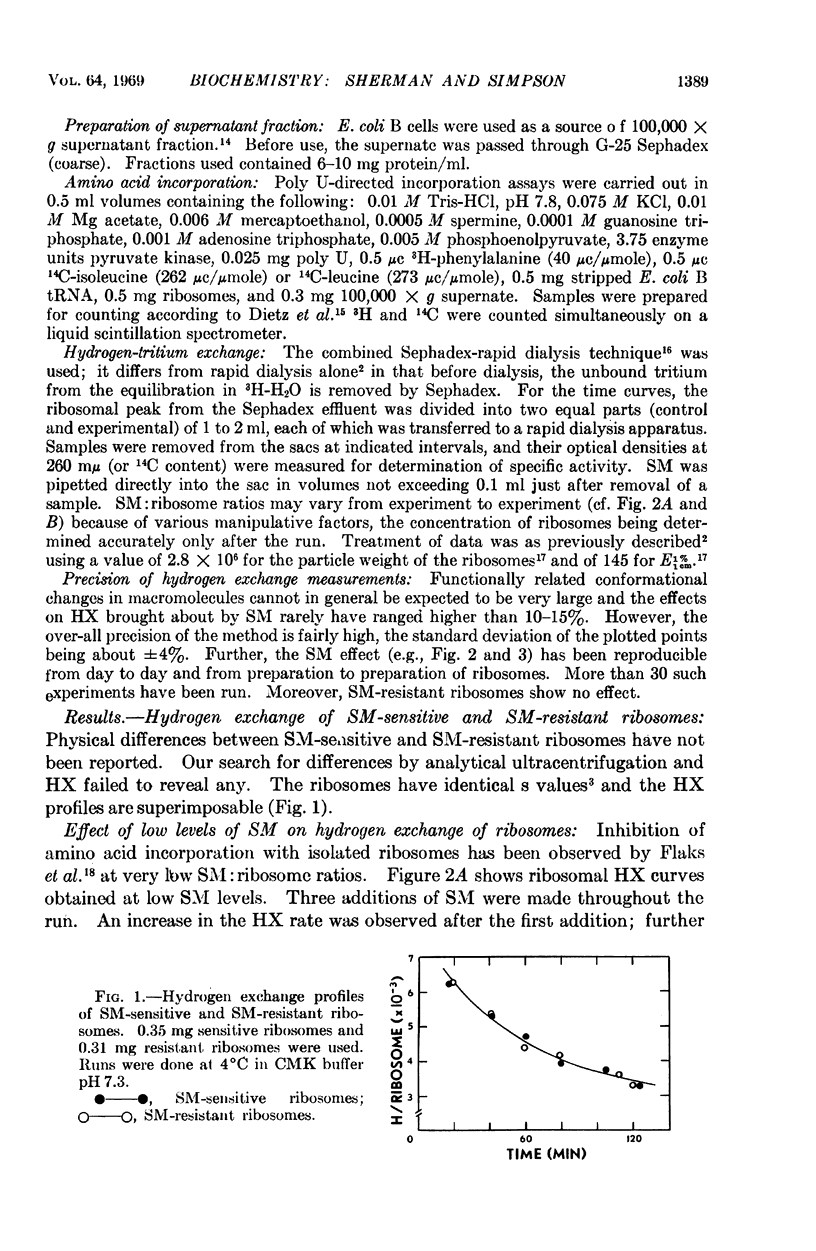
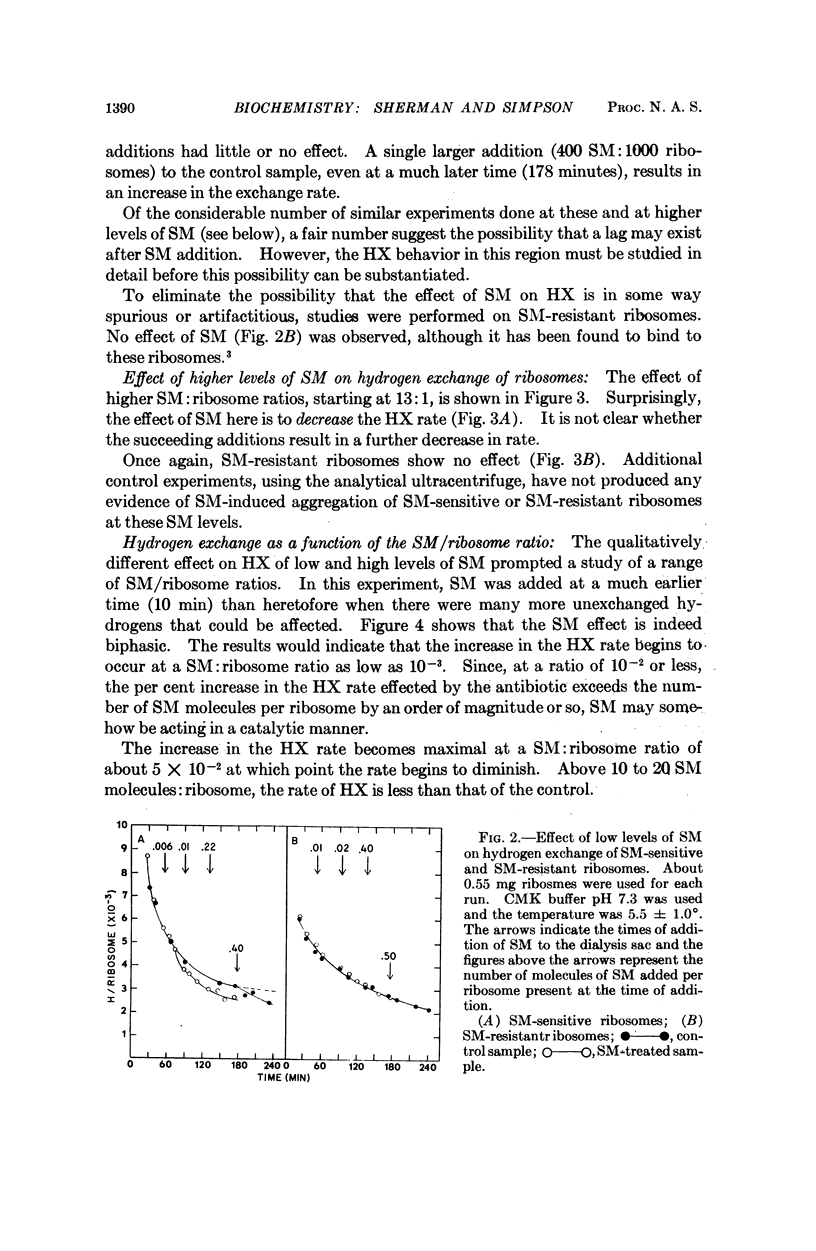
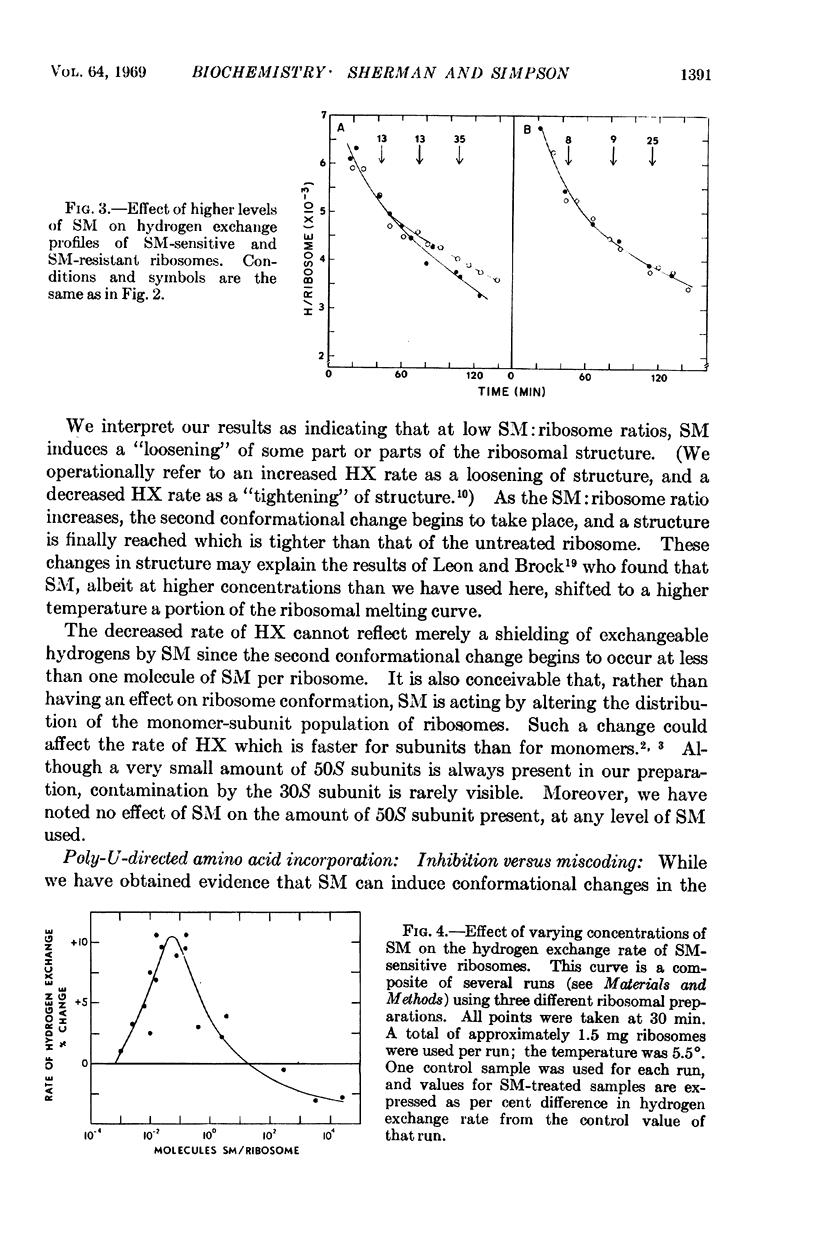
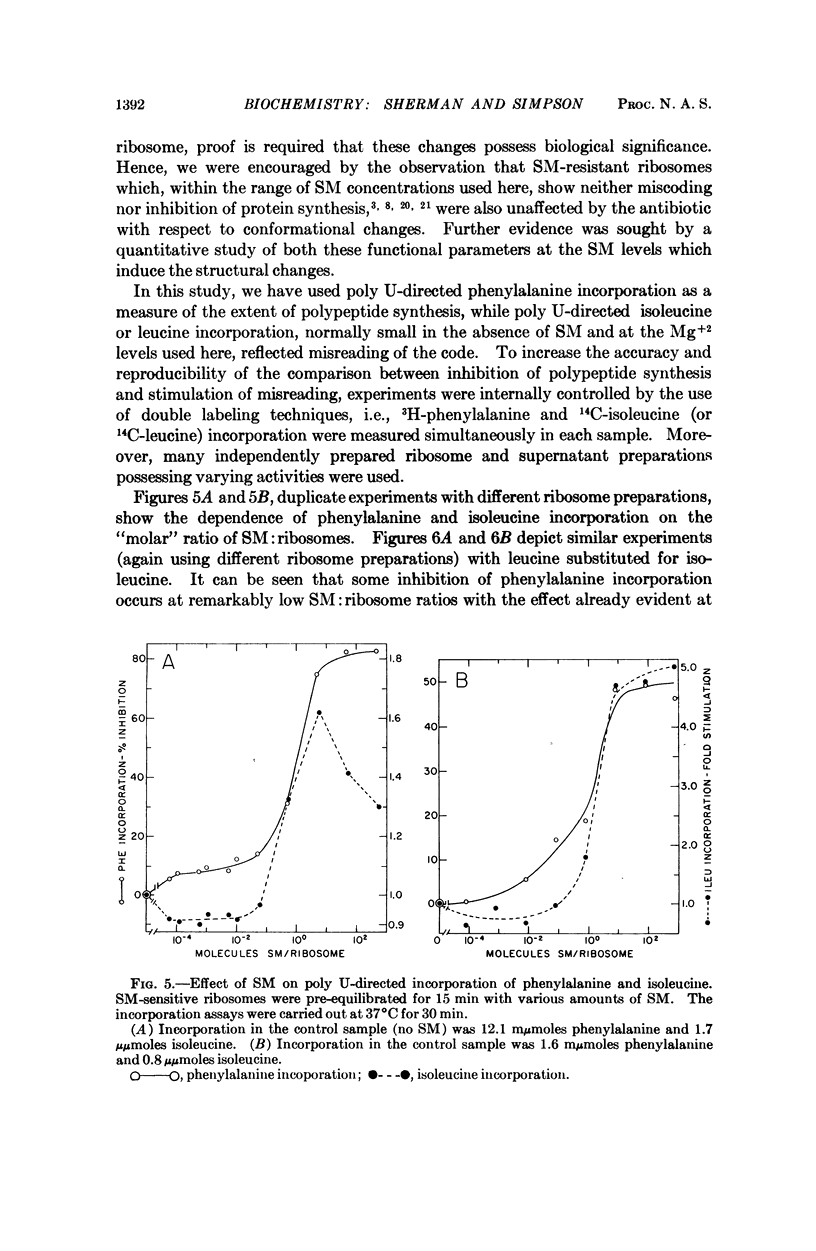
- Page L. A., Englander S. W., Simpson M. V. Hydrogen exchange studies on ribosomes. Biochemistry. 1967 Apr;6(4):968–977. doi: 10.1021/bi00856a003. [DOI] [PubMed] [Google Scholar]
- Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. I. The effect of streptomycin and ribosomal dissociation on 14-C-aminoacyl transfer ribonucleic acid binding to ribosomes. J Biol Chem. 1966 Jan 25;241(2):367–372. [PubMed] [Google Scholar]
- SPEYER J. F., LENGYEL P., BASILIO C. Ribosomal localization of streptomycin sensitivity. Proc Natl Acad Sci U S A. 1962 Apr 15;48:684–686. doi: 10.1073/pnas.48.4.684. [DOI] [PMC free article] [PubMed] [Google Scholar]



