Abstract
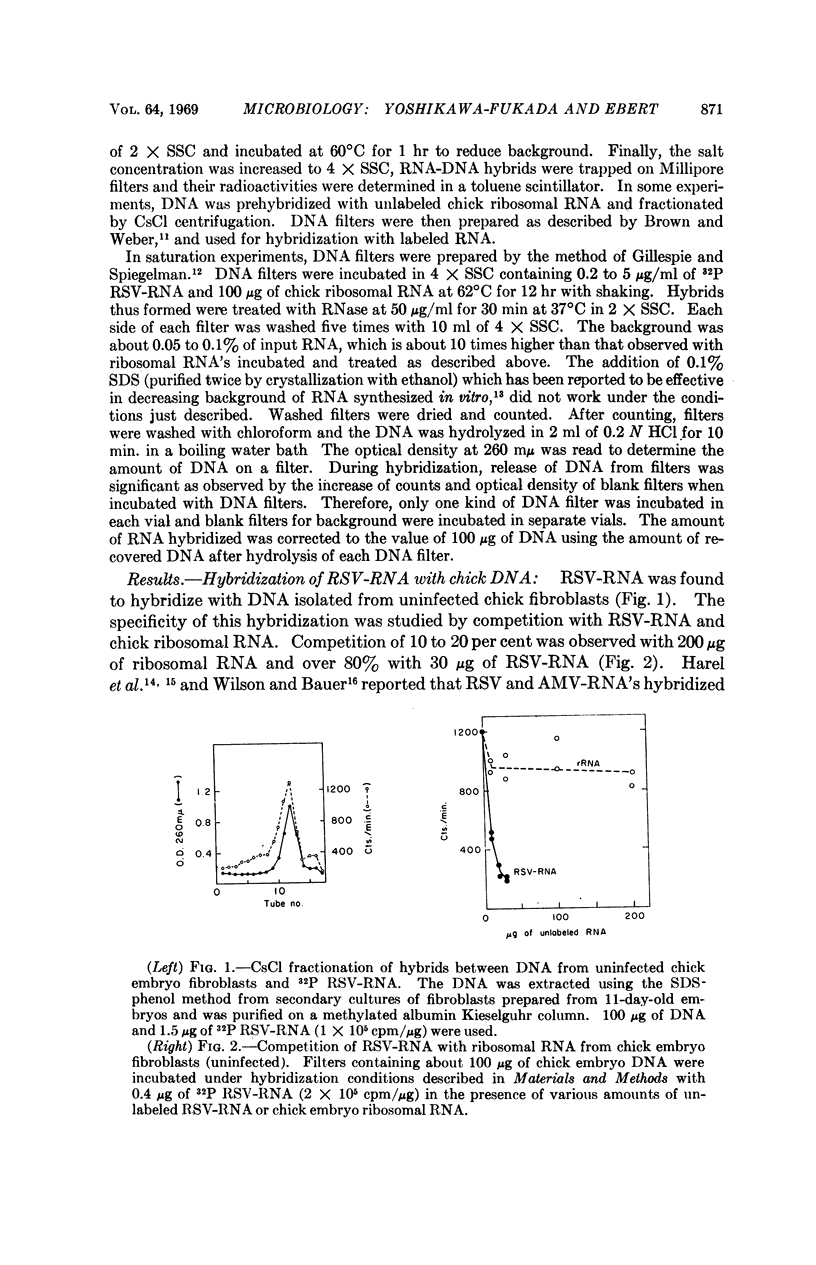
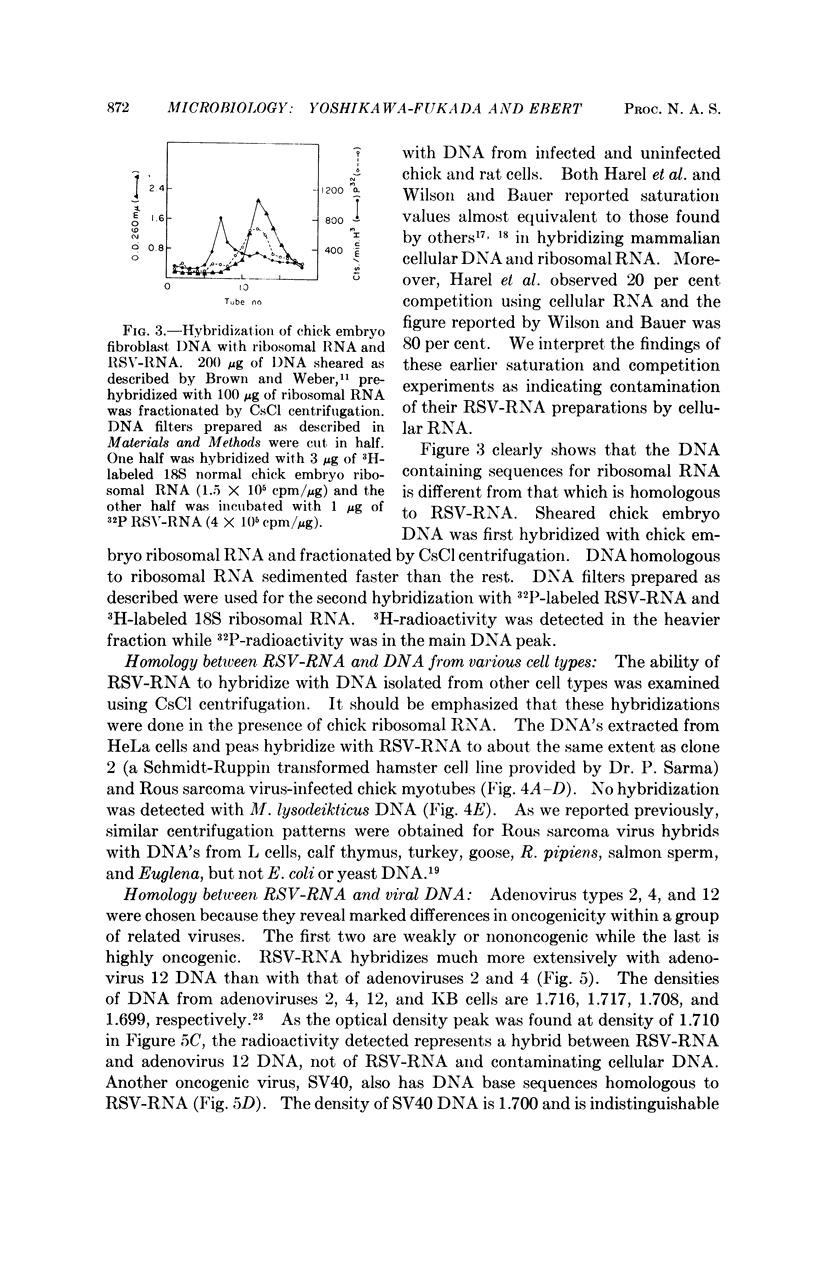
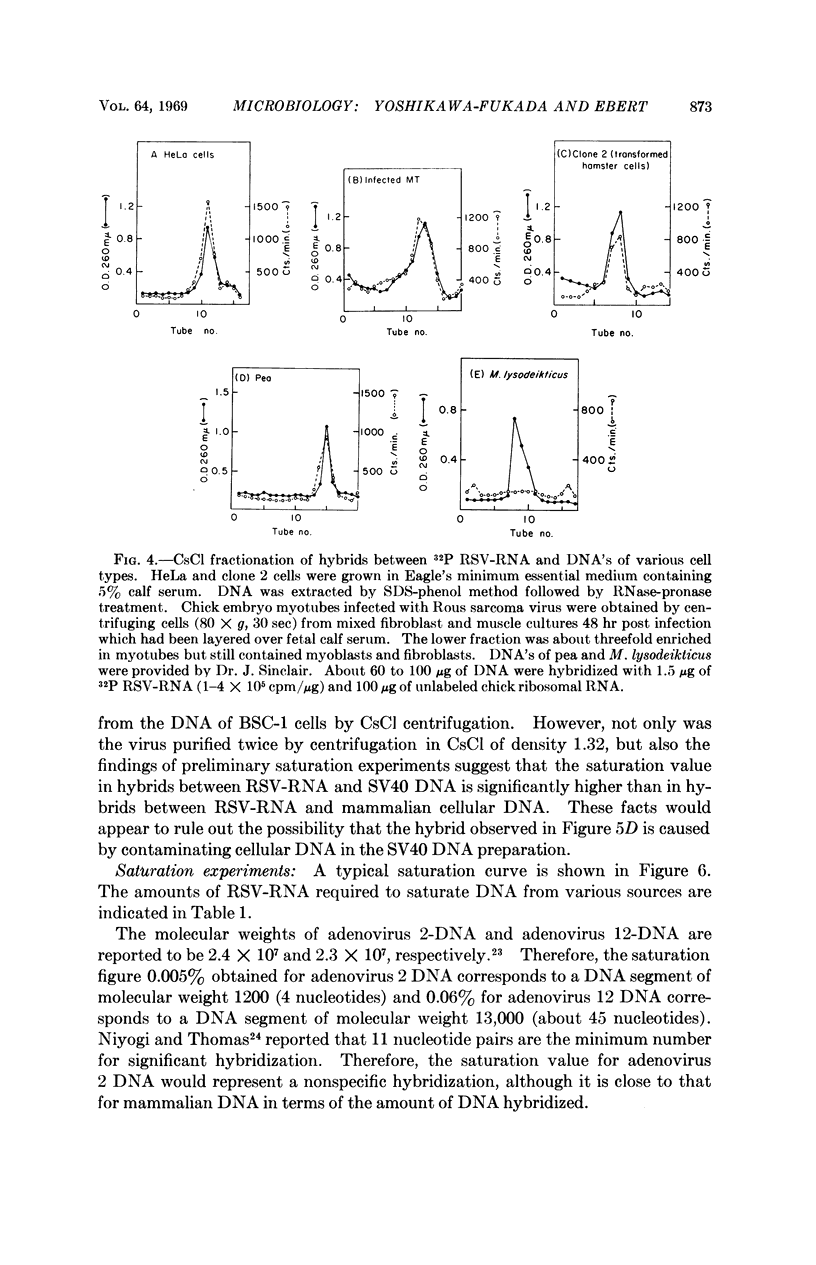
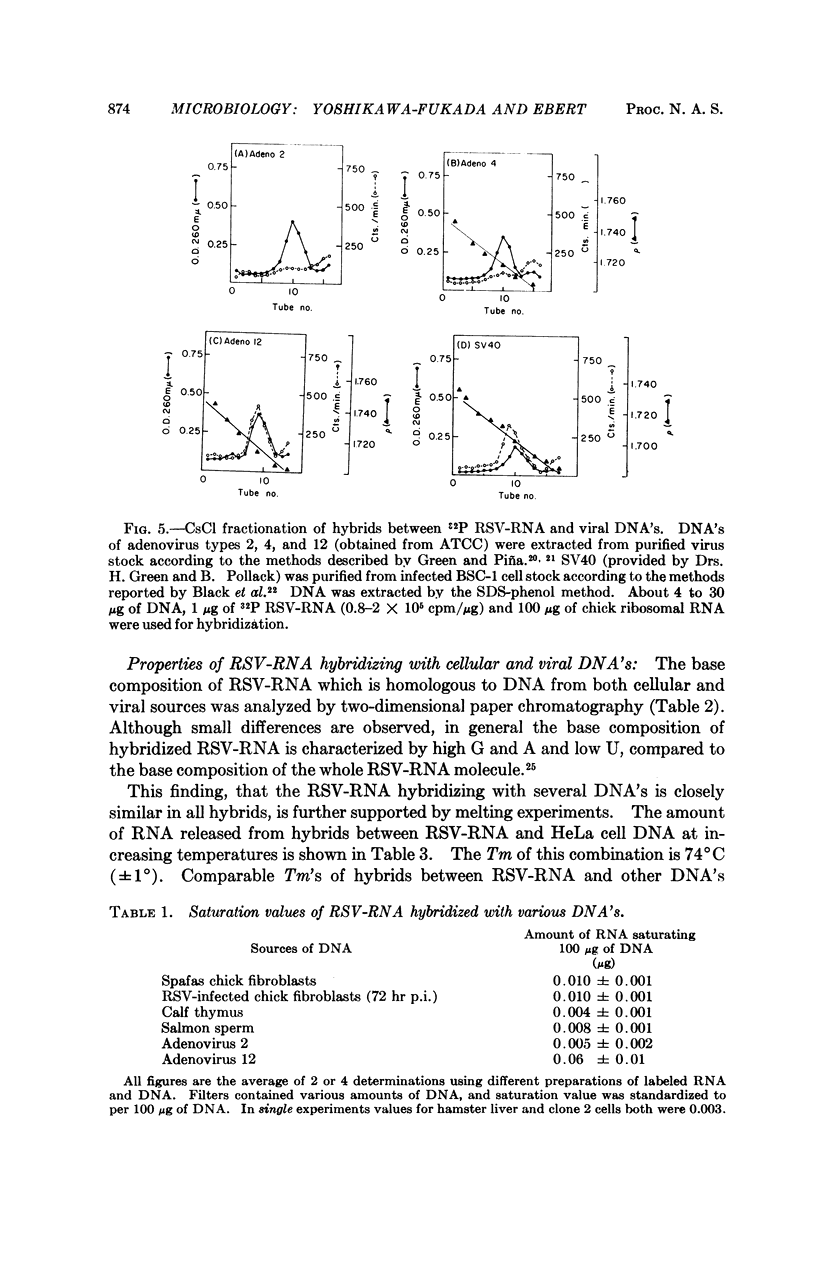
The RNA of Rous sarcoma virus (RSV) hybridizes with DNA's isolated from all eukaryotes tested (animal and plant) with the possible exception of yeast. Bacterial DNA's show no homology to RSV-RNA. DNA's from the oncogenic viruses, adenovirus type 12 and SV40 show significant homology with RSV-RNA, but those from nononcogenic adenoviruses types 2 and 4 are not homologous.
RSV-RNA hybridized to all of the DNA's examined has the same, or a closely similar, base composition. Moreover, the melting temperatures of all of the hybrids analyzed are very similar, further suggesting that a common base sequence in DNA functions in hybridization with RSV-RNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Huang P. C., Kabat S. Recognition of ribosomal RNA sites in DNA. II. The HeLa cell system. Proc Natl Acad Sci U S A. 1965 Jul;54(1):185–192. doi: 10.1073/pnas.54.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK P. H., CRAWFORD E. M., CRAWFORD L. V. THE PURIFICATION OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:381–387. doi: 10.1016/0042-6822(64)90175-8. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Weber C. S. Gene linkage by RNA-DNA hybridization. I. Unique DNA sequences homologous to 4 s RNA, 5 s RNA and ribosomal RNA. J Mol Biol. 1968 Jun 28;34(3):661–680. doi: 10.1016/0022-2836(68)90188-5. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., HARTWELL L. H., VOGT M. INDUCTION OF CELLULAR DNA SYNTHESIS BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Feb;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel L., Harel J., Lacour F., Huppert J. Homologie entre génome du virus de la myéloblastose aviare (AMV) et génome cellulaire. C R Acad Sci Hebd Seances Acad Sci D. 1966 Aug 8;263(6):616–619. [PubMed] [Google Scholar]
- Henry P., Black P. H., Oxman M. N., Weissman S. M. Stimulation of DNA synthesis in mouse cell line 3T3 by Simian virus 40. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1170–1176. doi: 10.1073/pnas.56.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson V., Earnhardt J., Chalkley R. A DNA-lipid protein containing material isolated from calf thymus nuclear chromatin. Biochem Biophys Res Commun. 1968 Oct 24;33(2):253–259. doi: 10.1016/0006-291x(68)90777-8. [DOI] [PubMed] [Google Scholar]
- Kaighn M. E., Ebert J. D., Stott P. M. The susceptibility of differentiating muscle clones to Rous sarcoma virus. Proc Natl Acad Sci U S A. 1966 Jul;56(1):133–140. doi: 10.1073/pnas.56.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubinski H., Rose J. A. Regions containing repeating base-pairs in DNA from some oncogenic and nononcogenic animal viruses. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1720–1725. doi: 10.1073/pnas.57.6.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. H., Kaighn M. E., Ebert J. D. Induction of thymidine-3H incorporation in multinucleated myotubes by Rous sarcoma virus. Int J Cancer. 1968 Jan 15;3(1):126–136. doi: 10.1002/ijc.2910030115. [DOI] [PubMed] [Google Scholar]
- MCCONKEY E. H., HOPKINS J. W. THE RELATIONSHIP OF THE NUCLEOLUS TO THE SYNTHESIS OF RIBOSOMAL RNA IN HELA CELLS. Proc Natl Acad Sci U S A. 1964 Jun;51:1197–1204. doi: 10.1073/pnas.51.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macieira-Coelho A., Pontén J. Induction of the division cycle in resting stage human fibroblasts after RSV infection. Biochem Biophys Res Commun. 1967 Nov 17;29(3):316–321. doi: 10.1016/0006-291x(67)90455-x. [DOI] [PubMed] [Google Scholar]
