Abstract
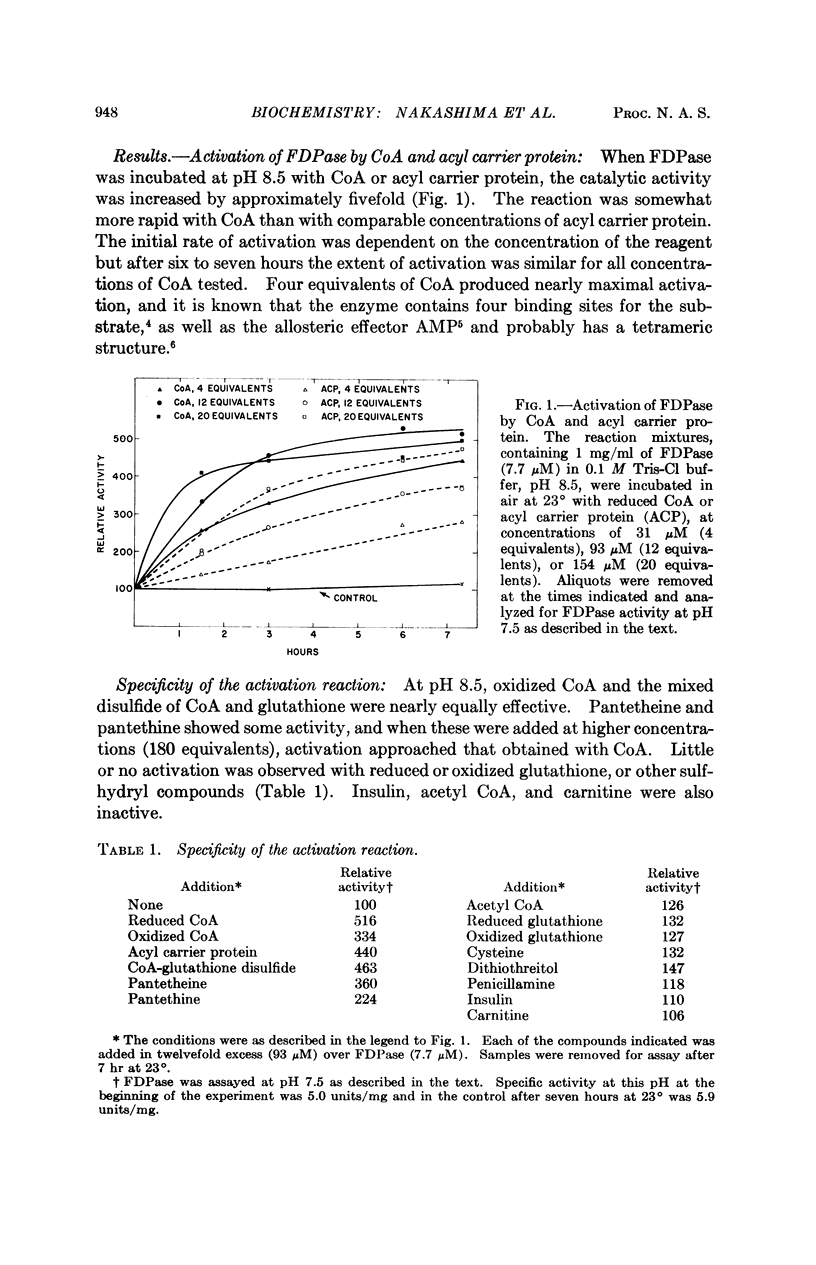
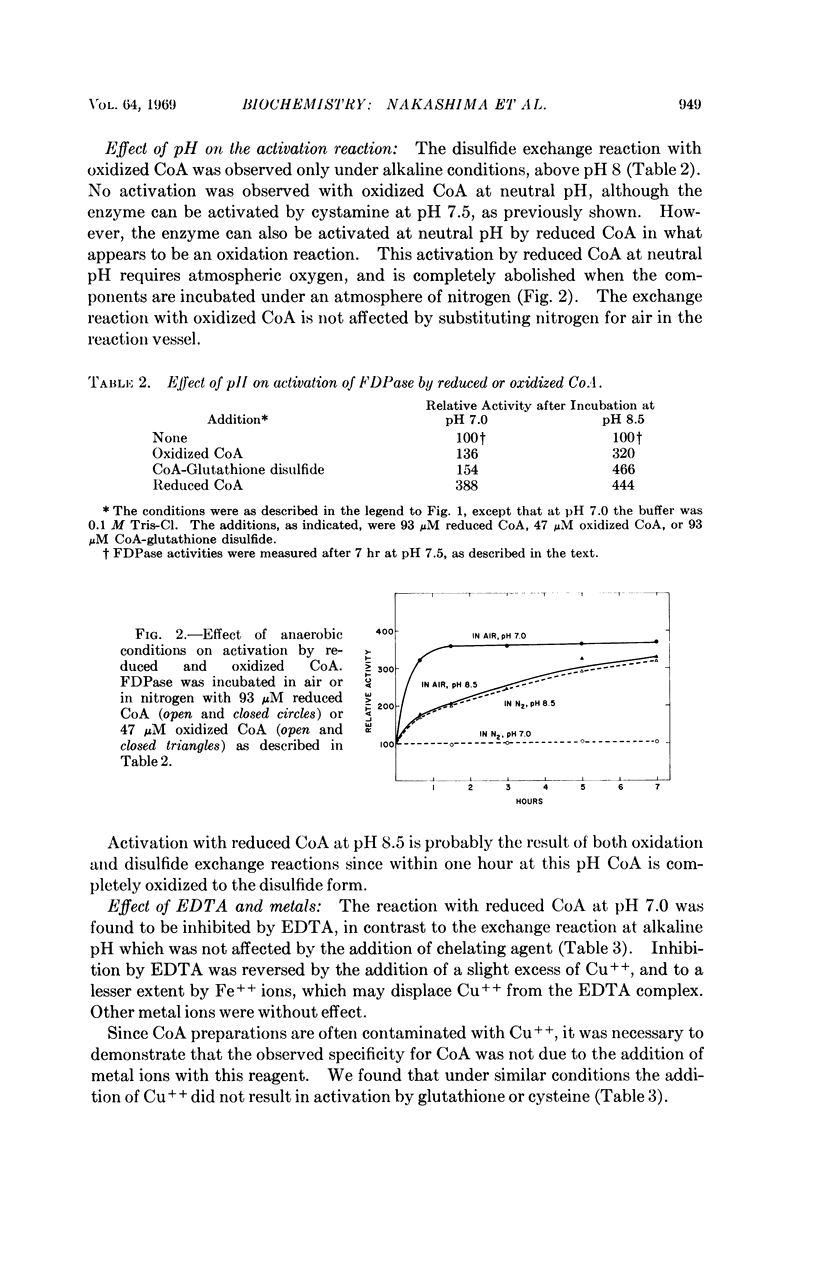
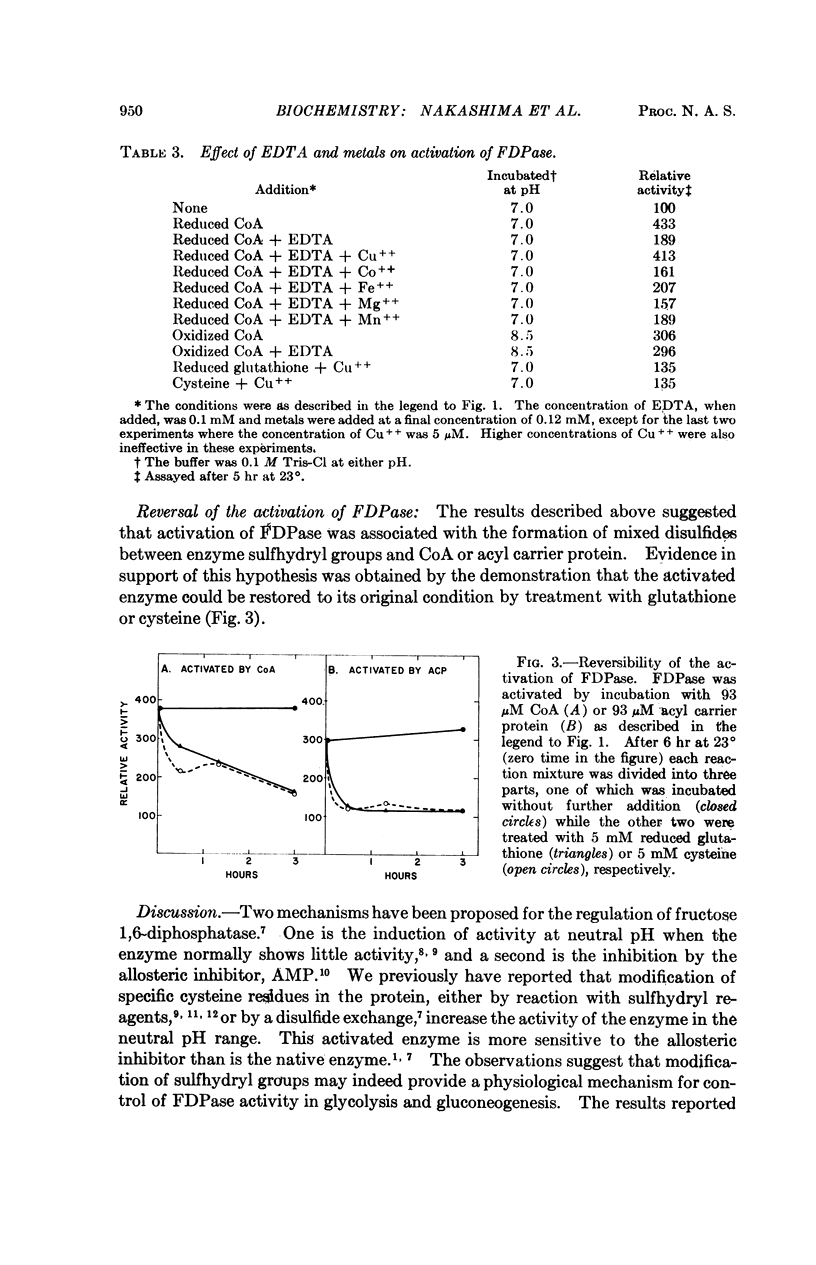
The catalytic activity of rabbit liver fructose diphosphatase is enhanced more than fourfold by treatment with coenzyme A or acyl carrier protein. Other sulfhydryl compounds, such as glutathione or cysteine, are without effect. Activation is reversed by reduced glutathione or cysteine, indicating that the enzyme and activator are linked by disulfide bridges. The activated enzyme derivative can be formed by a disulfide exchange reaction at alkaline pH or by an oxidation reaction which proceeds at neutral pH. The latter process requires O2 and Cu++, and is inhibited by EDTA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chesterton C. J., Butterworth P. H., Abramovitz A. S., Jacob E. J., Porter J. W. Incorporation of pantothenate-1-14C into pigeon liver fatty acid synthetase: 4'-phosphopantetheine, a prosthetic group of the multienzyme complex. Arch Biochem Biophys. 1968 Mar 20;124(1):386–391. doi: 10.1016/0003-9861(68)90342-1. [DOI] [PubMed] [Google Scholar]
