Abstract
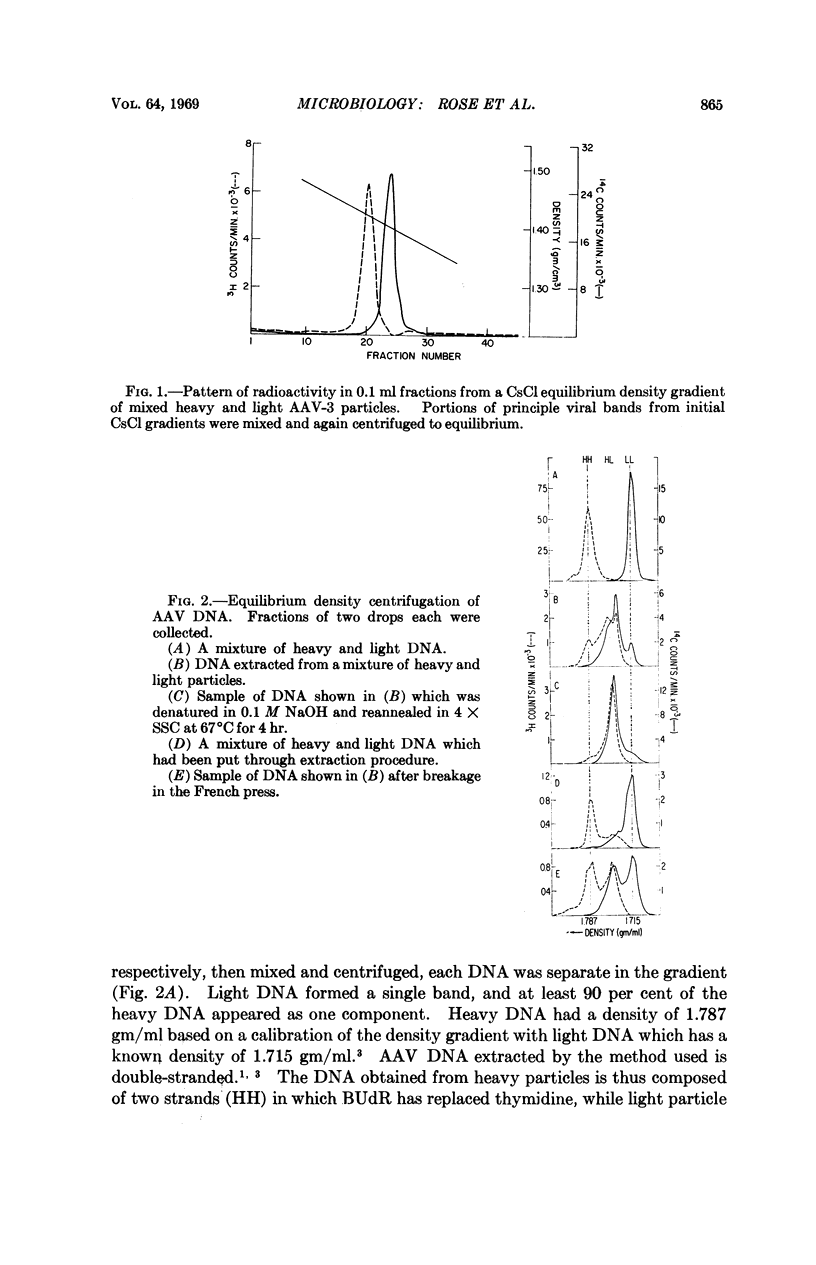
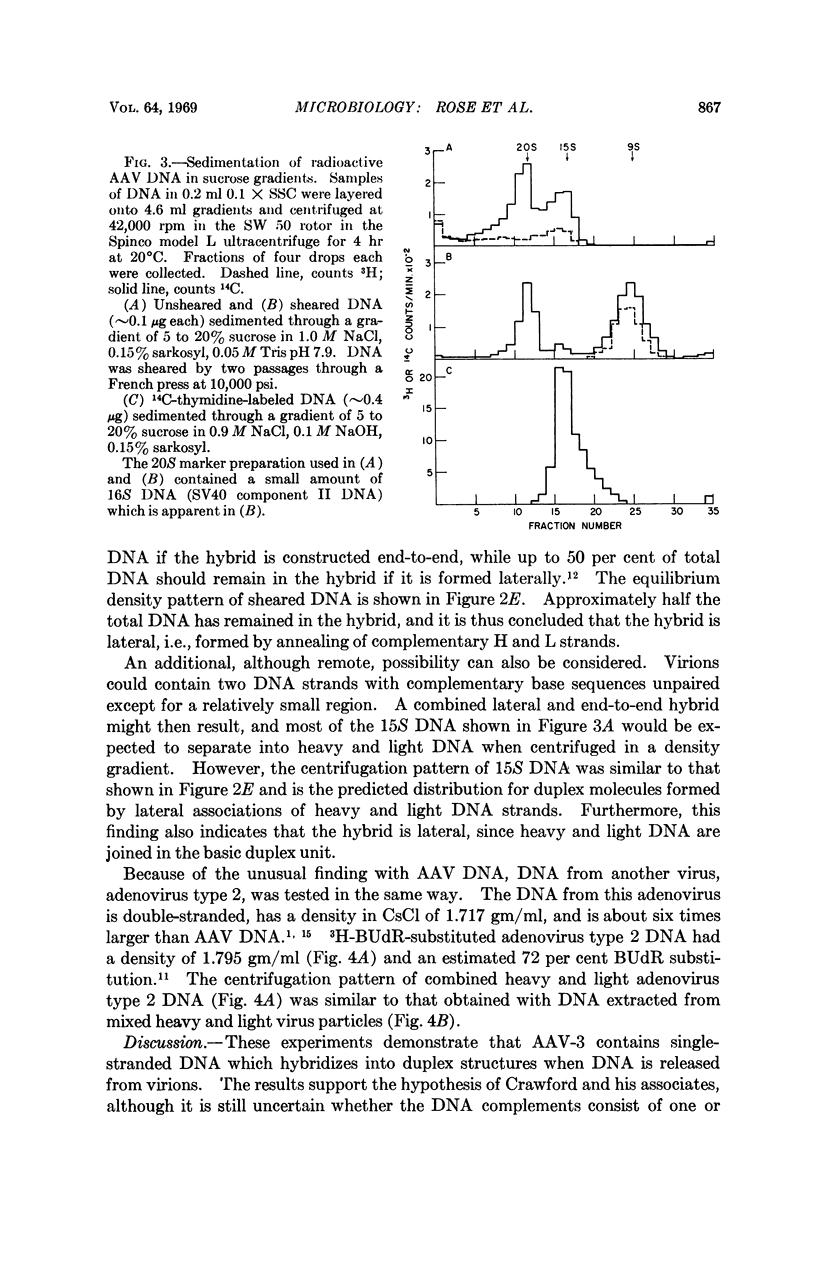
Extracted adenovirus-associated virus DNA is known to be double-stranded, and, therefore, it has been assumed that these virus particles contain a double-stranded genome. Recent findings, however, have suggested that the DNA in virus particles is equivalent to only half the molecular weight of extracted molecules. A density analysis of DNA extracted from a mixture of virus particles containing either bromodeoxyuridine-substituted or unsubstituted DNA shows that virions contain single-stranded DNA which, when released, forms duplex structures. A similar circumstance is as yet unknown among other viruses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- BALDWIN R. L., SHOOTER E. M. THE ALKALINE TRANSITION OF BU-CONTAINING DNA AND ITS BEARING ON THE REPLICATION OF DNA. J Mol Biol. 1963 Nov;7:511–526. doi: 10.1016/s0022-2836(63)80098-4. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Follett E. A., Burdon M. G., McGeoch D. J. The DNA of a minute virus of mice. J Gen Virol. 1969 Jan;4(1):37–46. doi: 10.1099/0022-1317-4-1-37. [DOI] [PubMed] [Google Scholar]
- Hershey A. D., Burgi E., Ingraham L. COHESION OF DNA MOLECULES ISOLATED FROM PHAGE LAMBDA. Proc Natl Acad Sci U S A. 1963 May;49(5):748–755. doi: 10.1073/pnas.49.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., Shatkin A. J., Blacklow N. R., Koczot F., Rose J. A. Helper-dependent infectious deoxyribonucleic acid from adenovirus-associated virus. J Virol. 