Abstract
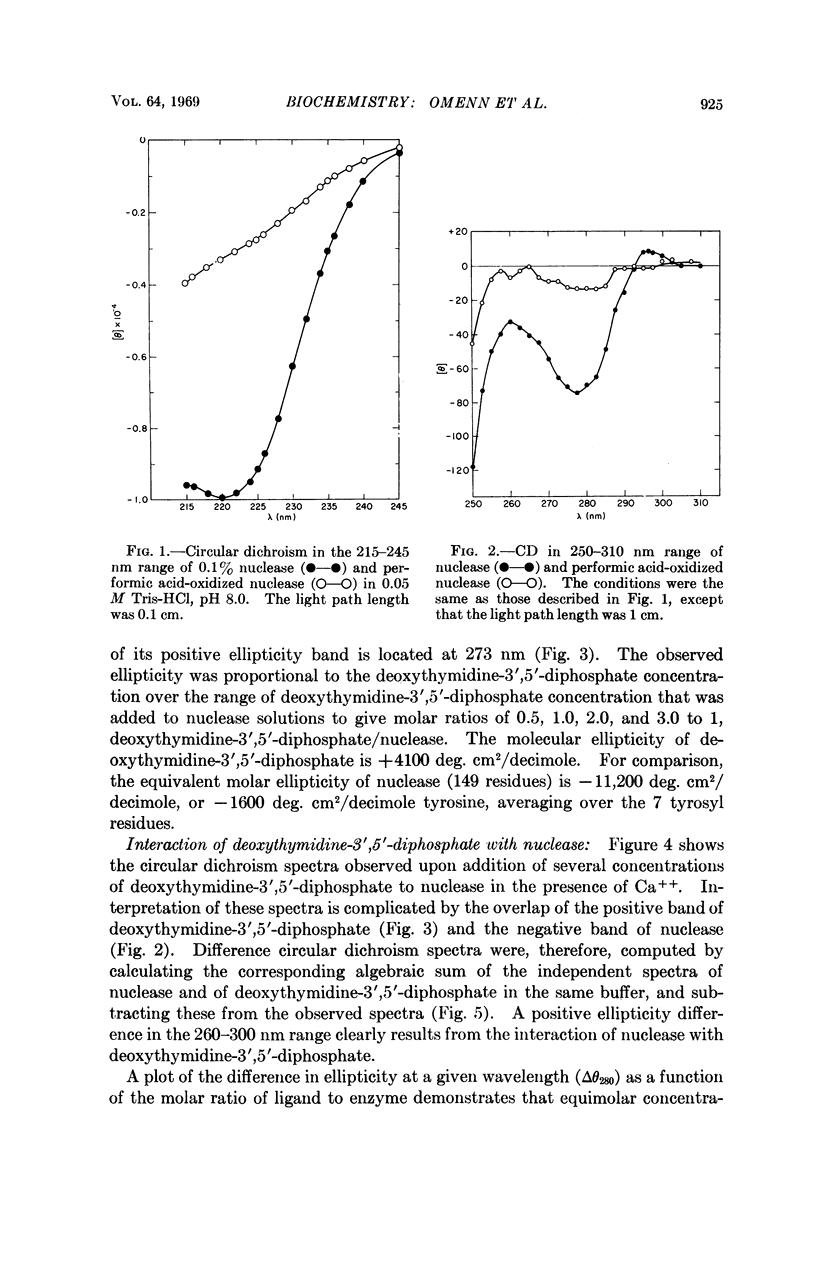
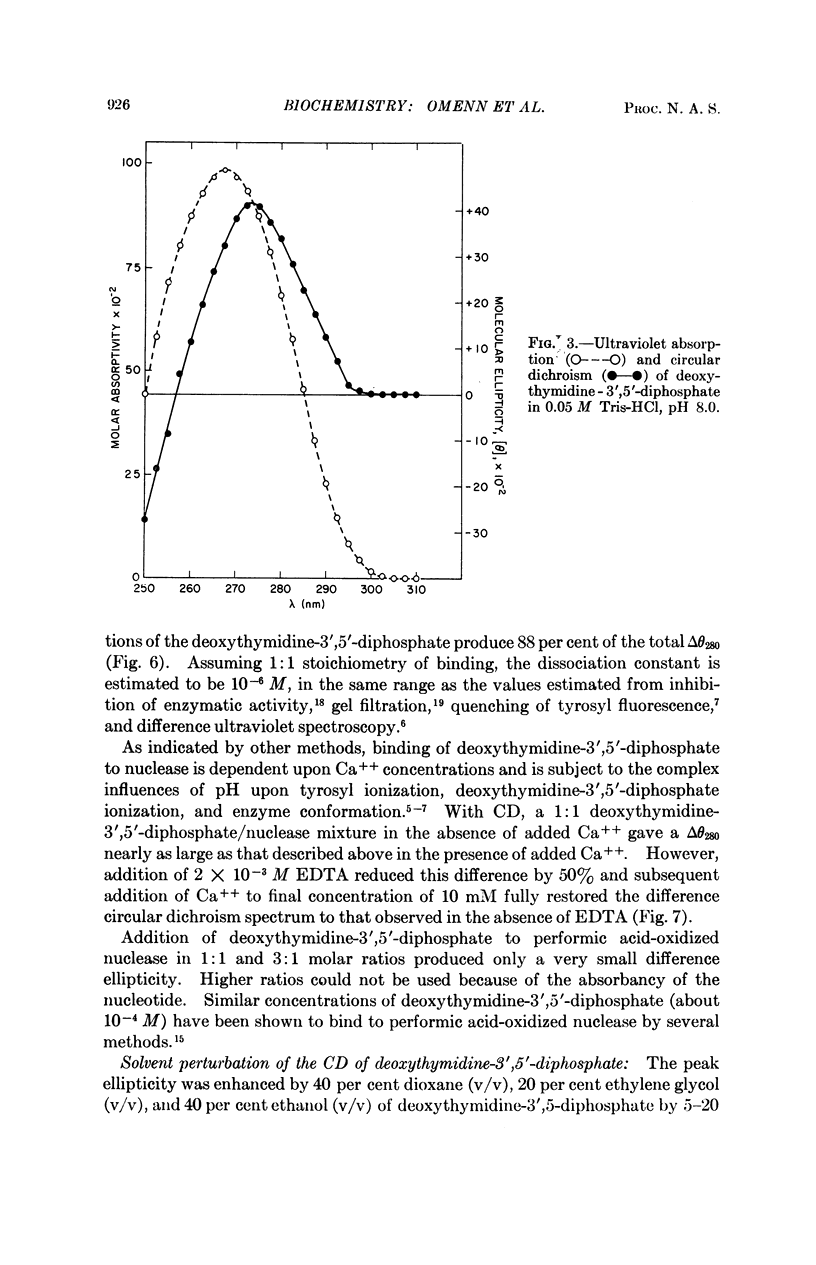
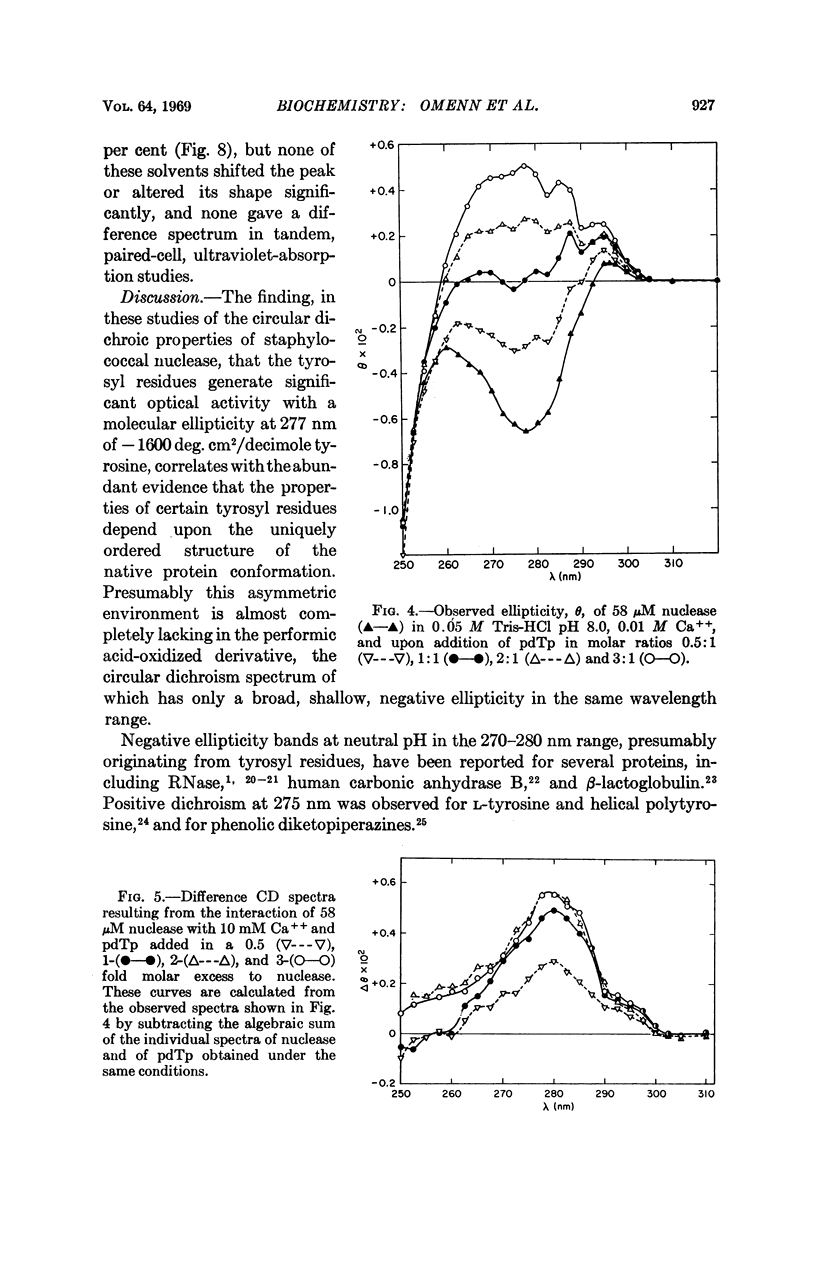
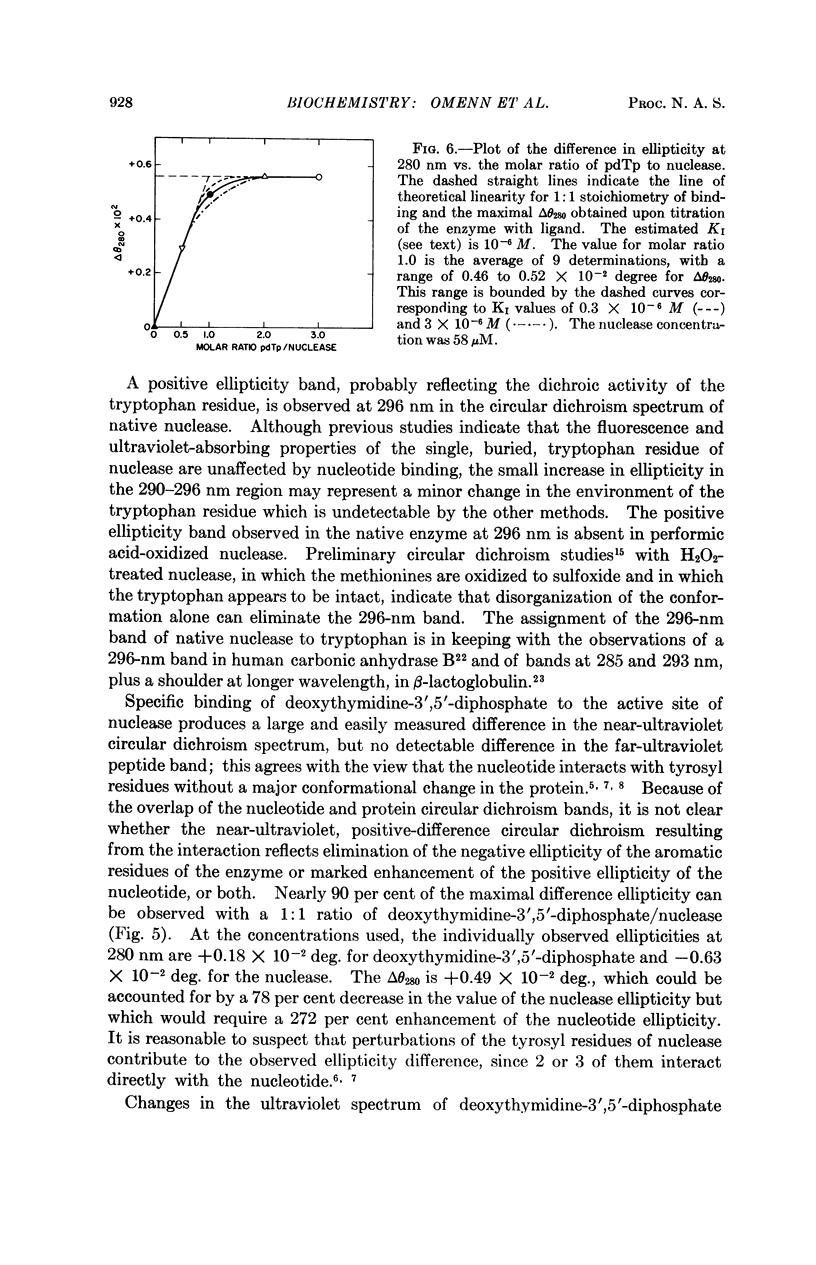
Specific contributions of tyrosyl and of tryptophanyl residues can be distinguished in the near-ultraviolet circular dichroic spectrum of staphylococcal nuclease. Upon binding of the inhibitor deoxythymidine 3′,5′-diphosphate in the presence of Ca++, a significant change in the circular dichroic spectrum results which has been used to characterize the interaction of ligand and enzyme. The data suggest that the asymmetric environment of certain tyrosyl residues is altered by binding of the nucleotide inhibitor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnone A., Bier C. J., Cotton F. A., Hazen E. E., Jr, Richardson D. C., Richardson J. S. The extracellular nuclease of Staphylococcus aureus: structures of the native enzyme and an enzyme-inhibitor complex at 4 A resolution. Proc Natl Acad Sci U S A. 1969 Oct;64(2):420–427. doi: 10.1073/pnas.64.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEYCHOK S., FASMAN G. D. CIRCULAR DICHROISM OF POLY-L-TYROSINE. Biochemistry. 1964 Nov;3:1675–1678. doi: 10.1021/bi00899a012. [DOI] [PubMed] [Google Scholar]