- Horecker B. L., Pontremoli S., Rosen O., Rosen R. Structure and function in fructose diphosphatase. Fed Proc. 1966 Sep-Oct;25(5):1521–1528. [PubMed] [Google Scholar]
- Larrabee A. R., McDaniel E. G., Bakerman H. A., Vagelos P. R. Acyl carrier protein. V. Identification of 4'-phosphopantetheine bound to a mammalian fatty acid synthetase preparation. Proc Natl Acad Sci U S A. 1965 Jul;54(1):267–273. doi: 10.1073/pnas.54.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Grazi E., Accorsi A. Fructose diphosphatase from rabbit liver. IX. Isolation and kinetic properties of the enzyme-substrate complex. Biochemistry. 1968 May;7(5):1655–1661. doi: 10.1021/bi00845a006. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Luppis B., Traniello S., Rippa M., Horecker B. L. Fructose diphosphatase from rabbit liver. IV. Sulfhydryl groups and their relation to the catalytic activity. Arch Biochem Biophys. 1965 Oct;112(1):7–15. doi: 10.1016/0003-9861(65)90003-2. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Luppis B., Traniello S., Wood W. A., Horecker B. L. Fructose diphosphatase from rabbit liver. 3. Nature of the groups reactive with dinitrofluorobenzene. J Biol Chem. 1965 Sep;240(9):3469–3472. [PubMed] [Google Scholar]