1968 Aug;2(8):850–851. doi: 10.1128/jvi.2.8.850-851.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor H. D., Melnick J. L. Small deoxyribonucleic acid-containing viruses (picodnavirus group). Nature. 1966 Apr 16;210(5033):331–332. doi: 10.1038/210331a0. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Green M., Piña M., Melnick J. L. Physicochemical characterization of adeno-associated satellite virus type 4 and its nucleic acid. J Virol. 1967 Oct;1(5):980–987. doi: 10.1128/jvi.1.5.980-987.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Melnick J. L., Rongey R., Mayor H. D. Physical assay and growth cycle studies of a defective adeno-satellite virus. J Virol. 1967 Feb;1(1):171–180. doi: 10.1128/jvi.1.1.171-180.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROLFE R. The molecular arrangement of the conserved subunits of DNA. J Mol Biol. 1962 Jan;4:22–30. doi: 10.1016/s0022-2836(62)80113-2. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Koczot F., Shatkin A. J. Genetic relatedness studies with adenovirus-associated viruses. J Virol. 1968 Oct;2(10):999–1005. doi: 10.1128/jvi.2.10.999-1005.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Shatkin A. J. Nucleic acid from an adeno-associated virus: chemical and physical studies. Proc Natl Acad Sci U S A. 1966 Jul;56(1):86–92. doi: 10.1073/pnas.56.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Gehle W. D., Thiel J. F. Properties of a small virus associated with adenovirus type 4. J Immunol. 1966 Dec;97(6):754–766. [PubMed] [Google Scholar]