- Beychok S., Armstrong J. M., Lindblow C., Edsall J. T. Optil rotatory dispersion and circular dichroism of human carbonic anhydrases B and C. J Biol Chem. 1966 Nov 10;241(21):5150–5160. [PubMed] [Google Scholar]
- Beychok S. Circular dichroism of biological macromolecules. Science. 1966 Dec 9;154(3754):1288–1299. doi: 10.1126/science.154.3754.1288. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Edelhoch H., Anfinsen C. B. Fluorescence studies of the interaction of nucleotides with the active site of the nuclease of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2043–2050. doi: 10.1073/pnas.58.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Fuchs S., Anfinsen C. B. Catalytic properties and specificity of the extracellular nuclease of Staphylococcus aureus. J Biol Chem. 1967 Apr 10;242(7):1541–1547. [PubMed] [Google Scholar]
- Cuatrecasas P., Fuchs S., Anfinsen C. B. The interaction of nucleotides with the active site of staphylococcal nuclease. Spectrophotometric studies. J Biol Chem. 1967 Oct 25;242(20):4759–4767. [PubMed] [Google Scholar]
- Cuatrecasas P., Fuchs S., Anfinsen C. B. The tyrosyl residues at the active site of staphylococcal nuclease. Modifications by tetranitromethane. J Biol Chem. 1968 Sep 25;243(18):4787–4798. [PubMed] [Google Scholar]
- Cuatrecasas P., Taniuchi H., Anfinsen C. B. The structural basis of the catalytic function of staphylococcal nuclease. Brookhaven Symp Biol. 1968 Jun;21(1):172–200. [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Affinity labeling of the active site of staphylococcal nuclease. Reactions with bromoacetylated substrate analogues. J Biol Chem. 1969 Aug 25;244(16):4316–4329. [PubMed] [Google Scholar]
- Edelhoch H., Lippoldt R. E., Wilchek M. The circular dichroism of tryosyl and tryptophanyl diketopiperazines. J Biol Chem. 1968 Sep 25;243(18):4799–4805. [PubMed] [Google Scholar]
- Gratzer W. B., Cowburn D. A. Optical activity of biopolymers. Nature. 1969 May 3;222(5192):426–431. doi: 10.1038/222426a0. [DOI] [PubMed] [Google Scholar]
- Markley J. L., Putter I., Jardetzky O. High-resolution nuclear magnetic resonance spectra of selectively deuterated staphylococcal nuclease. Science. 1968 Sep 20;161(3847):1249–1251. doi: 10.1126/science.161.3847.1249. [DOI] [PubMed] [Google Scholar]
- Morávek L., Anfinsen C. B., Cone J. L., Taniuchi H. The large scale preparation of an extracellular nuclease of Staphylococcus aureus. J Biol Chem. 1969 Jan 25;244(2):497–499. [PubMed] [Google Scholar]
- Schechter A. N., Morávek L., Anfinsen C. B. Suppression of hydrogen exchange in staphylococcal nuclease by ligands. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1478–1485. doi: 10.1073/pnas.61.4.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons N. S., Glazer A. N. An analysis of the tyrosine circular dichroism bands in ribonuclease. J Am Chem Soc. 1967 Sep 13;89(19):5040–5042. doi: 10.1021/ja00995a037. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Vallee B. L. Side-chain Cotton effects of ribonuclease. Biochemistry. 1966 Aug;5(8):2531–2538. doi: 10.1021/bi00872a007. [DOI] [PubMed] [Google Scholar]
- Taniuchi H., Anfinsen C. B. Steps in the formation of active derivatives of staphylococcal nuclease during trypsin digestion. J Biol Chem. 1968 Sep 25;243(18):4778–4786. [PubMed] [Google Scholar]