- Pontremoli S., Luppis B., Wood W. A., Traniello S., Horecker B. L. Fructose diphosphatase from rabbit liver. II. Changes in catalytic properties induced by dinitrofluorobenzene. J Biol Chem. 1965 Sep;240(9):3464–3468. [PubMed] [Google Scholar]
- Pontremoli S., Traniello S., Enser M., Shapiro S., Horecker B. L. Regulation of fructose diphosphatase activity by disulfide exchange. Proc Natl Acad Sci U S A. 1967 Jul;58(1):286–293. doi: 10.1073/pnas.58.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Traniello S., Luppis B., Wood W. A. Fructose diphosphatase from rabbit liver. I. Purification and properties. J Biol Chem. 1965 Sep;240(9):3459–3463. [PubMed] [Google Scholar]
- Sia C. L., Traniello S., Pontremoli S., Horecker B. L. Studies on the subunit structure of rabbit liver fructose diphosphatase. Arch Biochem Biophys. 1969 Jun;132(1):325–330. doi: 10.1016/0003-9861(69)90369-5. [DOI] [PubMed] [Google Scholar]
- TAKETA K., POGELL B. M. ALLOSTERIC INHIBITION OF RAT LIVER FRUCTOSE 1,6-DIPHOSPHATASE BY ADENOSINE 5'-MONOPHOSPHATE. J Biol Chem. 1965 Feb;240:651–662. [PubMed] [Google Scholar]