- Niyogi S. K., Thomas C. A., Jr The specific association of ribooligonucleotides of known chain length with denatured DNA. Biochem Biophys Res Commun. 1967 Jan 10;26(1):51–57. doi: 10.1016/0006-291x(67)90251-3. [DOI] [PubMed] [Google Scholar]
- Reich P. R., Black P. H., Weissman S. M. Nucleic acid homology studies of SV 40 virus-transformed and normal hamster cells. Proc Natl Acad Sci U S A. 1966 Jul;56(1):78–85. doi: 10.1073/pnas.56.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L. Isolation of noninfectious particles containing Rous sarcoma virus RNA from the medium of Rous sarcoma virus-transformed nonproducer cells. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1655–1662. doi: 10.1073/pnas.57.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer G., Defendi V. Stimulation of DNA synthesis and complement-fixing antigen production by SV40 in human diploid cell cultures: evidence for "abortive" infection. Proc Natl Acad Sci U S A. 1966 Aug;56(2):452–457. doi: 10.1073/pnas.56.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin R. DNA synthesis in rat embryo cells infected with polyoma virus. Virology. 1966 May;29(1):167–170. doi: 10.1016/0042-6822(66)90206-6. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. HOMOLOGY BETWEEN RNA FROM ROUS SARCOMA VIROUS AND DNA FROM ROUS SARCOMA VIRUS-INFECTED CELLS. Proc Natl Acad Sci U S A. 1964 Aug;52:323–329. doi: 10.1073/pnas.52.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M., Dulbecco R., Smith B. Induction of cellular DNA synthesis by polyoma virus. 3. Induction in productively infected cells. Proc Natl Acad Sci U S A. 1966 Apr;55(4):956–960. doi: 10.1073/pnas.55.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal H., Dulbecco R. Viral DNA in polyoma- and SV40-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1158–1165. doi: 10.1073/pnas.59.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E. On the apparent homology between DNA from polyoma virus and normal mouse synthetic RNA. Virology. 1967 Jan;31(1):15–28. doi: 10.1016/0042-6822(67)90003-7. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Gershon D. Multinucleated muscle fibres: induction of DNA synthesis and mitosis by polyoma virus infection. Nature. 1967 Jul 22;215(5099):421–424. doi: 10.1038/215421a0. [DOI] [PubMed] [Google Scholar]



